New Cardiovascular Risk Biomarkers in Rheumatoid Arthritis: Implications and Clinical Utility—A Narrative Review
Abstract
1. Introduction
2. Methods
3. Interleukin-32
3.1. Clinical Evidence on the Role of IL-32 in CVD
3.2. Clinical Evidence on the Role of IL-32 in RA
3.3. Mechanisms of Action of IL-32
3.4. Evidence Supporting IL-32 as a Biomarker of CVD Risk in RA
4. Dickkopf-1
4.1. Clinical Evidence on the Role of DKK-1 in CVD
4.2. Clinical Evidence on the Role of DKK-1 in RA
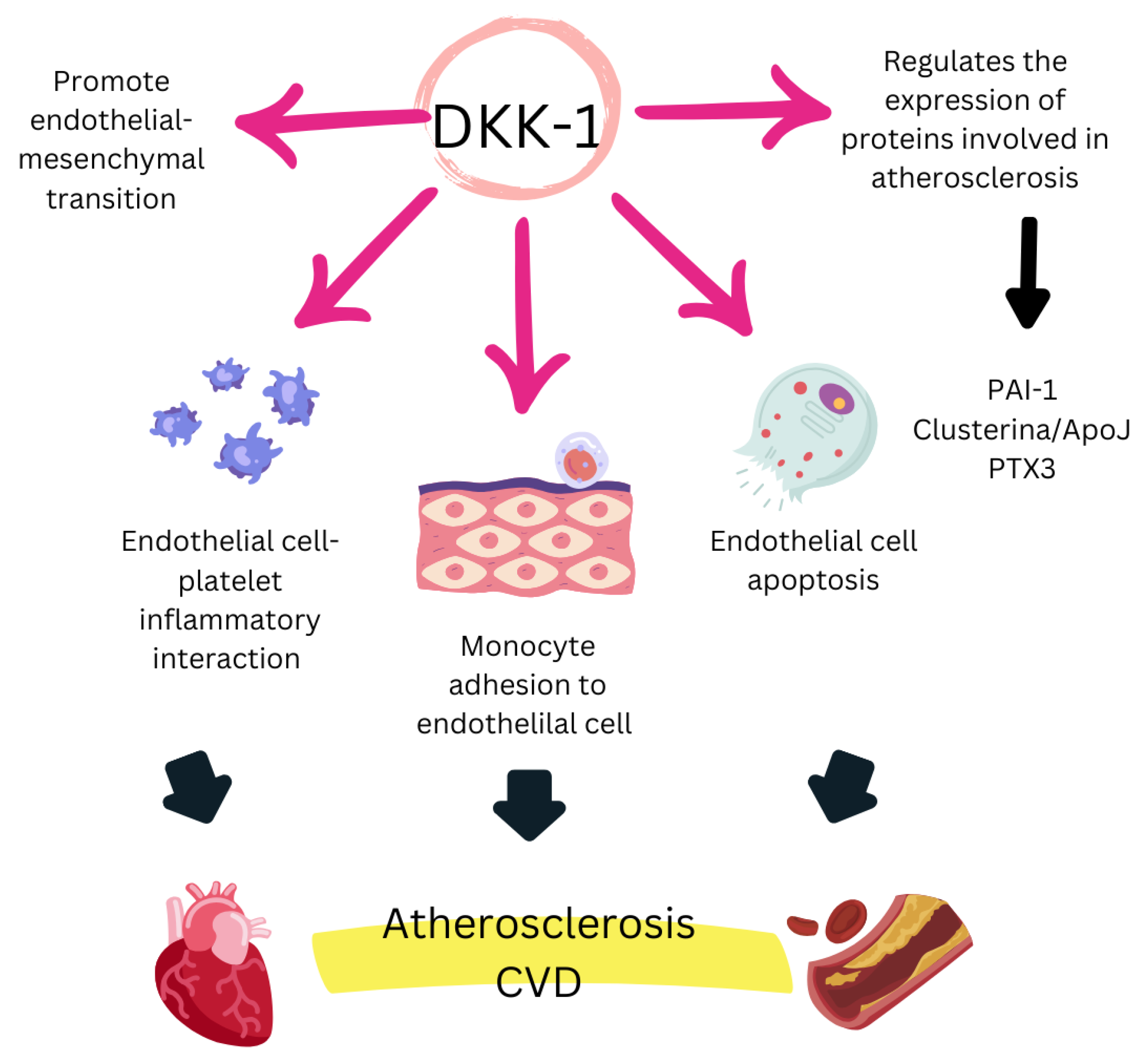
4.3. Mechanisms of Action of DKK-1
4.4. Evidence Supporting DKK-1 as a Biomarker of CVD Risk in RA
5. Galectin-3
5.1. Clinical Evidence on the Role of Gal-3 in CVD
5.2. Clinical Evidence on the Role of Gal-3 in RA
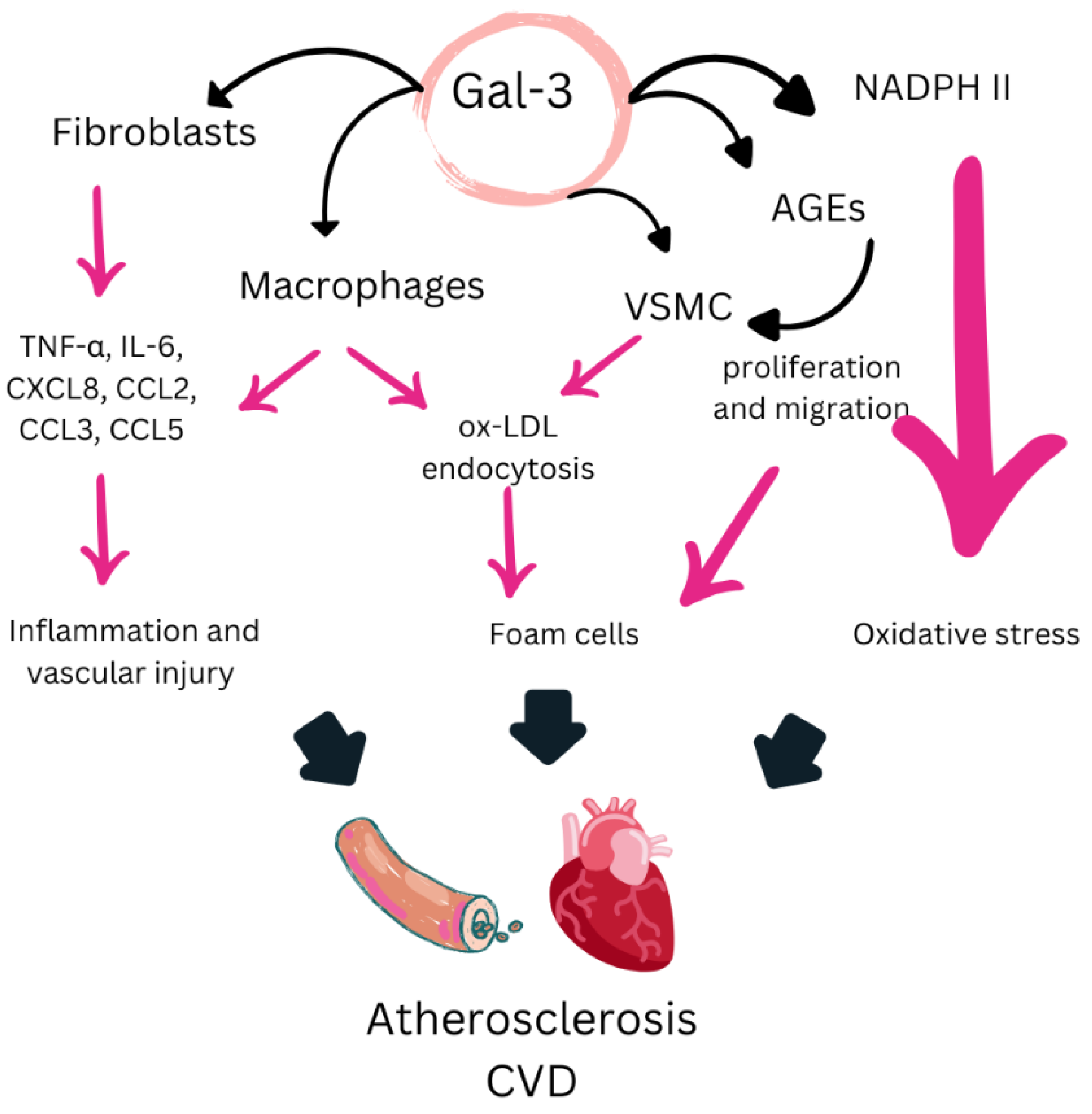
5.3. Mechanisms of Action of Gal-3
5.4. Evidence Supporting Gal-3 as a Biomarker of CVD Risk in RA
