MAST Kinases’ Function and Regulation: Insights from Structural Modeling and Disease Mutations
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylogenetic Analysis
2.2. Multiple Sequence Alignment
2.3. In Silico Structural Modeling
2.4. Mutational Consequence Estimation
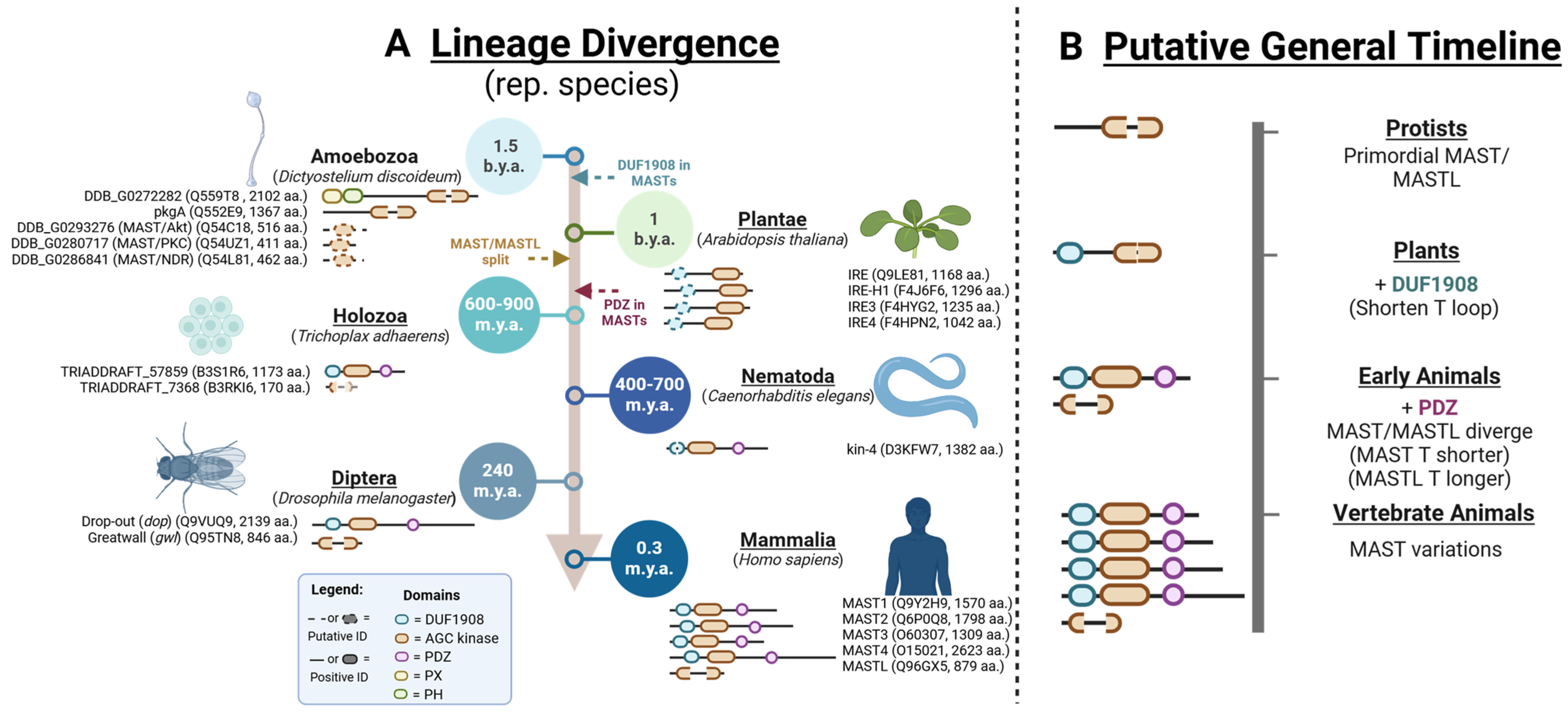
3. The Origins and Divergence of MAST Kinases
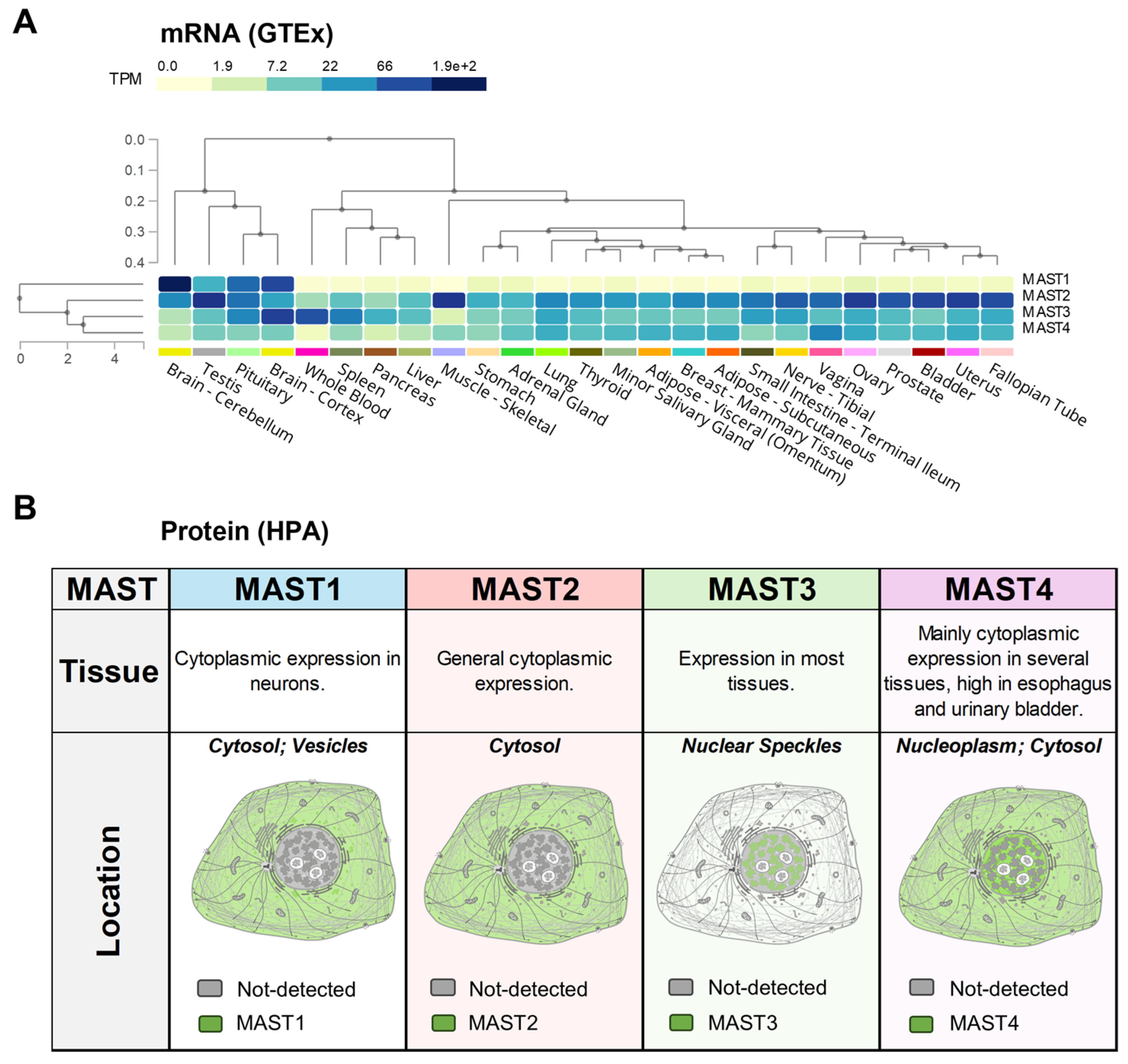
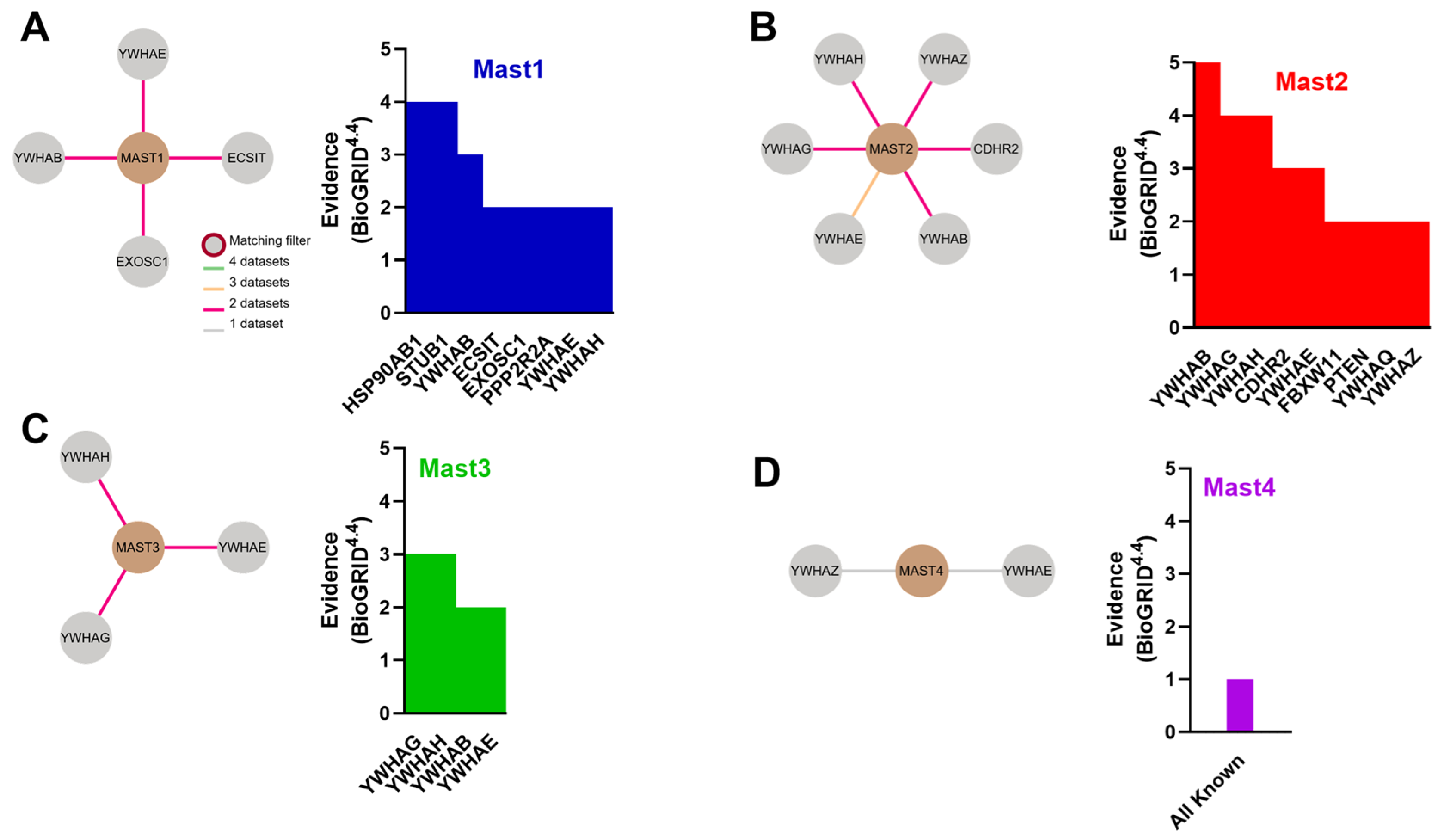
4. MAST Kinase Expression and Interactomes
5. Point Mutation In Silico Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schioth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Janne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Gizzio, J.; Thakur, A.; Haldane, A.; Levy, R.M. Evolutionary divergence in the conformational landscapes of tyrosine vs. serine/threonine kinases. eLife 2022, 11, e83368. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wilson, B.A.P.; Li, N.; Shah, R.; Dalilian, M.; Wang, D.; Smith, E.A.; Wamiru, A.; Goncharova, E.I.; Zhang, P.; et al. Discovery and Synthesis of a Naturally Derived Protein Kinase Inhibitor that Selectively Inhibits Distinct Classes of Serine/Threonine Kinases. J. Nat. Prod. 2023, 86, 2283–2293. [Google Scholar] [CrossRef]
- Maoz, A.; Ciccone, M.A.; Matsuzaki, S.; Coleman, R.L.; Matsuo, K. Emerging serine-threonine kinase inhibitors for treating ovarian cancer. Expert Opin. Emerg. Drugs 2019, 24, 239–253. [Google Scholar] [CrossRef]
- Kustatscher, G.; Collins, T.; Gingras, A.C.; Guo, T.; Hermjakob, H.; Ideker, T.; Lilley, K.S.; Lundberg, E.; Marcotte, E.M.; Ralser, M.; et al. Understudied proteins: Opportunities and challenges for functional proteomics. Nat. Methods 2022, 19, 774–779. [Google Scholar] [CrossRef]
- Kustatscher, G.; Collins, T.; Gingras, A.C.; Guo, T.; Hermjakob, H.; Ideker, T.; Lilley, K.S.; Lundberg, E.; Marcotte, E.M.; Ralser, M.; et al. An open invitation to the Understudied Proteins Initiative. Nat. Biotechnol. 2022, 40, 815–817. [Google Scholar] [CrossRef]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, J.M.; Pastor-Flores, D.; Bauer, A.F.; Schulze, J.O.; Biondi, R.M. AGC protein kinases: From structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim. Biophys. Acta 2013, 1834, 1302–1321. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Ackloo, S.; Al Chawaf, A.; Al-Lazikani, B.; Antolin, A.; Baell, J.B.; Beck, H.; Beedie, S.; Betz, U.A.K.; Bezerra, G.A.; et al. Target 2035—Update on the quest for a probe for every protein. RSC Med. Chem. 2022, 13, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, T.; Gerlach, M.; Morimoto, R.I.; Nunes Amaral, L.A. Large-scale investigation of the reasons why potentially important genes are ignored. PLoS Biol. 2018, 16, e2006643. [Google Scholar] [CrossRef]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2024, 53, D609–D617. [Google Scholar] [CrossRef]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2024, 53, D444–D456. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- wwPDB Consortium. Protein Data Bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019, 47, D520–D528. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Ferla, M.P.; Pagnamenta, A.T.; Damerell, D.; Taylor, J.C.; Marsden, B.D. MichelaNglo: Sculpting protein views on web pages without coding. Bioinformatics 2020, 36, 3268–3270. [Google Scholar] [CrossRef]
