TGF-β1 Improves Nerve Regeneration and Functional Recovery After Sciatic Nerve Injury by Alleviating Inflammation
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Animal Models
2.3. Real-Time Quantality PCR (RT-qPCR)
2.4. Western Blot
2.5. Primary DRG Neuron Cell Culture and Treatment
2.6. CCK8 Assay and TUNEL Staining
2.7. Immunofluorescence (IF) Staining
2.8. Lentivirus Injection
2.9. Hematoxylin and Eosin (H&E) Staining
2.10. Behavioral Tests
2.11. SFI Analysis
2.12. Thermal Pain Analysis
2.13. Electrophysiological Test
2.14. Transmission Electron Microscope Analysis
2.15. Statistical Analysis
3. Results
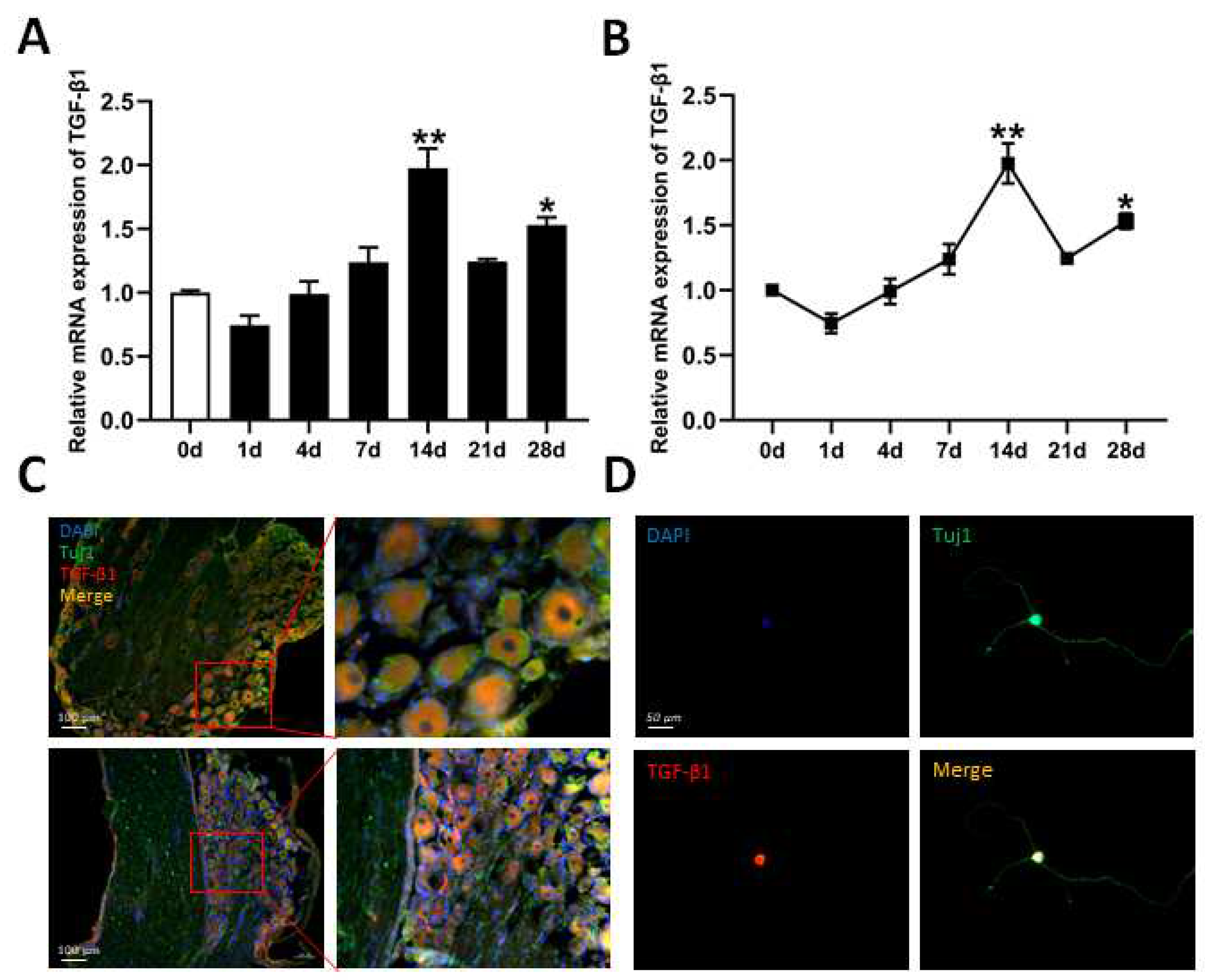
3.1. Expression of TGF-β1 in DRG After Sciatic Nerve Injury
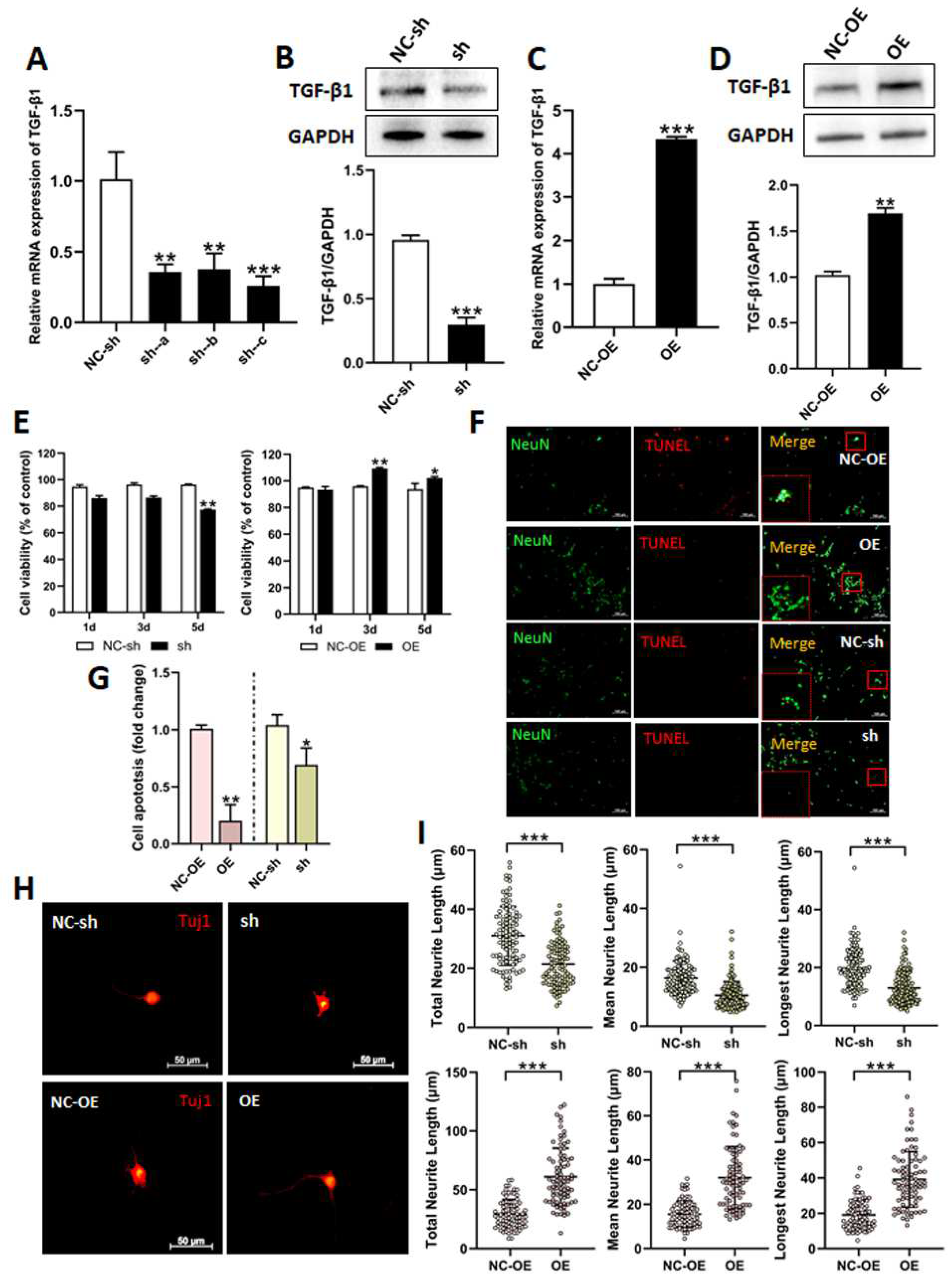
3.2. Effects of TGF-β1 on DRG Neuron Cells
3.3. Effects of TGF-β1 on Functional Recovery After Sciatic Nerve Injury
3.4. Effects of TGF-β1 on Nerve Regeneration After Sciatic Nerve Injury
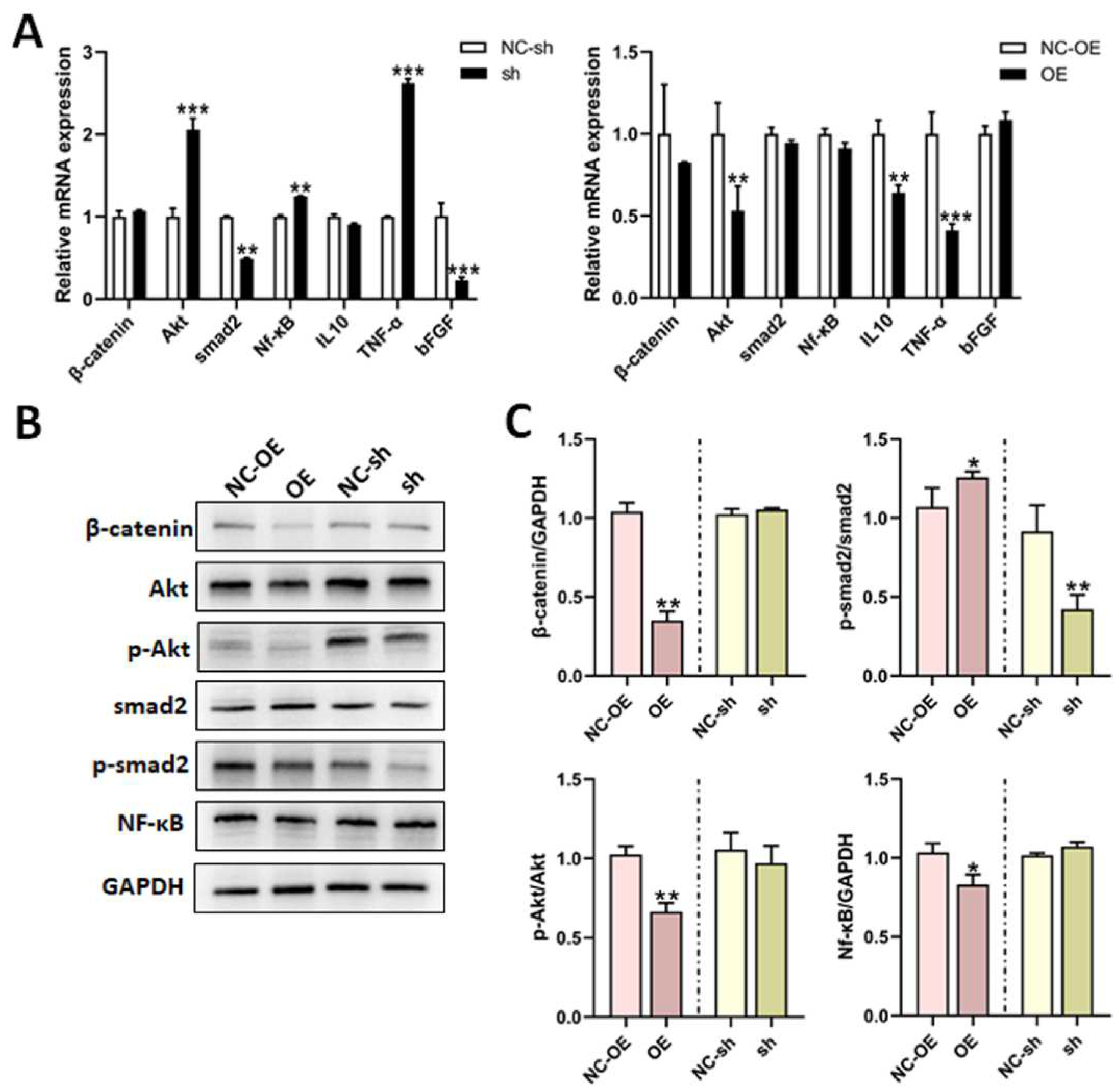
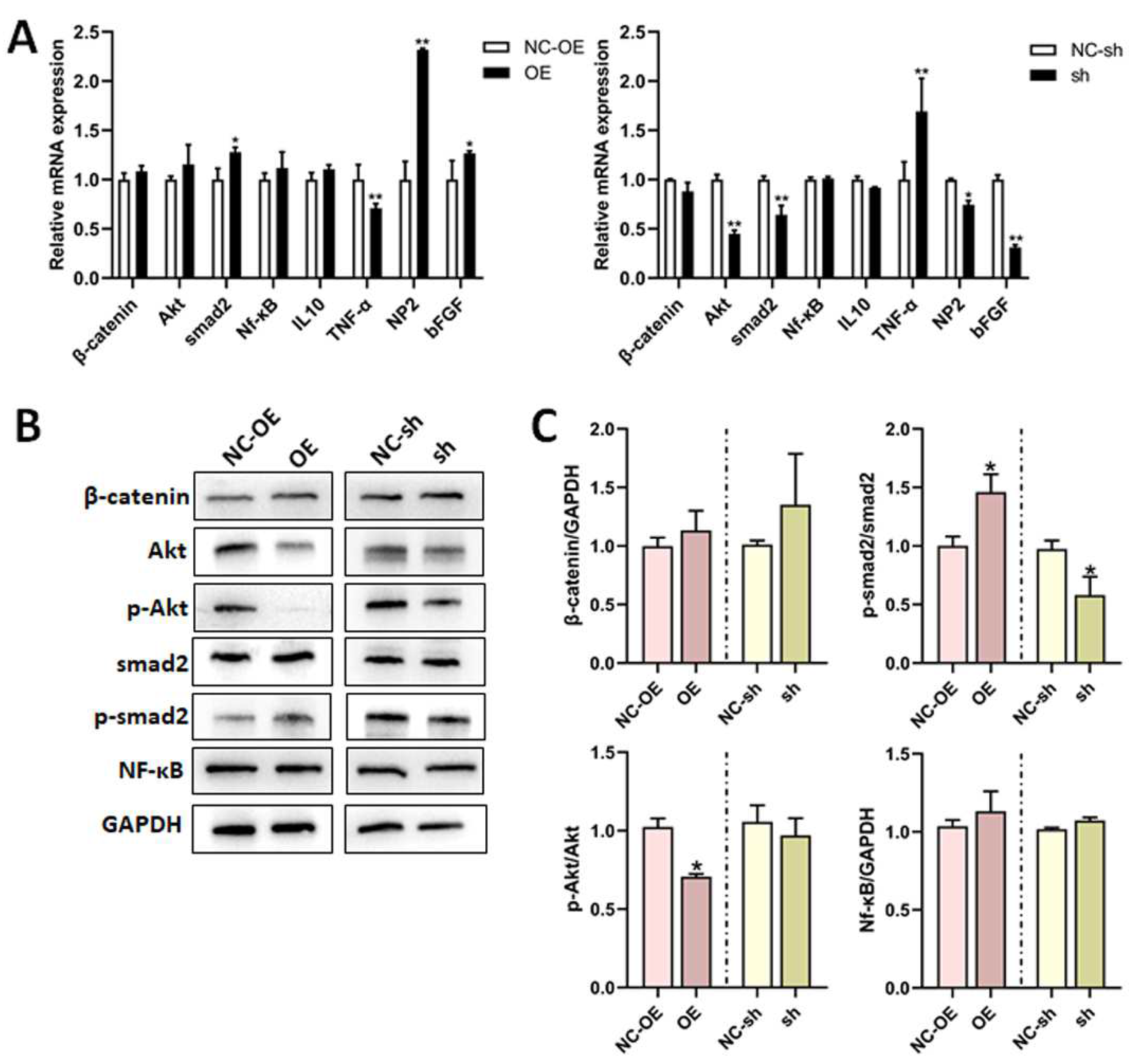
3.5. Mechanisms of TGF-β1 in Nerve Regeneration After Sciatic Nerve Injury
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juckett, L.; Saffari, T.M.; Ormseth, B.; Senger, J.-L.; Moore, A.M. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Divac, N.; Aksić, M.; Rasulić, L.; Jakovčevski, M.; Basailović, M.; Jakovčevski, I. Pharmacology of repair after peripheral nerve injury. Int. J. Clin. Pharmacol. Ther. 2021, 59, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp. Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef] [PubMed]
- O’brien, A.L.; West, J.M.; Saffari, T.M.; Nguyen, M.; Moore, A.M. Promoting Nerve Regeneration: Electrical Stimulation, Gene Therapy, and Beyond. Physiology 2022, 37, 302–310. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Reis, R.L.; Oliveira, J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv. Exp. Med. Biol. 2020, 1249, 173–201. [Google Scholar]