6. Catestatin
6.1. Clinical Evidence on the Role of CST in CVD
6.2. Clinical Evidence on the Role of CST in RA
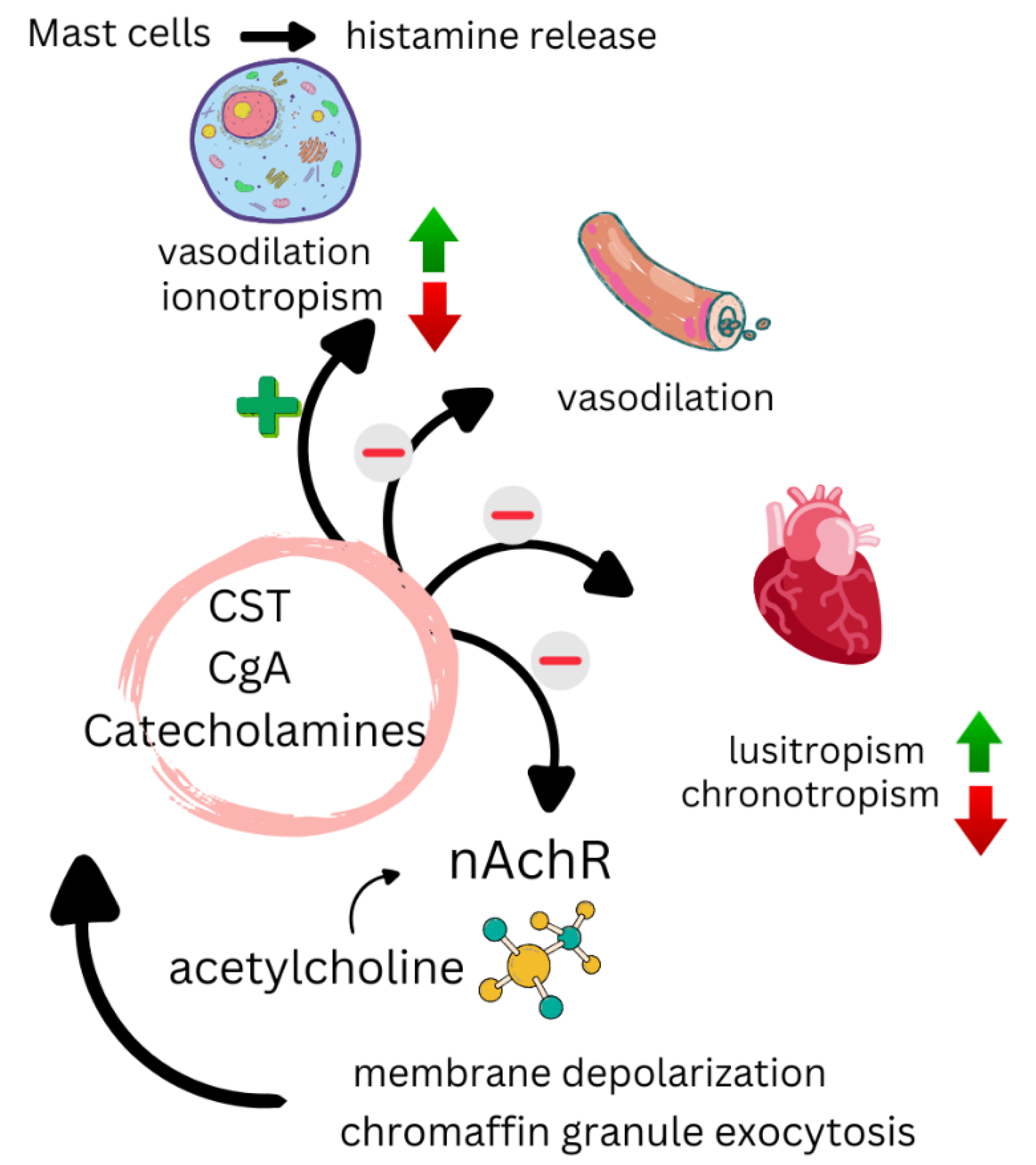
6.3. Mechanisms of Action of CST
6.4. Evidence Supporting CST as a Biomarker of CVD Risk in RA
7. Fetuin-A
7.1. Clinical Evidence on the Role of Fet-A in CVD
7.2. Clinical Evidence on the Role of Fet-A in RA
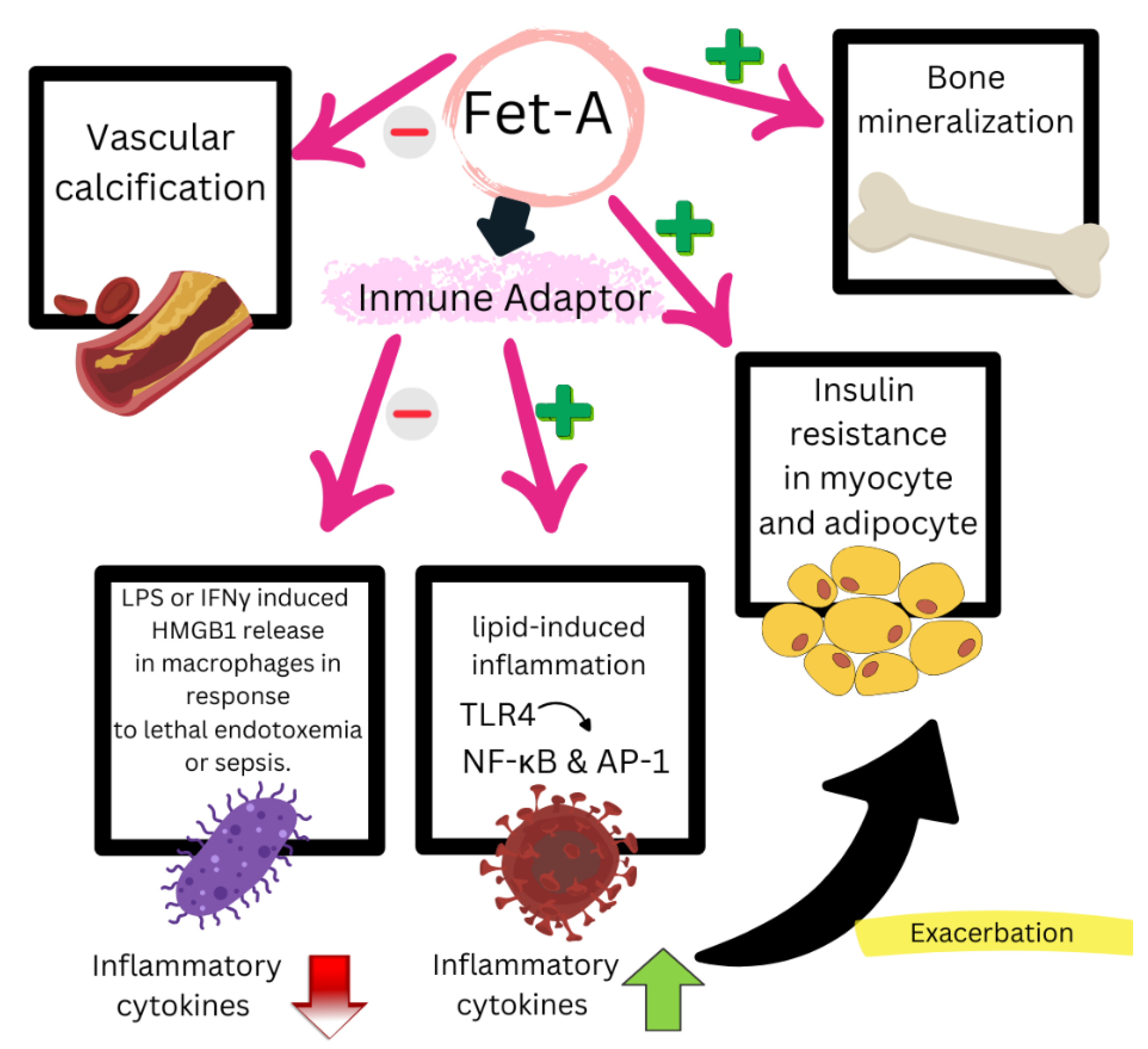
7.3. Mechanisms of Action of Fet-A
7.4. Evidence Supporting Fet-A as a Biomarker of CVD Risk in RA
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabriel, S.E.; Crowson, C.S.; Kremers, H.M.; Doran, M.F.; Turesson, C.; O’Fallon, W.M.; Matteson, E.L. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum. 2003, 48, 54–58. [Google Scholar] [PubMed]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Tanasescu, C.; Jurcut, C.; Jurcut, R.; Ginghina, C. Vascular