- Ferla, M.P.; Pagnamenta, A.T.; Koukouflis, L.; Taylor, J.C.; Marsden, B.D. Venus: Elucidating the Impact of Amino Acid Variants on Protein Function Beyond Structure Destabilisation. J. Mol. Biol. 2022, 434, 167567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pan, Q.; Pires, D.E.V.; Rodrigues, C.H.M.; Ascher, D.B. DDMut: Predicting effects of mutations on protein stability using deep learning. Nucleic Acids Res. 2023, 51, W122–W128. [Google Scholar] [CrossRef]
- Rumpf, M.; Pautz, S.; Drebes, B.; Herberg, F.W.; Muller, H.J. Microtubule-Associated Serine/Threonine (MAST) Kinases in Development and Disease. Int. J. Mol. Sci. 2023, 24, 11913. [Google Scholar] [CrossRef]
- Bogre, L.; Okresz, L.; Henriques, R.; Anthony, R.G. Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 2003, 8, 424–431. [Google Scholar] [CrossRef]
- Jiang, Y.; Meyers, T.J.; Emeka, A.A.; Cooley, L.F.; Cooper, P.R.; Lancki, N.; Helenowski, I.; Kachuri, L.; Lin, D.W.; Stanford, J.L.; et al. Genetic Factors Associated with Prostate Cancer Conversion from Active Surveillance to Treatment. HGG Adv. 2022, 3, 100070. [Google Scholar] [CrossRef]
- Adams, J.A. Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 2001, 101, 2271–2290. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.E.; Schulze, J.O.; Biondi, R.M. AGC kinases, mechanisms of regulation and innovative drug development. Semin. Cancer Biol. 2018, 48, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Yaron, T.M.; Huntsman, E.M.; Kerelsky, A.; Song, J.; Regev, A.; Lin, T.Y.; Liberatore, K.; Cizin, D.M.; Cohen, B.M.; et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature 2023, 613, 759–766. [Google Scholar] [CrossRef]
- An, S.W.A.; Choi, E.S.; Hwang, W.; Son, H.G.; Yang, J.S.; Seo, K.; Nam, H.J.; Nguyen, N.T.H.; Kim, E.J.E.; Suh, B.K.; et al. KIN-4/MAST kinase promotes PTEN-mediated longevity of Caenorhabditis elegans via binding through a PDZ domain. Aging Cell 2019, 18, e12906. [Google Scholar] [CrossRef]
- Hain, D.; Langlands, A.; Sonnenberg, H.C.; Bailey, C.; Bullock, S.L.; Muller, H.A. The Drosophila MAST kinase Drop out is required to initiate membrane compartmentalisation during cellularisation and regulates dynein-based transport. Development 2014, 141, 2119–2130. [Google Scholar] [CrossRef]
- Blake-Hodek, K.A.; Williams, B.C.; Zhao, Y.; Castilho, P.V.; Chen, W.; Mao, Y.; Yamamoto, T.M.; Goldberg, M.L. Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell Biol. 2012, 32, 1337–1353. [Google Scholar] [CrossRef]