- Meltzer, S.; Santiago, C.; Sharma, N.; Ginty, D.D. The cellular and molecular basis of somatosensory neuron development. Neuron 2021, 109, 3736–3757. [Google Scholar] [CrossRef]
- Lei, M.; Wang, W.; Zhang, H.; Gong, J.; Wang, Z.; Cai, H.; Yang, X.; Wang, S.; Ma, C. Cell-cell and cell-matrix adhesion regulated by Piezo1 is critical for stiffness-dependent DRG neuron aggregation. Cell Rep. 2023, 42, 113522. [Google Scholar] [CrossRef]
- Feng, R.; Saraswathy, V.M.; Mokalled, M.H.; Cavalli, V. Self-renewing macrophages in dorsal root ganglia contribute to promote nerve regeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2215906120. [Google Scholar] [CrossRef]
- An, S.; Shi, J.; Huang, J.; Li, Z.; Feng, M.; Cao, G. HIF-1α Induced by Hypoxia Promotes Peripheral Nerve Injury Recovery Through Regulating Ferroptosis in DRG Neuron. Mol. Neurobiol. 2024, 61, 6300–6311. [Google Scholar] [CrossRef]
- Avraham, O.; Feng, R.; Ewan, E.E.; Rustenhoven, J.; Zhao, G.; Cavalli, V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. eLife 2021, 10, e68457. [Google Scholar] [CrossRef]
- Terada, Y.; Morita-Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Shinjo, T.; Kawaguchi, M.; Wanaka, A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci. Lett. 2018, 686, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, L.-J.; Sun, Q.-L.; Gao, Y.-S.; Yang, Y.-D.; Tang, X.-S.; Zhao, D.-Y.; Xiong, Y.; Hu, Z.-G.; Li, C.-H.; et al. Identification of Key Pathways and Genes in L4 Dorsal Root Ganglion (DRG) After Sciatic Nerve Injury via Microarray Analysis. J. Investig. Surg. 2020, 33, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Oronsky, B.; Carter, C.A.; Oronsky, A.; Knox, S.J.; Sher, D.; Reid, T.R. TGF-beta: A master immune regulator. Expert Opin. Ther. Targets 2020, 24, 427–438. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Guo, W.; Liu, H.; Yan, Y.; Wu, D.; Yao, H.; Lin, K.; Li, X. Targeting the TGF-beta signaling pathway: An updated patent review (2021-present). Expert Opin. Ther. Pat. 2024, 34, 99–126. [Google Scholar] [CrossRef]
