B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Cell Lines
2.3. Flow Cytometry
2.4. Real-Time Quantitative PCR
2.5. Western Blotting
2.6. RNA Sequencing
2.7. Chemoresistance Experiment
2.8. Statistics
3. Results
3.1. HCL-c and HCL-v Cells Express Receptors of BAFF
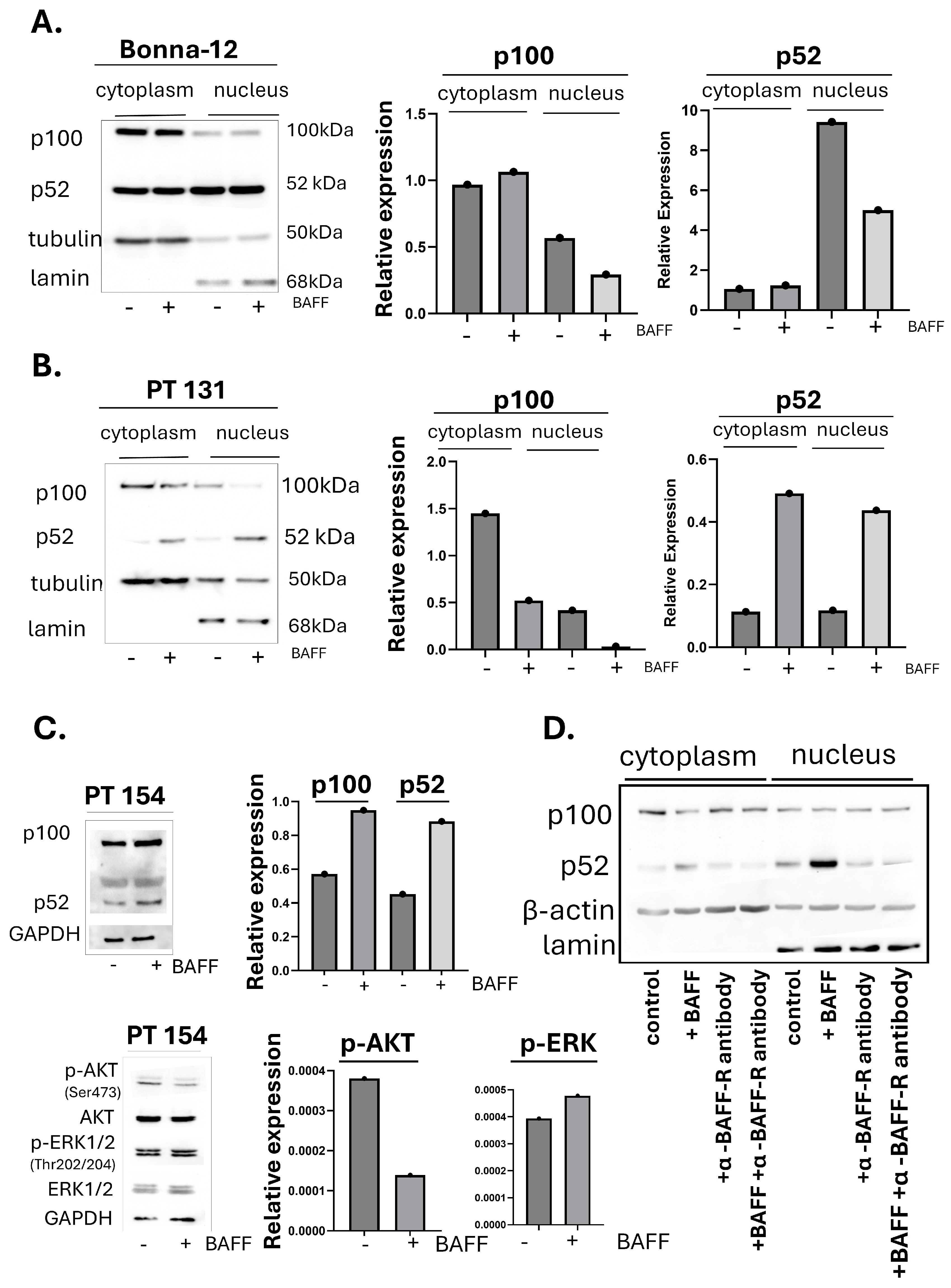
3.2. BAFF Preferentially Induces Signaling Through the Nonclassical NF-κB Pathway in HCL-v Cells
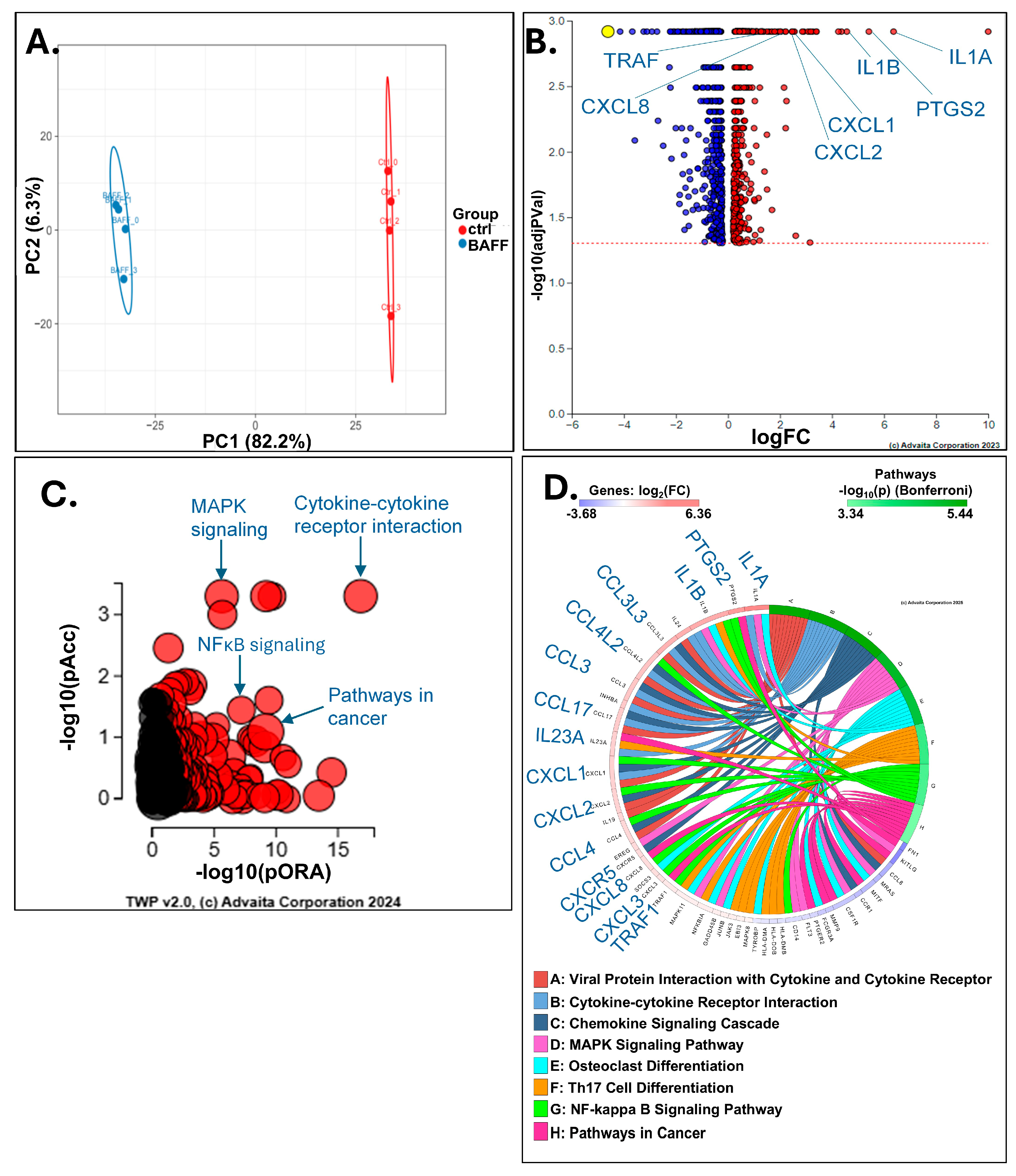
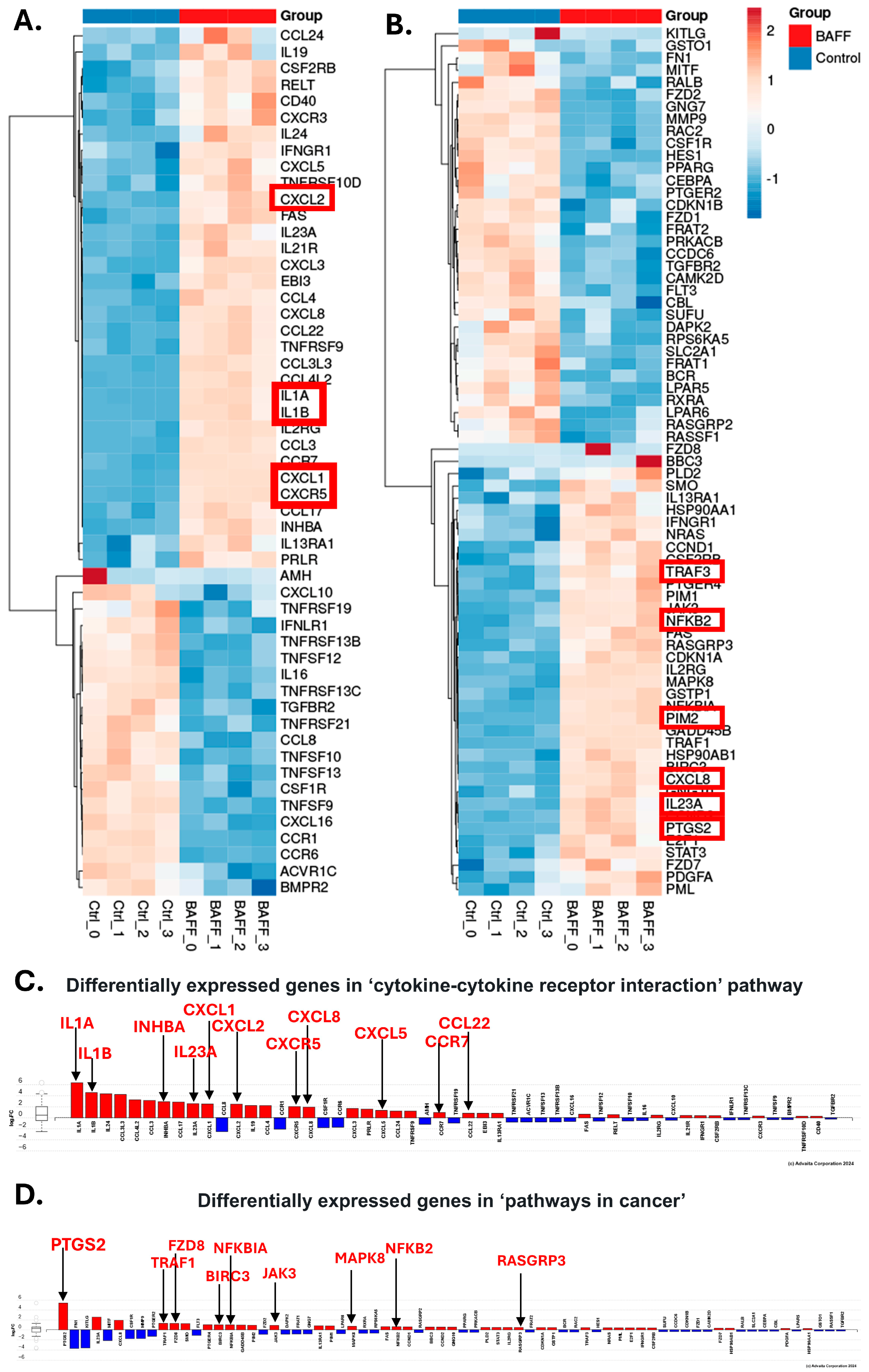
3.3. BAFF Induces Multiple Genes Involved in Chemoresistance in HCL-v Cells
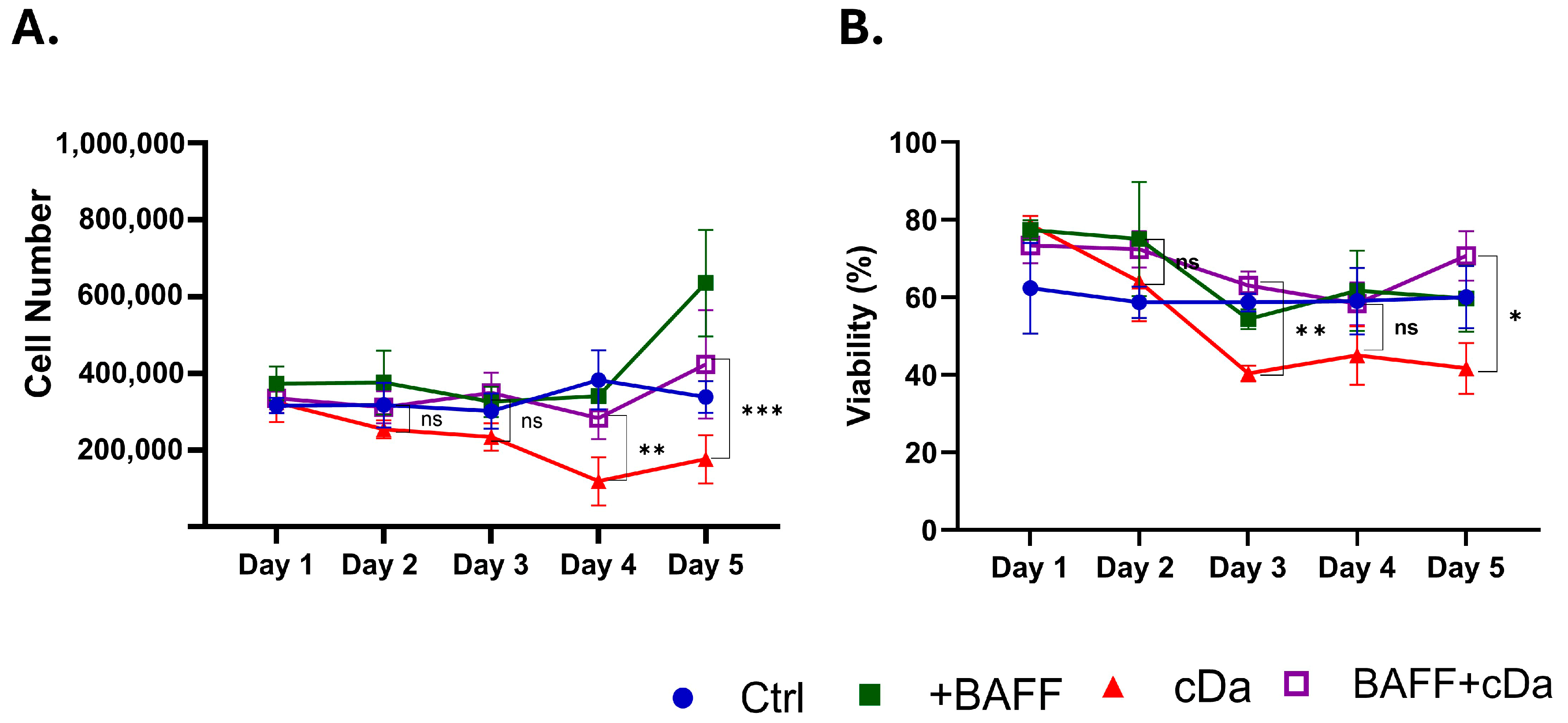
3.4. BAFF Promotes Chemoresistance in HCL-v Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paillassa, J.; Maitre, E.; Troussard, X. Hairy Cell Leukemia (HCL) and HCL Variant: Updates and Spotlights on Therapeutic Advances. Curr. Oncol. Rep. 2022, 24, 1133–1143. [Google Scholar] [CrossRef]
- Konkay, K.; Uppin, M.S.; Uppin, S.G.; Raghunadha Rao, D.; Geetha, C.; Paul, T.R. Hairy cell leukemia: Clinicopathological and immunophenotypic study. Indian. J. Hematol. Blood Transfus. 2014, 30, 180–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kreitman, R.J. Hairy cell leukemia: Present and future directions. Leuk. Lymphoma 2019, 60, 2869–2879. [Google Scholar] [CrossRef]
- Troussard, X.; Maitre, E.; Cornet, E. Hairy cell leukemia 2022: Update on diagnosis, risk-stratification, and treatment. Am. J. Hematol. 2022, 97, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Maitre, E.; Cornet, E.; Salaun, V.; Kerneves, P.; Cheze, S.; Repesse, Y.; Damaj, G.; Troussard, X. Immunophenotypic Analysis of Hairy Cell Leukemia (HCL) and Hairy Cell Leukemia-like (HCL-like) Disorders. Cancers 2022, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Maitre, E.; Paillassa, J.; Troussard, X. Novel targeted treatments in hairy cell leukemia and other hairy cell-like disorders. Front. Oncol. 2022, 12, 1068981. [Google Scholar] [CrossRef]