- Hermida, D.; Mortuza, G.B.; Pedersen, A.K.; Pozdnyakova, I.; Nguyen, T.; Maroto, M.; Williamson, M.; Ebersole, T.; Cazzamali, G.; Rand, K.; et al. Molecular Basis of the Mechanisms Controlling MASTL. Mol. Cell Proteom. 2020, 19, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Erguven, M.; Kilic, S.; Karaca, E.; Diril, M.K. Genetic complementation screening and molecular docking give new insight on phosphorylation-dependent Mastl kinase activation. J. Biomol. Struct. Dyn. 2023, 41, 8241–8253. [Google Scholar] [CrossRef]
- Marzec, K.; Burgess, A. The Oncogenic Functions of MASTL Kinase. Front. Cell Dev. Biol. 2018, 6, 162. [Google Scholar] [CrossRef]
- Archambault, V.; Zhao, X.; White-Cooper, H.; Carpenter, A.T.; Glover, D.M. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 2007, 3, e200. [Google Scholar] [CrossRef]
- Gharbi-Ayachi, A.; Labbe, J.C.; Burgess, A.; Vigneron, S.; Strub, J.M.; Brioudes, E.; Van-Dorsselaer, A.; Castro, A.; Lorca, T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010, 330, 1673–1677. [Google Scholar] [CrossRef]
- Marzec, K.A.; Rogers, S.; McCloy, R.; Parker, B.L.; James, D.E.; Watkins, D.N.; Burgess, A. SILAC kinase screen identifies potential MASTL substrates. Sci. Rep. 2022, 12, 10568. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Maslen, S.L.; Skehel, M.; Hunt, T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010, 330, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Terrien, E.; Chaffotte, A.; Lafage, M.; Khan, Z.; Prehaud, C.; Cordier, F.; Simenel, C.; Delepierre, M.; Buc, H.; Lafon, M.; et al. Interference with the PTEN-MAST2 interaction by a viral protein leads to cellular relocalization of PTEN. Sci. Signal 2012, 5, ra58. [Google Scholar] [CrossRef] [PubMed]
- Valiente, M.; Andres-Pons, A.; Gomar, B.; Torres, J.; Gil, A.; Tapparel, C.; Antonarakis, S.E.; Pulido, R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J. Biol. Chem. 2005, 280, 28936–28943. [Google Scholar] [CrossRef]
- Martinez-Rubio, D.; Hinarejos, I.; Argente-Escrig, H.; Marco-Marin, C.; Lozano, M.A.; Gorria-Redondo, N.; Lupo, V.; Marti-Carrera, I.; Miranda, C.; Vazquez-Lopez, M.; et al. Genetic Heterogeneity Underlying Phenotypes with Early-Onset Cerebellar Atrophy. Int. J. Mol. Sci. 2023, 24, 16400. [Google Scholar] [CrossRef]
- Yi, S.; Tang, X.; Chen, F.; Wang, L.; Chen, J.; Yang, Z.; Huang, M.; Yi, S.; Huang, L.; Yang, Q.; et al. A genetic variant in the MAST1 gene is associated with mega-corpus-callosum syndrome with hypoplastic cerebellar vermis, in a fetus. Mol. Genet. Genom. Med. 2024, 12, e2358. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Cao, Y.; Peng, Y.; Lian, A.; Chen, Y.; Wang, P.; Gu, W.; Xiao, B.; Yu, J.; et al. De novo variants in MAST4 related to neurodevelopmental disorders with developmental delay and infantile spasms: Genotype-phenotype association. Front. Mol. Neurosci. 2023, 16, 1097553. [Google Scholar] [CrossRef]