- Lomelí-Nieto, J.A.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Navarro-Zarza, J.E.; Godínez-Rubí, J.M.; García-Arellano, S.; Ramírez-Dueñas, M.G.; Parra-Rojas, I.; Villanueva-Pérez, A.; Hernández-Bello, J. Transforming growth factor beta isoforms and TGF-βR1 and TGF-βR2 expression in systemic sclerosis patients. Clin. Exp. Med. 2023, 23, 471–481. [Google Scholar] [CrossRef]
- Ye, Z.; Wei, J.; Zhan, C.; Hou, J. Role of Transforming Growth Factor Beta in Peripheral Nerve Regeneration: Cellular and Molecular Mechanisms. Front. Neurosci. 2022, 16, 917587. [Google Scholar] [CrossRef]
- Yao, D.; Li, M.; Shen, D.; Ding, F.; Lu, S.; Zhao, Q.; Gu, X. Gene expression profiling of the rat sciatic nerve in early Wallerian degeneration after injury. Neural Regen. Res. 2012, 7, 1285–1292. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Feng, Y.-M.; Shao, J.; Cai, M.; Zhou, Y.-Y.; Yao, Y.; Qian, J.-X.; Ding, Z.-H.; Jiang, M.-R.; Yao, D.-B. Long noncoding RNA H19 regulates degeneration and regeneration of injured peripheral nerves. Neural Regen. Res. 2023, 18, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, R.; Lyu, J.; Li, X.; Wang, W.; Wang, Z.; Sheng, H.; Zhang, W.; Karhausen, J.; Yang, W. MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation. J. Neuroinflamm. 2020, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Cheng, Q.; Su, W.; Wang, C.; Yang, Y.; Cao, Z.; Ding, F. The beneficial effect of chitooligosaccharides on cell behavior and function of primary schwann cells is accompanied by up-regulation of adhesion proteins and neurotrophins. Neurochem. Res. 2014, 39, 2047–2057. [Google Scholar] [CrossRef]