- Chihara, D.; Kreitman, R.J. Treatment of hairy cell leukemia. Expert. Rev. Hematol. 2020, 13, 1107–1117. [Google Scholar] [CrossRef]
- Falini, B.; De Carolis, L.; Tiacci, E. How I treat refractory/relapsed hairy cell leukemia with BRAF inhibitors. Blood 2022, 139, 2294–2305. [Google Scholar] [CrossRef]
- Jain, P.; Pemmaraju, N.; Ravandi, F. Update on the biology and treatment options for hairy cell leukemia. Curr. Treat. Options Oncol. 2014, 15, 187–209. [Google Scholar] [CrossRef]
- Tiacci, E.; De Carolis, L.; Simonetti, E.; Capponi, M.; Ambrosetti, A.; Lucia, E.; Antolino, A.; Pulsoni, A.; Ferrari, S.; Zinzani, P.L.; et al. Vemurafenib plus Rituximab in Refractory or Relapsed Hairy-Cell Leukemia. N. Engl. J. Med. 2021, 384, 1810–1823. [Google Scholar] [CrossRef]
- Tiacci, E.; De Carolis, L.; Simonetti, E.; Merluzzi, M.; Bennati, A.; Perriello, V.M.; Pucciarini, A.; Santi, A.; Venanzi, A.; Pettirossi, V.; et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: A pilot phase-2 clinical trial. Leukemia 2021, 35, 3314–3318. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.M.; Wang, H.W.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.; et al. Randomized Phase II Study of First-Line Cladribine With Concurrent or Delayed Rituximab in Patients With Hairy Cell Leukemia. J. Clin. Oncol. 2020, 38, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Fanta, P.T.; Saven, A. Hairy cell leukemia. Cancer Treat. Res. 2008, 142, 193–209. [Google Scholar] [CrossRef]
- Shao, H.; Calvo, K.R.; Gronborg, M.; Tembhare, P.R.; Kreitman, R.J.; Stetler-Stevenson, M.; Yuan, C.M. Distinguishing hairy cell leukemia variant from hairy cell leukemia: Development and validation of diagnostic criteria. Leuk. Res. 2013, 37, 401–409. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Kumar Upadhyay, A.; Kumar, M.; Prasad, A.; Shekhar, S.; Singh, R. A Case of Hairy Cell Leukemia Variant: Literature Analysis With Focus on Unmet Needs. Cureus 2023, 15, e47085. [Google Scholar] [CrossRef] [PubMed]