- Ringrose, J.H.; van den Toorn, H.W.; Eitel, M.; Post, H.; Neerincx, P.; Schierwater, B.; Altelaar, A.F.; Heck, A.J. Deep proteome profiling of Trichoplax adhaerens reveals remarkable features at the origin of metazoan multicellularity. Nat. Commun. 2013, 4, 1408. [Google Scholar] [CrossRef]
- Chudinova, E.M.; Karpov, P.A.; Fokin, A.I.; Yemets, A.I.; Lytvyn, D.I.; Nadezhdina, E.S.; Blume, Y.B. MAST-like protein kinase IREH1 from Arabidopsis thaliana co-localizes with the centrosome when expressed in animal cells. Planta 2017, 246, 959–969. [Google Scholar] [CrossRef]
- Fey, P.; Dodson, R.J.; Basu, S.; Chisholm, R.L. One stop shop for everything Dictyostelium: dictyBase and the Dicty Stock Center in 2012. Methods Mol. Biol. 2013, 983, 59–92. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.M.; Manning, G.; Liu, A.; Fey, P.; Pilcher, K.E.; Xu, Y.; Smith, J.L. The dictyostelium kinome--analysis of the protein kinases from a simple model organism. PLoS Genet. 2006, 2, e38. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.; Dunbrack, R.L., Jr. Defining a new nomenclature for the structures of active and inactive kinases. Proc. Natl. Acad. Sci. USA 2019, 116, 6818–6827. [Google Scholar] [CrossRef]
- Levinson, N.M.; Kuchment, O.; Shen, K.; Young, M.A.; Koldobskiy, M.; Karplus, M.; Cole, P.A.; Kuriyan, J. A Src-like inactive conformation in the abl tyrosine kinase domain. PLoS Biol. 2006, 4, e144. [Google Scholar] [CrossRef]
- Miller, C.J.; Turk, B.E. Homing in: Mechanisms of Substrate Targeting by Protein Kinases. Trends Biochem. Sci. 2018, 43, 380–394. [Google Scholar] [CrossRef]
- Garland, P.; Quraishe, S.; French, P.; O’Connor, V. Expression of the MAST family of serine/threonine kinases. Brain Res. 2008, 1195, 12–19. [Google Scholar] [CrossRef]
- Tripathy, R.; Leca, I.; van Dijk, T.; Weiss, J.; van Bon, B.W.; Sergaki, M.C.; Gstrein, T.; Breuss, M.; Tian, G.; Bahi-Buisson, N.; et al. Mutations in MAST1 Cause Mega-Corpus-Callosum Syndrome with Cerebellar Hypoplasia and Cortical Malformations. Neuron 2018, 100, 1354–1368 e1355. [Google Scholar] [CrossRef]
- Karapurkar, J.K.; Colaco, J.C.; Suresh, B.; Tyagi, A.; Woo, S.H.; Jo, W.J.; Ko, N.; Singh, V.; Hong, S.H.; Oh, S.J.; et al. USP28 promotes tumorigenesis and cisplatin resistance by deubiquitinating MAST1 protein in cancer cells. Cell Mol. Life Sci. 2024, 81, 145. [Google Scholar] [CrossRef]
- Pan, C.; Chun, J.; Li, D.; Boese, A.C.; Li, J.; Kang, J.; Umano, A.; Jiang, Y.; Song, L.; Magliocca, K.R.; et al. Hsp90B enhances MAST1-mediated cisplatin resistance by protecting MAST1 from proteosomal degradation. J. Clin. Investig. 2019, 129, 4110–4123. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, J.; Wang, W.; Ma, H.; Yang, Y. E3 Ubiquitin Ligase CHIP Inhibits the Interaction between Hsp90β and MAST1 to Repress Radiation Resistance in Non-Small-Cell Lung Cancer Stem Cells. Stem Cells Int. 2022, 2022, 2760899. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Kaushal, K.; Chandrasekaran, A.P.; Sarodaya, N.; Das, S.; Park, C.H.; Hong, S.H.; Kim, K.S.; Ramakrishna, S. CRISPR/Cas9-based genome-wide screening for deubiquitinase subfamily identifies USP1 regulating MAST1-driven cisplatin-resistance in cancer cells. Theranostics 2022, 12, 5949–5970. [Google Scholar] [CrossRef]