- Henley, R.; Chandrasekaran, V.; Giulivi, C. Computing neurite outgrowth and arborization in superior cervical ganglion neurons. Brain Res. Bull. 2019, 144, 194–199. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Y.; Feng, Y.; Qiu, Z.; Luo, S.; Shi, X.; Gu, D.; Jiang, M.; Cai, M.; Yao, D. Fas ligand regulate nerve injury and repair by affecting AKT, β-catenin, and NF-κB pathways. IBRO Neurosci. Rep. 2024, 16, 455–467. [Google Scholar] [CrossRef]
- Jin, G.-H.; Zhao, H.-Y.; Zhang, S.-T.; Cheng, X.; Li, H.-M.; Zhang, L.; He, H.; Qin, J.-B.; Zhang, W.-Y.; Sun, Y. Long non-coding RNA GAS5 promotes PC12 cells differentiation into Tuj1-positive neuron-like cells and induces cell cycle arrest. Neural Regen. Res. 2019, 14, 2118–2125. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Mi, D.; Gu, X.; Hu, W. Periodical assessment of electrophysiological recovery following sciatic nerve crush via surface stimulation in rats. Neurol. Sci. 2015, 36, 449–456. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Pei, X.; Zheng, P.; Miao, L.; Li, L.; Luo, C.; Zhang, P.; Jiang, B.; Teng, J.; et al. SCG10 is required for peripheral axon maintenance and regeneration in mice. J. Cell Sci. 2023, 136, jcs260490. [Google Scholar] [CrossRef]
- Gu, D.; Xia, Y.; Ding, Z.; Qian, J.; Gu, X.; Bai, H.; Jiang, M.; Yao, D. Inflammation in the Peripheral Nervous System after Injury. Biomedicines 2024, 12, 1256. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Li, C.; Xu, W.; Guan, Y.; Li, X.; Cheng, H.; Chen, S.; Yang, B.; Liu, Y.; et al. Electrical stimulation accelerates Wallerian degeneration and promotes nerve regeneration after sciatic nerve injury. Glia 2023, 71, 758–774. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, J.; Li, R.; Peng, Y.; Huang, J.; Huang, L. Basic fibroblast growth factor accelerates myelin debris clearance through activating autophagy to facilitate early peripheral nerve regeneration. J. Cell. Mol. Med. 2021, 25, 2596–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Huffman, L.D.; Hafner, H.; Athaiya, M.; Finneran, M.C.; Kalinski, A.L.; Kohen, R.; Flynn, C.; Passino, R.; Johnson, C.N.; et al. The injured sciatic nerve atlas (iSNAT), insights into the cellular and molecular basis of neural tissue degeneration and regeneration. Elife 2022, 11, e80881. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wen, J.; Fu, L.; Liao, L.; Zou, Y.; Zhang, J.; Deng, J.; Zhang, H.; Liu, J.; Wang, X.; et al. Macrophage-specific RhoA knockout delays Wallerian degeneration after peripheral nerve injury in mice. J. Neuroinflamm. 2021, 18, 234. [Google Scholar] [CrossRef]
- Lanier, S.T.; Hill, J.R.; Dy, C.J.; Brogan, D.M. Evolving Techniques in Peripheral Nerve Regeneration. J. Hand Surg. Am. 2021, 46, 695–701. [Google Scholar]
- Yao, D.; Li, M.; Shen, D.; Ding, F.; Lu, S.; Zhao, Q.; Gu, X. Expression changes and bioinformatic analysis of Wallerian degeneration after sciatic nerve injury in rat. Neurosci. Bull. 2013, 29, 321–332. [Google Scholar] [CrossRef]
- Xie, L.; Yin, Y.; Jayakar, S.; Kawaguchi, R.; Wang, Q.; Peterson, S.; Shi, C.; Turnes, B.L.; Zhang, Z.; Oses-Prieto, J.; et al. The oncomodulin receptor ArmC10 enables axon regeneration in mice after nerve injury and neurite outgrowth in human iPSC–derived sensory neurons. Sci. Transl. Med. 2023, 15, eadg6241. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, M.; Chen, D.; Deng, C.; Zhang, Q.; Gu, X.; Ding, F. Chitosan Nerve Grafts Incorporated with SKP-SC-EVs Induce Peripheral Nerve Regeneration. Tissue Eng. Regen. Med. 2023, 20, 309–322. [Google Scholar] [CrossRef]