- Paillassa, J.; Safa, F.; Troussard, X. Updates in hairy cell leukemia (HCL) and variant-type HCL (HCL-V): Rationale for targeted treatments with a focus on ibrutinib. Ther. Adv. Hematol. 2022, 13, 20406207221090886. [Google Scholar] [CrossRef]
- Rihacek, M.; Bienertova-Vasku, J.; Valik, D.; Sterba, J.; Pilatova, K.; Zdrazilova-Dubska, L. B-Cell Activating Factor as a Cancer Biomarker and Its Implications in Cancer-Related Cachexia. Biomed. Res. Int. 2015, 2015, 792187. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Garcia-Carmona, Y.; Fribourg, M.; Sowa, A.; Cerutti, A.; Cunningham-Rundles, C. TACI and endogenous APRIL in B cell maturation. Clin. Immunol. 2023, 253, 109689. [Google Scholar] [CrossRef]
- Sevdali, E.; Block, V.; Lataretu, M.; Li, H.; Smulski, C.R.; Briem, J.S.; Heitz, Y.; Fischer, B.; Ramirez, N.J.; Grimbacher, B.; et al. BAFFR activates PI3K/AKT signaling in human naive but not in switched memory B cells through direct interactions with B cell antigen receptors. Cell Rep. 2022, 39, 111019. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Mackay, F. The BAFF-APRIL System in Cancer. Cancers 2023, 15, 1791. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.J.; Grote, D.M.; Stenson, M.; Ziesmer, S.C.; Witzig, T.E.; Habermann, T.M.; Harder, B.; Ristow, K.M.; Bram, R.J.; Jelinek, D.F.; et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: Correlation with disease activity and patient outcome. Blood 2004, 104, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Georgakis, G.V.; Migone, T.S.; Kwak, L.W.; Younes, A. Prognostic significance of serum B-lymphocyte stimulator in Hodgkin’s lymphoma. Haematologica 2007, 92, 269–270. [Google Scholar] [CrossRef]
- Onda, K.; Iijima, K.; Katagiri, Y.U.; Okita, H.; Saito, M.; Shimizu, T.; Kiyokawa, N. Differential effects of BAFF on B cell precursor acute lymphoblastic leukemia and Burkitt lymphoma. Int. J. Hematol. 2010, 91, 808–819. [Google Scholar] [CrossRef]
- Zhang, K.; Roy, N.K.; Vicioso, Y.; Woo, J.; Beck, R.; de Lima, M.; Caimi, P.; Feinberg, D.; Parameswaran, R. BAFF receptor antibody for mantle cell lymphoma therapy. Oncoimmunology 2021, 10, 1893501. [Google Scholar] [CrossRef]