- Walden, P.D.; Cowan, N.J. A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol. Cell Biol. 1993, 13, 7625–7635. [Google Scholar] [CrossRef]
- Huang, N.; Wen, Y.; Guo, X.; Li, Z.; Dai, J.; Ni, B.; Yu, J.; Lin, Y.; Zhou, W.; Yao, B.; et al. A Screen for Genomic Disorders of Infertility Identifies MAST2 Duplications Associated with Nonobstructive Azoospermia in Humans. Biol. Reprod. 2015, 93, 61. [Google Scholar] [CrossRef] [PubMed]
- Delhommel, F.; Chaffotte, A.; Terrien, E.; Raynal, B.; Buc, H.; Delepierre, M.; Cordier, F.; Wolff, N. Deciphering the unconventional peptide binding to the PDZ domain of MAST2. Biochem. J. 2015, 469, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, J.; Zhang, W.; Pan, L.; Zhang, D.; Zhao, P.; Wang, F.; Luo, H.; He, J.; Qin, Y.; et al. Young female patients with multiple myeloma have low occurrence of osteolytic lesion. Bone 2018, 110, 21–28. [Google Scholar] [CrossRef]
- Shu, L.; Xiao, N.; Qin, J.; Tian, Q.; Zhang, Y.; Li, H.; Liu, J.; Li, Q.; Gu, W.; Wang, P.; et al. The Role of Microtubule Associated Serine/Threonine Kinase 3 Variants in Neurodevelopmental Diseases: Genotype-Phenotype Association. Front. Mol. Neurosci. 2021, 14, 775479. [Google Scholar] [CrossRef]
- Spinelli, E.; Christensen, K.R.; Bryant, E.; Schneider, A.; Rakotomamonjy, J.; Muir, A.M.; Giannelli, J.; Littlejohn, R.O.; Roeder, E.R.; Schmidt, B.; et al. Pathogenic MAST3 Variants in the STK Domain Are Associated with Epilepsy. Ann. Neurol. 2021, 90, 274–284. [Google Scholar] [CrossRef]
- Andrade, E.C.; Musante, V.; Horiuchi, A.; Matsuzaki, H.; Brody, A.H.; Wu, T.; Greengard, P.; Taylor, J.R.; Nairn, A.C. ARPP-16 Is a Striatal-Enriched Inhibitor of Protein Phosphatase 2A Regulated by Microtubule-Associated Serine/Threonine Kinase 3 (Mast 3 Kinase). J. Neurosci. 2017, 37, 2709–2722. [Google Scholar] [CrossRef]
- Musante, V.; Li, L.; Kanyo, J.; Lam, T.T.; Colangelo, C.M.; Cheng, S.K.; Brody, A.H.; Greengard, P.; Le Novere, N.; Nairn, A.C. Reciprocal regulation of ARPP-16 by PKA and MAST3 kinases provides a cAMP-regulated switch in protein phosphatase 2A inhibition. eLife 2017, 6, e24998. [Google Scholar] [CrossRef]