- Hausott, B.; Klimaschewski, L. Promotion of Peripheral Nerve Regeneration by Stimulation of the Extracellular Signal-Regulated Kinase (ERK) Pathway. Anat. Rec. 2019, 302, 1261–1267. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef]
- Liu, X.; Zou, D.; Hu, Y.; He, Y.; Lu, J. Research Progress of Low-Intensity Pulsed Ultrasound in the Repair of Peripheral Nerve Injury. Tissue Eng. Part B Rev. 2023, 29, 414–428. [Google Scholar] [CrossRef]
- Meng, Q.; Burrell, J.C.; Zhang, Q.; Le, A.D. Potential Application of Orofacial MSCs in Tissue Engineering Nerve Guidance for Peripheral Nerve Injury Repair. Stem Cell Rev. Rep. 2023, 19, 2612–2631. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Parkinson, D.B.; Dun, X. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Huang, Q.-M.; Hu, D.-X.; Zhang, W.-J. Therapeutic effect of exosomes derived from Schwann cells in the repair of peripheral nerve injury. Life Sci. 2024, 357, 123086. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar]
- Chen, J.; Zhu, Y.; Gao, H.; Chen, X.; Yi, D.; Li, M.L.; Wang, L.; Xing, G.; Chen, S.; Tang, J.; et al. HucMSCs Delay Muscle Atrophy After Peripheral Nerve Injury Through Exosomes by Repressing Muscle-Specific Ubiquitin Ligases. Stem Cells 2024, 42, 460–474. [Google Scholar] [CrossRef]
- Mehrotra, P.; Jablonski, J.; Toftegaard, J.; Zhang, Y.; Shahini, S.; Wang, J.; Hung, C.W.; Ellis, R.; Kayal, G.; Rajabian, N.; et al. Skeletal muscle reprogramming enhances reinnervation after peripheral nerve injury. Nat. Commun. 2024, 15, 9218. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury. Prog. Neurobiol. 2019, 173, 102–121. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Zou, Y.; Fu, L.; Ma, X.; Zhang, H.; Xu, Y.; Xu, J.; Zhang, J.; Li, M.; et al. Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 2022, 17, 673–681. [Google Scholar]