- Tandler, C.; Schmidt, M.; Heitmann, J.S.; Hierold, J.; Schmidt, J.; Schneider, P.; Dorfel, D.; Walz, J.; Salih, H.R. Neutralization of B-Cell Activating Factor (BAFF) by Belimumab Reinforces Small Molecule Inhibitor Treatment in Chronic Lymphocytic Leukemia. Cancers 2020, 12, 2725. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Reme, T.; Lugagne, C.; Moine, P.; Rossi, J.F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef]
- McWilliams, E.M.; Lucas, C.R.; Chen, T.; Harrington, B.K.; Wasmuth, R.; Campbell, A.; Rogers, K.A.; Cheney, C.M.; Mo, X.; Andritsos, L.A.; et al. Anti-BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 2019, 3, 447–460. [Google Scholar] [CrossRef]
- Otipoby, K.L.; Sasaki, Y.; Schmidt-Supprian, M.; Patke, A.; Gareus, R.; Pasparakis, M.; Tarakhovsky, A.; Rajewsky, K. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12435–12438. [Google Scholar] [CrossRef]
- Sun, S.C. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Tegowski, M.; Baldwin, A. Noncanonical NF-kappaB in Cancer. Biomedicines 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Stanam, A.; Gibson-Corley, K.N.; Love-Homan, L.; Ihejirika, N.; Simons, A.L. Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 76087–76100. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Rodia, M.T.; Pucci, M.; Dall’Ora, M.; Santi, S.; Solmi, R.; Roth, L.; Lindzen, M.; Bonafe, M.; Bertotti, A.; et al. A module of inflammatory cytokines defines resistance of colorectal cancer to EGFR inhibitors. Oncotarget 2016, 7, 72167–72183. [Google Scholar] [CrossRef]
- Singh, S.; Xiao, Z.; Bavisi, K.; Roszik, J.; Melendez, B.D.; Wang, Z.; Cantwell, M.J.; Davis, R.E.; Lizee, G.; Hwu, P.; et al. IL-1alpha Mediates Innate and Acquired Resistance to Immunotherapy in Melanoma. J. Immunol. 2021, 206, 1966–1975. [Google Scholar] [CrossRef]