- Kumm, E.J.; Pagel, O.; Gambaryan, S.; Walter, U.; Zahedi, R.P.; Smolenski, A.; Jurk, K. The Cell Cycle Checkpoint System MAST(L)-ENSA/ARPP19-PP2A is Targeted by cAMP/PKA and cGMP/PKG in Anucleate Human Platelets. Cells 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Castillo, B.; Hurtado, B.; Vara-Ciruelos, D.; El Bakkali, A.; Hermida, D.; Salvador-Barbero, B.; Martinez-Alonso, D.; Gonzalez-Martinez, J.; Santiveri, C.; Campos-Olivas, R.; et al. The MASTL/PP2A cell cycle kinase-phosphatase module restrains PI3K-Akt activity in an mTORC1-dependent manner. EMBO J. 2023, 42, e110833. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, F.; Zhang, D.; Huang, J.; Yang, Y.; Xu, J.; Gao, Y.; Ding, H.; Qu, Y.; Zhang, W.; et al. Estrogen-Responsive Gene MAST4 Regulates Myeloma Bone Disease. J. Bone Miner. Res. 2022, 37, 711–723. [Google Scholar] [CrossRef]
- Kim, P.; Park, J.; Lee, D.J.; Mizuno, S.; Shinohara, M.; Hong, C.P.; Jeong, Y.; Yun, R.; Park, H.; Park, S.; et al. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Sakaji, K.; Ebrahimiazar, S.; Harigae, Y.; Ishibashi, K.; Sato, T.; Yoshikawa, T.; Atsumi, G.I.; Sung, C.H.; Saito, M. MAST4 promotes primary ciliary resorption through phosphorylation of Tctex-1. Life Sci. Alliance 2023, 6, e202301947. [Google Scholar] [CrossRef]
- Obsilova, V.; Obsil, T. Structural insights into the functional roles of 14-3-3 proteins. Front. Mol. Biosci. 2022, 9, 1016071. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Tinti, M.; Wood, N.T.; Campbell, D.G.; Toth, R.; Dubois, F.; Geraghty, K.M.; Wong, B.H.; Brown, L.J.; Tyler, J.; et al. Visualization and biochemical analyses of the emerging mammalian 14-3-3-phosphoproteome. Mol. Cell Proteom. 2011, 10, M110.005751. [Google Scholar] [CrossRef]
- Madeira, F.; Tinti, M.; Murugesan, G.; Berrett, E.; Stafford, M.; Toth, R.; Cole, C.; MacKintosh, C.; Barton, G.J. 14-3-3-Pred: Improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics 2015, 31, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Johnson, C.; Crowther, S.; Stafford, M.J.; Campbell, D.G.; Toth, R.; MacKintosh, C. Bioinformatic and experimental survey of 14-3-3-binding sites. Biochem. J. 2010, 427, 69–78. [Google Scholar] [CrossRef]
- Necci, M.; Piovesan, D.; Clementel, D.; Dosztanyi, Z.; Tosatto, S.C.E. MobiDB-lite 3.0: Fast consensus annotation of intrinsic disorder flavors in proteins. Bioinformatics 2021, 36, 5533–5534. [Google Scholar] [CrossRef] [PubMed]
- Herrington, N.B.; Li, Y.C.; Stein, D.; Pandey, G.; Schlessinger, A. A comprehensive exploration of the druggable conformational space of protein kinases using AI-predicted structures. PLoS Comput. Biol. 2024, 20, e1012302. [Google Scholar] [CrossRef]
- Nussinov, R.; Zhang, M.; Liu, Y.; Jang, H. AlphaFold, allosteric, and orthosteric drug discovery: Ways forward. Drug Discov. Today 2023, 28, 103551. [Google Scholar] [CrossRef]
- Sora, V.; Laspiur, A.O.; Degn, K.; Arnaudi, M.; Utichi, M.; Beltrame, L.; De Menezes, D.; Orlandi, M.; Stoltze, U.K.; Rigina, O.; et al. RosettaDDGPrediction for high-throughput mutational scans: From stability to binding. Protein Sci. 2023, 32, e4527. [Google Scholar] [CrossRef]
- Hernandez, I.M.; Dehouck, Y.; Bastolla, U.; Lopez-Blanco, J.R.; Chacon, P. Predicting protein stability changes upon mutation using a simple orientational potential. Bioinformatics 2023, 39, btad011. [Google Scholar] [CrossRef]
