Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Lung Organoids
2.2. Generation of Recombinant AAV Vectors
2.3. Generation of a Recombinant Adenoviral Vector
2.4. rAAV and rAdV5 Infection and Measurement of GFP and mCherry-Positive Cells
2.5. Immunofluorescence Staining
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results
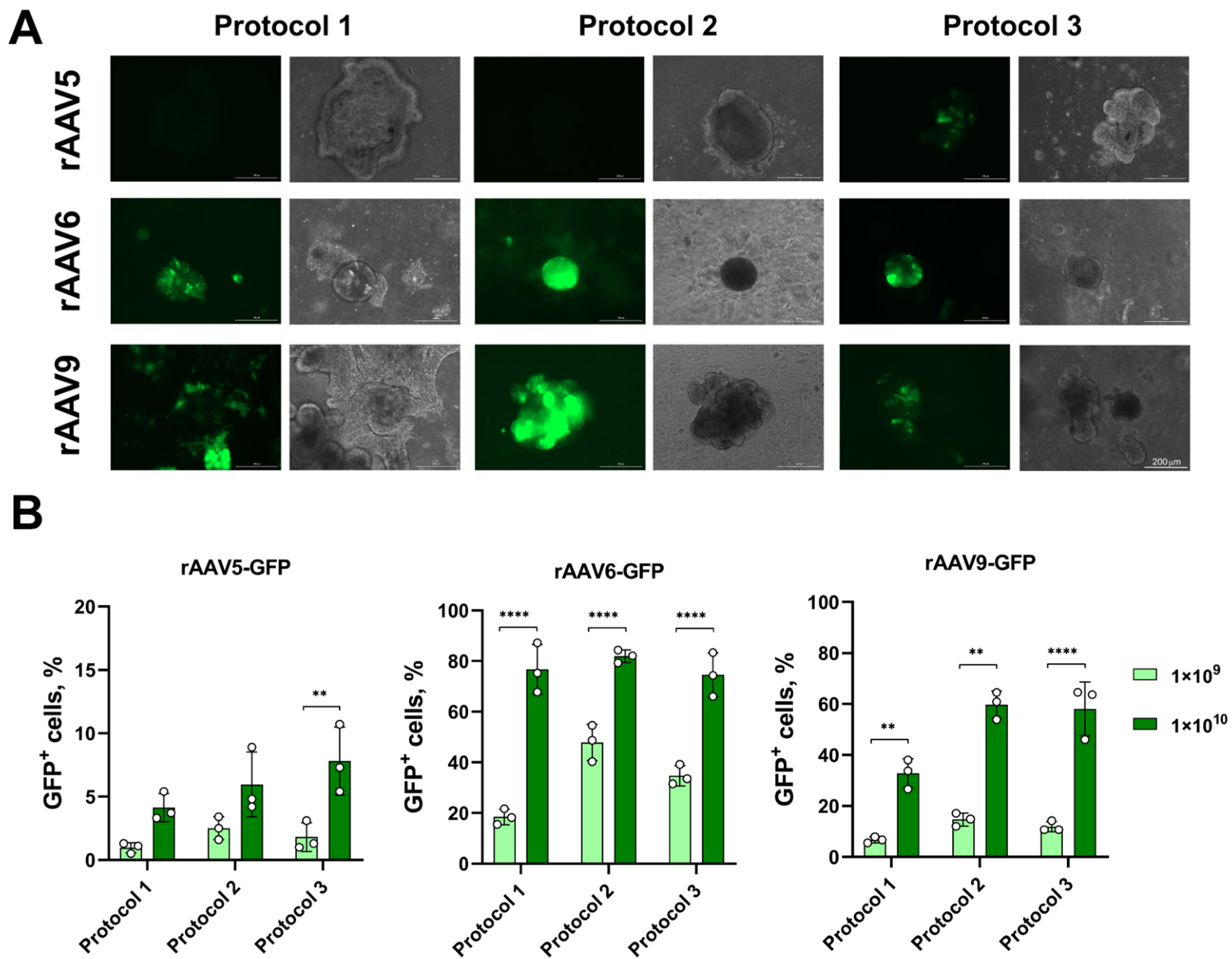
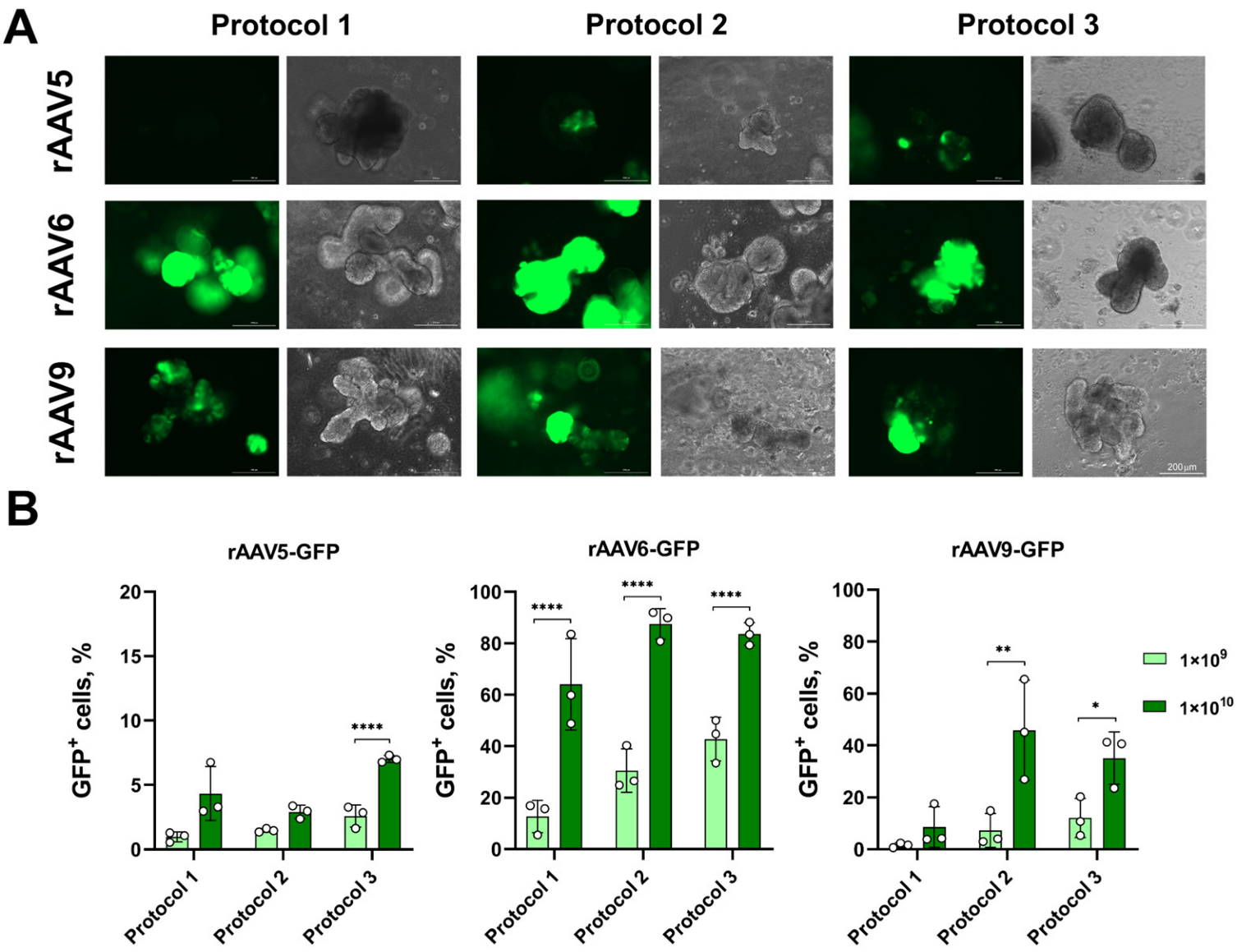
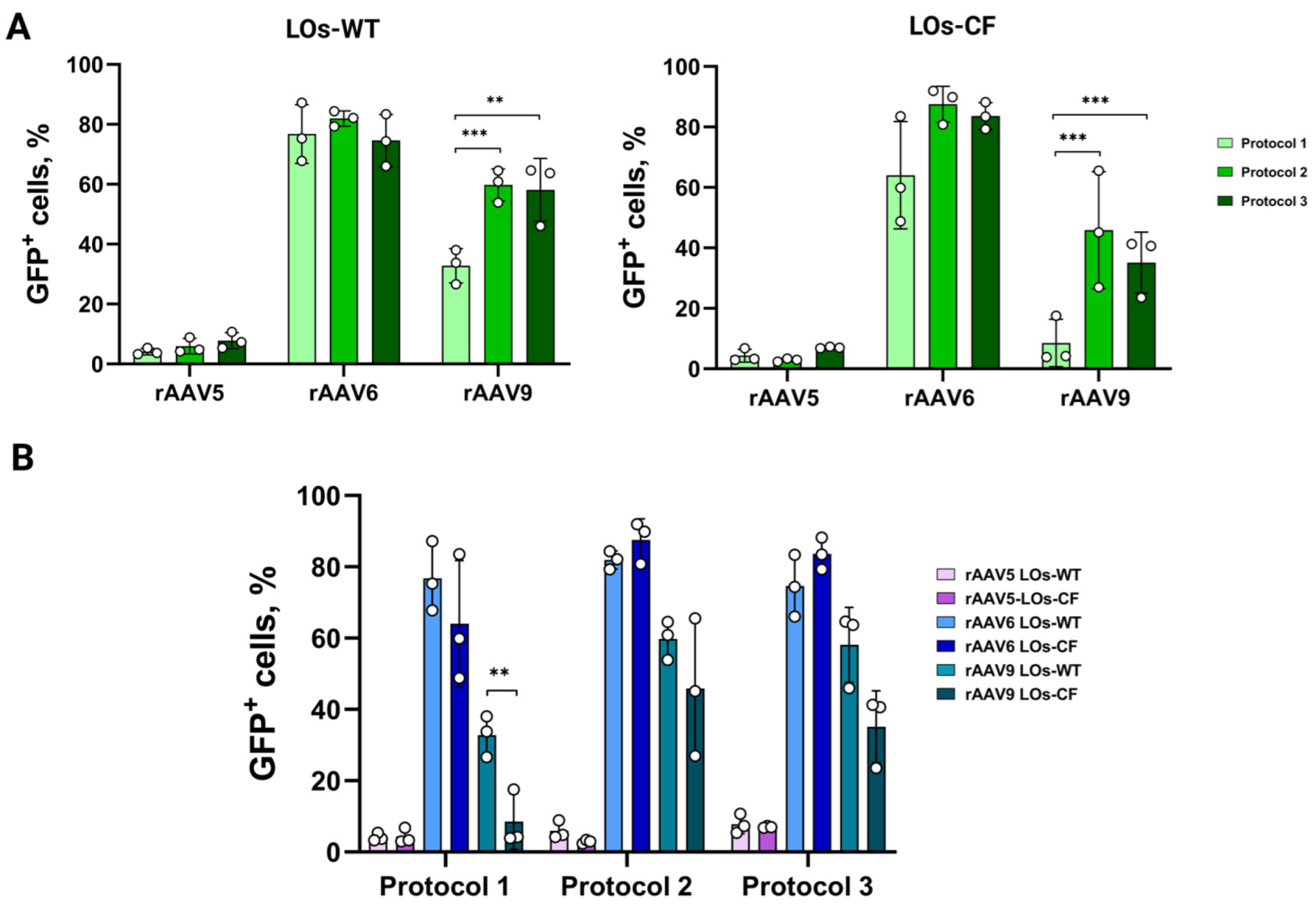
3.1. Transduction of LOs Using rAAV Vectors Serotypes 5, 6 and 9
3.2. Transduction of LOs Using rAdV5 Vector
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| AdV | Adenovirus |
| AAV | Adeno-associated virus |
| hiPSC | Human-induced pluripotent stem cells |
| LO | Lung organoid |
| HSPG | Heparan sulfate proteoglycan |
| MOI | Multiplicity of infection |
| PFU | Plaque-forming unit |
| SCGB3A2 | Secretoglobin Family 3A, Member 2 |
| TP63 | Tumor protein p63 |
References
- Bierlaagh, M.C.; Muilwijk, D.; Beekman, J.M.; van der Ent, C.K. A New Era for People with Cystic Fibrosis. Eur. J. Pediatr. 2021, 180, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Ruffin, M.; Corvol, H.; Guillot, L. Gene Therapy: A Possible Alternative to CFTR Modulators? Front. Pharmacol. 2021, 12, 648203. [Google Scholar] [CrossRef]
- Fellmann, C.; Gowen, B.G.; Lin, P.-C.; Doudna, J.A.; Corn, J.E. Cornerstones of CRISPR–Cas in Drug Discovery and Therapy. Nat. Rev. Drug Discov. 2017, 16, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Smirnikhina, S.A. Perspectives on Genetic Medicine for Cystic Fibrosis. Curr. Gene Ther. 2022, 22, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kochergin-Nikitsky, K.; Belova, L.; Lavrov, A.; Smirnikhina, S. Tissue and Cell-Type-Specific Transduction Using rAAV Vectors in Lung Diseases. J. Mol. Med. 2021, 99, 1057–1071. [Google Scholar] [CrossRef]