- Liu, B.; Xin, W.; Tan, J.-R.; Zhu, R.-P.; Li, T.; Wang, D.; Kan, S.-S.; Xiong, D.-K.; Li, H.-H.; Zhang, M.-M.; et al. Myelin sheath structure and regeneration in peripheral nerve injury repair. Proc. Natl. Acad. Sci. USA 2019, 116, 22347–22352. [Google Scholar] [CrossRef]
- Pottorf, T.S.; Rotterman, T.M.; McCallum, W.M.; Haley-Johnson, Z.A.; Alvarez, F.J. The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury. Cells 2022, 11, 2083. [Google Scholar] [CrossRef]
- Faroni, A.; Martin, S.L.; Reid, A.J.; Verkhratsky, A.; Magnaghi, V. Gene expression changes in dorsal root ganglia following peripheral nerve injury: Roles in inflammation, cell death and nociception. Neural Regen. Res. 2019, 14, 939–947. [Google Scholar] [CrossRef]
- Kalinski, A.L.; Yoon, C.; Huffman, L.D.; Duncker, P.C.; Kohen, R.; Passino, R.; Hafner, H.; Johnson, C.; Kawaguchi, R.; Carbajal, K.S.; et al. Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement. eLife 2020, 9, e60223. [Google Scholar] [CrossRef]




| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | TGGAGTCTACTGGCGTCTT |
| Reverse | TGTCATATTTCTCGTGGTTCA | |
| TGF-β1 | Forward | GGCTGAACCAAGGAGACGG |
| Reverse | CCTCGACGTTTGGGACTGAT | |
| TNF-α | Forward | ATGGGCTCCCTCTCATCAGT |
| Reverse | GCTTGGTGGTTTGCTACGAC | |
| IL-10 | Forward | GGGAGAGAAGCTGAAGACCC |
| Reverse | ACACCTTTGTCTTGGAGCTTATTA | |
| Akt | Forward | CCGGTGAACTCTGACCCTTG |
| Reverse | GGCCGCAGCGTCTTCAT | |
| β-catenin | Forward | GGAGCTAAAATGGCAGTGCG |
| Reverse | GGCCAGAATGATGAGCTTGC | |
| bFGF | Forward | CCCGCACCCTATCCCTTCACAGC |
| Reverse | CACAACGACCAGCCTTCCACCCAAA | |
| NF-κB | Forward | ACAATAACCCCTTTCAAGTTCCC |
| Reverse | AATCGGATGCGAGAGGACAG | |
| Smad2 | Forward | GCCGCCCGAAGGGTAGAT |
| Reverse | AGACCCACCGGCTGATTTTT | |
| NP2 | Forward | GAGAAGTCCCTGCTCCACAA |
| Reverse | TTGAATGCACTGTTGCCTCTCT |
| Antibody | Sources | Catalogue Number |
|---|---|---|
| GAPDH | Proteintech | 60004-1-Ig |
| TGF-β1 | Abcam | Ab215715 |