- Akimov, V.; Barrio-Hernandez, I.; Hansen, S.V.F.; Hallenborg, P.; Pedersen, A.K.; Bekker-Jensen, D.B.; Puglia, M.; Christensen, S.D.K.; Vanselow, J.T.; Nielsen, M.M.; et al. UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat. Struct. Mol. Biol. 2018, 25, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.J.; Deery, E.C.; Walker, C.E.; Wilkie, S.E.; Srinivasan, N.; Hunt, D.M.; Bhattacharya, S.S.; Warren, M.J. The destabilization of human GCAP1 by a proline to leucine mutation might cause cone-rod dystrophy. Hum. Mol. Genet. 2001, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gizzio, J.; Thakur, A.; Haldane, A.; Post, C.B.; Levy, R.M. Evolutionary sequence and structural basis for the distinct conformational landscapes of Tyr and Ser/Thr kinases. Nat. Commun. 2024, 15, 6545. [Google Scholar] [CrossRef]
- Vijayan, R.S.; He, P.; Modi, V.; Duong-Ly, K.C.; Ma, H.; Peterson, J.R.; Dunbrack, R.L., Jr.; Levy, R.M. Conformational analysis of the DFG-out kinase motif and biochemical profiling of structurally validated type II inhibitors. J. Med. Chem. 2015, 58, 466–479. [Google Scholar] [CrossRef]
- Bjorklund, A.K.; Ekman, D.; Elofsson, A. Expansion of protein domain repeats. PLoS Comput. Biol. 2006, 2, e114. [Google Scholar] [CrossRef]
- Buljan, M.; Bateman, A. The evolution of protein domain families. Biochem. Soc. Trans. 2009, 37, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Baljuls, A.; Reinders, J.; Nekhoroshkova, E.; Sibilski, C.; Metz, R.; Albert, S.; Rajalingam, K.; Hekman, M.; Rapp, U.R. Regulation of RAF activity by 14-3-3 proteins: RAF kinases associate functionally with both homo- and heterodimeric forms of 14-3-3 proteins. J. Biol. Chem. 2009, 284, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.L.; Chan, T.Y.; Torres, M.P.; Andersen, J.L. The dynamic and stress-adaptive signaling hub of 14-3-3: Emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 2018, 37, 5587–5604. [Google Scholar] [CrossRef] [PubMed]
- Obsilova, V.; Obsil, T. The 14-3-3 Proteins as Important Allosteric Regulators of Protein Kinases. Int. J. Mol. Sci. 2020, 21, 8824. [Google Scholar] [CrossRef]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- Kopra, K.; Valtonen, S.; Mahran, R.; Kapp, J.N.; Hassan, N.; Gillette, W.; Dennis, B.; Li, L.; Westover, K.D.; Pluckthun, A.; et al. Thermal Shift Assay for Small GTPase Stability Screening: Evaluation and Suitability. Int. J. Mol. Sci. 2022, 23, 7095. [Google Scholar] [CrossRef]
- Tolvanen, T.A. Current Advances in CETSA. Front. Mol. Biosci. 2022, 9, 866764. [Google Scholar] [CrossRef]



| Isoform | Mutation, Disease [28,46,47,48] | Location | ΔΔG * (kcal/mol) (VENUS) | -(ΔΔG) ** (kcal/mol) (DDMut) | Interpretation |
|---|---|---|---|---|---|
| MAST1 (Q9Y2H9) | S81Y, cancer S93L, neuronal disability | DUF DUF | −0.7 −1.8 | −0.05 −0.06 | Neutral, altered phosphosite Neutral, loss of phosphosite |