- Zhao, S.; Xing, S.; Wang, L.; Ouyang, M.; Liu, S.; Sun, L.; Yu, H. IL-1beta is involved in docetaxel chemoresistance by regulating the formation of polyploid giant cancer cells in non-small cell lung cancer. Sci. Rep. 2023, 13, 12763. [Google Scholar] [CrossRef]
- Xuan, Y.; Wang, Y.N. Hypoxia/IL-1alpha axis promotes gastric cancer progression and drug resistance. J. Dig. Dis. 2017, 18, 511–520. [Google Scholar] [CrossRef]
- Liu, S.; Lee, J.S.; Jie, C.; Park, M.H.; Iwakura, Y.; Patel, Y.; Soni, M.; Reisman, D.; Chen, H. HER2 Overexpression Triggers an IL1alpha Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer Res. 2018, 78, 2040–2051. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef]
- de Mooij, C.E.M.; Netea, M.G.; van der Velden, W.; Blijlevens, N.M.A. Targeting the interleukin-1 pathway in patients with hematological disorders. Blood 2017, 129, 3155–3164. [Google Scholar] [CrossRef]
- Zhang, B.; Chu, S.; Agarwal, P.; Campbell, V.L.; Hopcroft, L.; Jorgensen, H.G.; Lin, A.; Gaal, K.; Holyoake, T.L.; Bhatia, R. Inhibition of interleukin-1 signaling enhances elimination of tyrosine kinase inhibitor-treated CML stem cells. Blood 2016, 128, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012, 150, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Cabioglu, N.; Onder, S.; Karatay, H.; Bayram, A.; Oner, G.; Tukenmez, M.; Muslumanoglu, M.; Igci, A.; Dinccag, A.; Ozmen, V.; et al. New Emerging Chemokine Receptors: CCR5 or CXCR5 on Tumor Is Associated with Poor Response to Chemotherapy and Poor Prognosis in Locally Advanced Triple-Negative Breast Cancer. Cancers 2024, 16, 2388. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Peng, C.; Xia, B.; Gan, L. CXCL8 Facilitates the Survival and Paclitaxel-Resistance of Triple-Negative Breast Cancers. Clin. Breast Cancer 2022, 22, e191–e198. [Google Scholar] [CrossRef]