- MacNeil, K.M.; Dodge, M.J.; Evans, A.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Adenoviruses in Medicine: Innocuous Pathogen, Predator, or Partner. Trends Mol. Med. 2023, 29, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Belova, L.; Demchenko, A.; Kochergin-Nikitsky, K.; Kondrateva, E.; Slesarenko, Y.; Salikhova, D.; Lavrov, A.; Efremova, A.; Bukharova, T.; Goldshtein, D.; et al. Recombinant Adeno-Associated Viral Vectors Serotypes 6 and 9 Are Able to Transduce Human Tracheal Epithelial Cells but Not Human Induced Pluripotent Stem Cells. Mol. Biotechnol. 2023, 65, 1539–1546. [Google Scholar] [CrossRef]
- San Martín, C. Latest Insights on Adenovirus Structure and Assembly. Viruses 2012, 4, 847–877. [Google Scholar] [CrossRef]
- Belova, L.; Lavrov, A.; Smirnikhina, S. Organoid Transduction Using Recombinant Adeno-Associated Viral Vectors: Challenges and Opportunities. BioEssays 2022, 44, 2200055. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In Vitro Generation of Human Pluripotent Stem Cell Derived Lung Organoids. eLife 2015, 2015, e05098. [Google Scholar] [CrossRef]
- Leibel, S.L.; McVicar, R.N.; Winquist, A.M.; Niles, W.D.; Snyder, E.Y. Generation of Complete Multi−Cell Type Lung Organoids From Human Embryonic and Patient-Specific Induced Pluripotent Stem Cells for Infectious Disease Modeling and Therapeutics Validation. Curr. Protoc. Stem Cell Biol. 2020, 54, e118. [Google Scholar] [CrossRef]
- Joo, H.; Min, S.; Cho, S.-W. Advanced Lung Organoids for Respiratory System and Pulmonary Disease Modeling. J. Tissue Eng. 2024, 15, 20417314241232502. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, F.; Liang, X.; Duan, L.; Li, Q.; Pan, G.; Ma, C.; Liu, M.; Li, M.; Wang, P.; et al. iPSC-Derived Airway Epithelial Cells: Progress, Promise, and Challenges. Stem Cells 2023, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, D.; Carlon, M.S.; Cunha, M.F.D.; Dekkers, J.F.; Hollenhorst, M.I.; Bijvelds, M.J.C.; Ramalho, A.S.; Haute, C.V.D.; Ferrante, M.; Baekelandt, V.; et al. RAAV-CFTRDR Rescues the Cystic Fibrosis Phenotype in Human Intestinal Organoids and Cystic Fibrosis Mice. Am. J. Respir. Crit. Care Med. 2016, 193, 288–298. [Google Scholar] [CrossRef]