| L232P, MCCCHCM C291F, cancer | DUF DUF | 4.6 0.1 | 3.01 0.48 | Destabilizing Neutral, loss of di-sulfide bond | |
| V316E, cancer P500L, neuronal disability G517S, MCCCHCM G522E, MCCCHCM V558L, MCCCHCM P1177R, neuronal disability L1180R, neuronal disability | DUF Catalytic-HRD+3 Catalytic-G of DFG Catalytic-DFG+5 Catalytic-APE+1 IDR IDR | −0.1 0.6 −0.9 2.4 0.7 0.3 −0.2 | 0.47 0.93 0.06 2.66 0.55 −0.07 −0.06 | Neutral, altered hydrophobicity/Ub site Neutral, altered RD pocket flexibility Neutral, may stabilize DFGin Destabilizing Neutral, may alter DFGin/DFGout shift Neutral, altered electrostatics Neutral, altered electrostatics | |
| MAST2 (Q6P0Q8) | R89Q, vascular disease A1463T, TII-diabetes | IDR IDR | −2.3 0.3 | 0.21 0 | Possibly stabilizing Neutral, gain of phosphosite |
| MAST3 (O60307) | S101F, neuronal disability | DUF | −9.8 | −0.05 | Possibly stabilizing |
| S104L, neuronal disability G510S, neuronal disability | DUF Catalytic-G of DFG | 0.7 −1.0 | −0.06 0.16 | Neutral, loss of phosphosite Neutral, may stabilize DFGin | |
| G515S, neuronal disability | Catalytic-DFG+5 | −0.3 | 0.66 | Neutral, may alter DFGin/DFGout shift | |
| L516P, neuronal disability G861S, IBS | Catalytic-DFG+6 IDR | −0.3 −4.4 | 0.59 −0.04 | Neutral, may alter DFGin/DFGout shift Possibly stabilizing | |
| MAST4 (O15021) | T347M, neuronal disability I898T, neuronal disability | DUF Catalytic-HM motif | −1.6 0.3 | −0.01 1.37 | Neutral, loss of phosphosite Neutral, reduced activity |
| P1201R, neuronal disability T1471I, neuronal disability S2552W, neuronal disability | PDZ IDR IDR | 1.2 −2.7 0.7 | −0.15 −0.07 −0.05 | Neutral, altered PDZ flexibility Possibly stabilizing Neutral, loss of phosphosite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemke, M.C.; Avala, N.R.; Rader, M.T.; Hargett, S.R.; Lank, D.S.; Seltzer, B.D.; Harris, T.E. MAST Kinases’ Function and Regulation: Insights from Structural Modeling and Disease Mutations. Biomedicines 2025, 13, 925. https://doi.org/10.3390/biomedicines13040925
Lemke MC, Avala NR, Rader MT, Hargett SR, Lank DS, Seltzer BD, Harris TE. MAST Kinases’ Function and Regulation: Insights from Structural Modeling and Disease Mutations. Biomedicines. 2025; 13(4):925. https://doi.org/10.3390/biomedicines13040925
Chicago/Turabian StyleLemke, Michael C., Nithin R. Avala, Michael T. Rader, Stefan R. Hargett, Daniel S. Lank, Brandon D. Seltzer, and Thurl E. Harris. 2025. "MAST Kinases’ Function and Regulation: Insights from Structural Modeling and Disease Mutations" Biomedicines 13, no. 4: 925. https://doi.org/10.3390/biomedicines13040925
APA StyleLemke, M. C., Avala, N. R., Rader, M. T., Hargett, S. R., Lank, D. S., Seltzer, B. D., & Harris, T. E. (2025). MAST Kinases’ Function and Regulation: Insights from Structural Modeling and Disease Mutations. Biomedicines, 13(4), 925. https://doi.org/10.3390/biomedicines13040925