- Lin, X.M.; Luo, W.; Wang, H.; Li, R.Z.; Huang, Y.S.; Chen, L.K.; Wu, X.P. The Role of Prostaglandin-Endoperoxide Synthase-2 in Chemoresistance of Non-Small Cell Lung Cancer. Front. Pharmacol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Qi, C.; Yu, C.; Li, Y.; Huan, W.; Wang, R.; Luo, W.; Shen, D.; Ding, L.; et al. N(6)-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol. Cancer 2022, 21, 111. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, G.; Zhang, T.; Yao, K.; Chen, H.; Park, M.H.; Yamamoto, H.; Wang, K.; Ma, W.; Malakhova, M.; et al. TRAF1 Is Critical for Regulating the BRAF/MEK/ERK Pathway in Non-Small Cell Lung Carcinogenesis. Cancer Res. 2018, 78, 3982–3994. [Google Scholar] [CrossRef]
- Durkop, H.; Hirsch, B.; Hahn, C.; Foss, H.D.; Stein, H. Differential expression and function of A20 and TRAF1 in Hodgkin lymphoma and anaplastic large cell lymphoma and their induction by CD30 stimulation. J. Pathol. 2003, 200, 229–239. [Google Scholar] [CrossRef]
- Liu, Q.; Harris, N.; Epperla, N.; Andritsos, L.A. Current and Emerging Therapeutic Options for Hairy Cell Leukemia Variant. Onco Targets Ther. 2021, 14, 1797–1805. [Google Scholar] [CrossRef]
- Morgan, D.; Garg, M.; Tergaonkar, V.; Tan, S.Y.; Sethi, G. Pharmacological significance of the non-canonical NF-kappaB pathway in tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188449. [Google Scholar] [CrossRef]
- Nuan-Aliman, S.; Bordereaux, D.; Thieblemont, C.; Baud, V. The Alternative RelB NF-kB Subunit Exerts a Critical Survival Function upon Metabolic Stress in Diffuse Large B-Cell Lymphoma-Derived Cells. Biomedicines 2022, 10, 348. [Google Scholar] [CrossRef]
- Marshall, L.A.; Marubayashi, S.; Jorapur, A.; Jacobson, S.; Zibinsky, M.; Robles, O.; Hu, D.X.; Jackson, J.J.; Pookot, D.; Sanchez, J.; et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J. Immunother. Cancer 2020, 8, e000764. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Gu, L.H.; Tong, Y.L.; Chen, R.Q.; Chen, S.Y.; Yu, X.L.; Liu, N.; Lu, J.L.; Si, Y.; Sun, J.H.; et al. INHBA promotes tumor growth and induces resistance to PD-L1 blockade by suppressing IFN-gamma signaling. Acta Pharmacol. Sin. 2024, 46, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Su, Y.; Zhu, H.; Wang, X.; Li, X.; Dai, C.; Xu, C.; Zheng, T.; Mao, C.; Chen, D. Interleukin-23 receptor signaling mediates cancer dormancy and radioresistance in human esophageal squamous carcinoma cells via the Wnt/Notch pathway. J. Mol. Med. 2019, 97, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Bodaar, K.; Yamagata, N.; Barthe, A.; Landrigan, J.; Chonghaile, T.N.; Burns, M.; Stevenson, K.E.; Devidas, M.; Loh, M.L.; Hunger, S.P.; et al. JAK3 mutations and mitochondrial apoptosis resistance in T-cell acute lymphoblastic leukemia. Leukemia 2022, 36, 1499–1507. [Google Scholar] [CrossRef]
- Geraldo, L.H.; Garcia, C.; Xu, Y.; Leser, F.S.; Grimaldi, I.; de Camargo Magalhaes, E.S.; Dejaegher, J.; Solie, L.; Pereira, C.M.; Correia, A.H.; et al. CCL21-CCR7 signaling promotes microglia/macrophage recruitment and chemotherapy resistance in glioblastoma. Cell Mol. Life Sci. 2023, 80, 179. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Zhang, L.; Zhang, J.; Xiao, Y.; Wu, Q.; Wang, Y.; Zhan, Q. Tumor-associated macrophage (TAM)-secreted CCL22 confers cisplatin resistance of esophageal squamous cell carcinoma (ESCC) cells via regulating the activity of diacylglycerol kinase alpha (DGKalpha)/NOX4 axis. Drug Resist. Updat. 2024, 73, 101055. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, G.; Hou, S.; Sha, L.G. MAPK8 mediates resistance to temozolomide and apoptosis of glioblastoma cells through MAPK signaling pathway. Biomed. Pharmacother. 2018, 106, 1419–1427. [Google Scholar] [CrossRef]
- Yin, S.; Xu, L.; Bonfil, R.D.; Banerjee, S.; Sarkar, F.H.; Sethi, S.; Reddy, K.B. Tumor-initiating cells and FZD8 play a major role in drug resistance in triple-negative breast cancer. Mol. Cancer Ther. 2013, 12, 491–498. [Google Scholar] [CrossRef]
- Yang, D.; Kedei, N.; Li, L.; Tao, J.; Velasquez, J.F.; Michalowski, A.M.; Toth, B.I.; Marincsak, R.; Varga, A.; Biro, T.; et al. RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 2010, 70, 7905–7917. [Google Scholar] [CrossRef]