- Meyer-Berg, H.; Yang, L.Z.; de Lucas, M.P.; Zambrano, A.; Hyde, S.C.; Gill, D.R. Identification of AAV Serotypes for Lung Gene Therapy in Human Embryonic Stem Cell-Derived Lung Organoids. Stem Cell Res. Ther. 2020, 11, 448. [Google Scholar] [CrossRef]
- Garita-Hernandez, M.; Routet, F.; Guibbal, L.; Khabou, H.; Toualbi, L.; Riancho, L.; Reichman, S.; Duebel, J.; Sahel, J.A.; Goureau, O.; et al. AAV-Mediated Gene Delivery to 3D Retinal Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 994. [Google Scholar] [CrossRef]
- Achberger, K.; Cipriano, M.; Düchs, M.; Schön, C.; Michelfelder, S.; Stierstorfer, B.; Lamla, T.; Kauschke, S.G.; Chuchuy, J.; Roosz, J.; et al. Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors. Stem Cell Rep. 2021, 16, 2242–2256. [Google Scholar]
- Depla, J.A.; Sogorb-Gonzalez, M.; Mulder, L.A.; Heine, V.M.; Konstantinova, P.; van Deventer, S.J.; Wolthers, K.C.; Pajkrt, D.; Sridhar, A.; Evers, M.M. Cerebral Organoids: A Human Model for AAV Capsid Selection and Therapeutic Transgene Efficacy in the Brain. Mol. Ther. Methods Clin. Dev. 2020, 18, 167–175. [Google Scholar] [CrossRef]
- Wei, J.; Ran, G.; Wang, X.; Jiang, N.; Liang, J.; Lin, X.; Ling, C.; Zhao, B. Gene Manipulation in Liver Ductal Organoids by Optimized Recombinant Adeno-Associated Virus Vectors. J. Biol. Chem. 2019, 294, 14096–14104. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Gershenson, Z.T.; Giles, A.R.; Holzbaur, E.L.F.; Wolfe, J.H. Adeno-Associated Virus Serotypes 1, 8, and 9 Share Conserved Mechanisms for Anterograde and Retrograde Axonal Transport. Hum. Gene Ther. 2014, 25, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cordero, A.; Goh, D.; Kruczek, K.; Naeem, A.; Fernando, M.; Holthaus, S.M.K.; Takaaki, M.; Blackford, S.J.I.; Kloc, M.; Agundez, L.; et al. Assessment of AAV Vector Tropisms for Mouse and Human Pluripotent Stem Cell-Derived RPE and Photoreceptor Cells. Hum. Gene Ther. 2018, 29, 1124–1139. [Google Scholar] [CrossRef]