| smad2 | Abcam | Ab40855 |
| p-smad2 | Abcam | Ab188334 |
| AKT | Cell signaling | 4691T |
| p-AKT | Cell signaling | 4060T |
| β-catenin | Cell signaling | 8480T |
| NF-kB | Cell signaling | 8214T |
| F4/80 | Abcam | Ab307470 |
| TUBB3 (Tuj1) | Abways | AB0043 |
| TUBB3 (Tuj1) | Proteintech | 66375-1-Ig |
| STMN2 (SCG10) | Proteintech | 10586-1-AP |
| NeuN | Abcam | Ab177487 |
| Goat Anti-Mouse IgG HRP | Abways | AB0102 |
| Goat Anti-Rabbit IgG HRP | Abways | AB0101 |
| Cy3-conjugated Goat Anti-Rabbit | Proteintech | SA00009-2 |
| CoraLite488-conjugated Goat Anti-Rabbit | Proteintech | SA00013-2 |
| CoraLite488-conjugated Goat Anti-Mouse | Proteintech | SA00013-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Ding, Z.; Huang, Y.; Jiang, T.; Xia, Y.; Gu, D.; Gu, X.; Bai, H.; Yao, D. TGF-β1 Improves Nerve Regeneration and Functional Recovery After Sciatic Nerve Injury by Alleviating Inflammation. Biomedicines 2025, 13, 872. https://doi.org/10.3390/biomedicines13040872
Jiang M, Ding Z, Huang Y, Jiang T, Xia Y, Gu D, Gu X, Bai H, Yao D. TGF-β1 Improves Nerve Regeneration and Functional Recovery After Sciatic Nerve Injury by Alleviating Inflammation. Biomedicines. 2025; 13(4):872. https://doi.org/10.3390/biomedicines13040872
Chicago/Turabian StyleJiang, Maorong, Zihan Ding, Yuxiao Huang, Taoran Jiang, Yiming Xia, Dandan Gu, Xi Gu, Huiyuan Bai, and Dengbing Yao. 2025. "TGF-β1 Improves Nerve Regeneration and Functional Recovery After Sciatic Nerve Injury by Alleviating Inflammation" Biomedicines 13, no. 4: 872. https://doi.org/10.3390/biomedicines13040872
APA StyleJiang, M., Ding, Z., Huang, Y., Jiang, T., Xia, Y., Gu, D., Gu, X., Bai, H., & Yao, D. (2025). TGF-β1 Improves Nerve Regeneration and Functional Recovery After Sciatic Nerve Injury by Alleviating Inflammation. Biomedicines, 13(4), 872. https://doi.org/10.3390/biomedicines13040872