- Cao, H.; Tadros, V.; Hiramoto, B.; Leeper, K.; Hino, C.; Xiao, J.; Pham, B.; Kim, D.H.; Reeves, M.E.; Chen, C.S.; et al. Targeting TKI-Activated NFKB2-MIF/CXCLs-CXCR2 Signaling Pathways in FLT3 Mutated Acute Myeloid Leukemia Reduced Blast Viability. Biomedicines 2022, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Vicioso, Y.; Gram, H.; Beck, R.; Asthana, A.; Zhang, K.; Wong, D.P.; Letterio, J.; Parameswaran, R. Combination Therapy for Treating Advanced Drug-Resistant Acute Lymphoblastic Leukemia. Cancer Immunol. Res. 2019, 7, 1106–1119. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence |
|---|---|
| BAFF—forward | GGAGGCAACTCCAGTCAG |
| BAFF—reverse | CAGTGCAGTCCCAAACTACCAGGACTT |
| TACI—forward | GAGCAAGGCAAGTTCTATGACC |
| TACI—reverse | CCTTCCCGAGTTGTCTGAATTG |
| BCMA—forward | ACCTTGTCAACTTCGATGTTCTT |
| BCMA—reverse | CAGAGAATCGCATTCGTTCCTT |
| Actin—forward | TCCACGAAminACTACCTTCAACTC |
| Actin—reverse | GTCATACTCCTGCTTGCTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, C.; Feinberg, D.; Radhakrishnan, A.; Klatt, K.; Chan, E.R.; Rock, P.; Burack, R.; Parameswaran, R. B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant. Biomedicines 2025, 13, 890. https://doi.org/10.3390/biomedicines13040890
Fritz C, Feinberg D, Radhakrishnan A, Klatt K, Chan ER, Rock P, Burack R, Parameswaran R. B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant. Biomedicines. 2025; 13(4):890. https://doi.org/10.3390/biomedicines13040890
Chicago/Turabian StyleFritz, Claire, Daniel Feinberg, Akshaya Radhakrishnan, Kayla Klatt, E. Ricky Chan, Philip Rock, Richard Burack, and Reshmi Parameswaran. 2025. "B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant" Biomedicines 13, no. 4: 890. https://doi.org/10.3390/biomedicines13040890
APA StyleFritz, C., Feinberg, D., Radhakrishnan, A., Klatt, K., Chan, E. R., Rock, P., Burack, R., & Parameswaran, R. (2025). B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant. Biomedicines, 13(4), 890. https://doi.org/10.3390/biomedicines13040890