- Latour, Y.L.; Yoon, R.; Thomas, S.E.; Grant, C.; Li, C.; Sena-Esteves, M.; Allende, M.L.; Proia, R.L.; Tifft, C.J. Human GLB1 Knockout Cerebral Organoids: A Model System for Testing AAV9-Mediated GLB1 Gene Therapy for Reducing GM1 Ganglioside Storage in GM1 Gangliosidosis. Mol. Genet. Metab. Rep. 2019, 21, 100513. [Google Scholar] [CrossRef]
- Raimondi, G.; Mato-Berciano, A.; Pascual-Sabater, S.; Rovira-Rigau, M.; Cuatrecasas, M.; Fondevila, C.; Sánchez-Cabús, S.; Begthel, H.; Boj, S.F.; Clevers, H.; et al. Patient-Derived Pancreatic Tumour Organoids Identify Therapeutic Responses to Oncolytic Adenoviruses. EBioMedicine 2020, 56, 102786. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, H.; Zhang, B.-Q.; Liu, W.; Zhang, Z.; Qiao, M.; Zhang, H.; Deng, F.; Wu, N.; Chen, X.; et al. Adenovirus-Mediated Efficient Gene Transfer into Cultured Three-Dimensional Organoids. PLoS ONE 2014, 9, e93608. [Google Scholar] [CrossRef]
- Pascual-Sabater, S.; Raimondi, G.; Mato-Berciano, A.; Vaquero, E.C.; Ausania, F.; Fillat, C. Preclinical Testing of Oncolytic Adenovirus Sensitivity in Patient-Derived Tumor Organoids. STAR Protoc. 2021, 2, 101017. [Google Scholar] [CrossRef]
- Sharma, A.; Li, X.; Bangari, D.S.; Mittal, S.K. Adenovirus Receptors and Their Implications in Gene Delivery. Virus Res. 2009, 143, 184–194. [Google Scholar] [CrossRef]
- Salikhova, D.; Bukharova, T.; Cherkashova, E.; Namestnikova, D.; Leonov, G.; Nikitina, M.; Gubskiy, I.; Akopyan, G.; Elchaninov, A.; Midiber, K.; et al. Therapeutic Effects of hiPSC-Derived Glial and Neuronal Progenitor Cells-Conditioned Medium in Experimental Ischemic Stroke in Rats. Int. J. Mol. Sci. 2021, 22, 4694. [Google Scholar] [CrossRef]
- Kondrateva, E.; Adilgereeva, E.; Amelina, E.; Tabakov, V.; Demchenko, A.; Ustinov, K.; Yasinovsky, M.; Voronina, E.; Lavrov, A.; Smirnikhina, S. Generation of Induced Pluripotent Stem Cell Line (RCMGi001-A) from Human Skin Fibroblasts of a Cystic Fibrosis Patient with p.F508del Mutation. Stem Cell Res. 2020, 48, 101933. [Google Scholar] [CrossRef]
- Demchenko, A.; Kondrateva, E.; Tabakov, V.; Efremova, A.; Salikhova, D.; Bukharova, T.; Goldshtein, D.; Balyasin, M.; Bulatenko, N.; Amelina, E.; et al. Airway and Lung Organoids from Human-Induced Pluripotent Stem Cells Can Be Used to Assess CFTR Conductance. Int. J. Mol. Sci. 2023, 24, 6293. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Obata, Y.; Ishibashi, K.; Tamura, N.; Kikuchi, I.; Aoyama, K.; Hattori, Y.; Tsuda, K.; Nakayama, Y.; Yamaguchi, N. Cost-Effective Gene Transfection by DNA Compaction at pH 4.0 Using Acidified, Long Shelf-Life Polyethylenimine. Cytotechnology 2010, 62, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Aurnhammer, C.; Haase, M.; Muether, N.; Hausl, M.; Rauschhuber, C.; Huber, I.; Nitschko, H.; Busch, U.; Sing, A.; Ehrhardt, A.; et al. Universal Real-Time {PCR} for the Detection and Quantification of Adeno-Associated Virus Serotype 2-Derived Inverted Terminal Repeat Sequences. Hum. Gene Ther. Methods 2012, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Alieva, M.; Wellens, L.M.; Ariese, H.C.R.; Jamieson, P.R.; Vonk, A.M.; Amatngalim, G.D.; Hu, H.; Oost, K.C.; Snippert, H.J.G.; et al. High-Resolution 3D Imaging of Fixed and Cleared Organoids. Nat. Protoc. 2019, 14, 1756–1771. [Google Scholar] [CrossRef]
- Parekh, K.R.; Nawroth, J.; Pai, A.; Busch, S.M.; Senger, C.N.; Ryan, A.L. Stem Cells and Lung Regeneration. Am. J. Physiol. Cell Physiol. 2020, 319, C675–C693. [Google Scholar] [CrossRef]
- Khare, R.; Chen, C.Y.; Weaver, E.A.; Barry, M.A. Advances and Future Challenges in Adenoviral Vector Pharmacology and Targeting. Curr. Gene Ther. 2011, 11, 241–258. [Google Scholar] [CrossRef]
- Srivastava, A. In Vivo Tissue-Tropism of Adeno-Associated Viral Vectors. Curr. Opin. Virol. 2016, 21, 75–80. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Zabner, J.; Seiler, M.; Walters, R.; Kotin, R.M.; Fulgeras, W.; Davidson, B.L.; Chiorini, J.A. Adeno-Associated Virus Type 5 (AAV5) but Not AAV2 Binds to the Apical Surfaces of Airway Epithelia and Facilitates Gene Transfer. J. Virol. 2000, 74, 3852–3858. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belova, L.; Demchenko, A.; Erofeeva, A.; Kochergin-Nikitsky, K.; Zubkova, O.; Popova, O.; Ozharovskaia, T.; Salikhova, D.; Efremova, A.; Lavrov, A.; et al. Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors. Biomedicines 2025, 13, 879. https://doi.org/10.3390/biomedicines13040879
Belova L, Demchenko A, Erofeeva A, Kochergin-Nikitsky K, Zubkova O, Popova O, Ozharovskaia T, Salikhova D, Efremova A, Lavrov A, et al. Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors. Biomedicines. 2025; 13(4):879. https://doi.org/10.3390/biomedicines13040879
Chicago/Turabian StyleBelova, Lyubava, Anna Demchenko, Anastasia Erofeeva, Konstantin Kochergin-Nikitsky, Olga Zubkova, Olga Popova, Tatiana Ozharovskaia, Diana Salikhova, Anna Efremova, Alexander Lavrov, and et al. 2025. "Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors" Biomedicines 13, no. 4: 879. https://doi.org/10.3390/biomedicines13040879
APA StyleBelova, L., Demchenko, A., Erofeeva, A., Kochergin-Nikitsky, K., Zubkova, O., Popova, O., Ozharovskaia, T., Salikhova, D., Efremova, A., Lavrov, A., & Smirnikhina, S. (2025). Lung Organoids from hiPSCs Can Be Efficiently Transduced by Recombinant Adeno-Associated Viral and Adenoviral Vectors. Biomedicines, 13(4), 879. https://doi.org/10.3390/biomedicines13040879