disease in rheumatoid arthritis: From subclinical lesions to cardiovascular risk. Eur. J. Intern. Med. 2009, 20, 348–354. [Google Scholar]
- Castañeda, S.; Martín-Martínez, M.A.; González-Juanatey, C.; Llorca, J.; García-Yébenes, M.J.; Pérez-Vicente, S.; Sánchez-Costa, J.T.; Díaz-Gonzalez, F.; González-Gay, M.A. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project. Semin. Arthritis Rheum. 2015, 44, 618–626. [Google Scholar]
- van Halm, V.P.; Peters, M.J.L.; Voskuyl, A.E.; Boers, M.; Lems, W.F.; Visser, M.; Stehouwer, C.D.A.; Spijkerman, A.M.W.; Dekker, J.M.; Nijpels, G.; et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE Investigation. Ann. Rheum. Dis. 2009, 68, 1395–1400. [Google Scholar] [CrossRef]
- Meune, C.; Touzé, E.; Trinquart, L.; Allanore, Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch. Cardiovasc. Dis. 2010, 103, 253–261. [Google Scholar]
- Taverner, D.; Vallvé, J.C.; Ferré, R.; Paredes, S.; Masana, L.; Castro, A. Variables associated with subclinical atherosclerosis in a cohort of rheumatoid arthritis patients: Sex-specific associations and differential effects of disease activity and age. PLoS ONE 2018, 13, e0193690. [Google Scholar]
- Taverner, D.; Paredes, S.; Ferré, R.; Masana, L.; Castro, A.; Vallvé, J.C. Assessment of arterial stiffness variables in patients with rheumatoid arthritis: A mediation analysis. Sci. Rep. 2019, 9, 4543. [Google Scholar]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Yoshitomi, H. Regulation of Immune Responses and Chronic Inflammation by Fibroblast-Like Synoviocytes. Front. Immunol. 2019, 10, 1395. [Google Scholar]
- Stevens, R.J.; Douglas, K.M.J.; Saratzis, A.N.; Kitas, G.D. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev. Mol. Med. 2005, 7, 1–24. [Google Scholar] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.N.; Giles, J.T.; Liao, K.P. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat. Rev. Rheumatol. 2023, 19, 417–428. [Google Scholar]
- Paredes, S.; Girona, J.; Hurt-Camejo, E.; Vallvé, J.C.; Olivé, S.; Heras, M.; Benito, P.; Masana, L. Antioxidant vitamins and lipid peroxidation in patients with rheumatoid arthritis: Association with inflammatory markers. J. Rheumatol. 2002, 29, 2271–2277. [Google Scholar] [CrossRef]
- Vallvé, J.C.; Paredes, S.; Girona, J.; Uliaque, K.; Ribalta, J.; Hurt-Camejo, E.; Masana, L. Tumor necrosis factor-α-1031 T/C polymorphism is associated with smaller and more proatherogenic low density lipoprotein particles in patients with rheumatoid arthritis. J. Rheumatol. 2008, 35, 1697–1703. [Google Scholar]
- Baghdadi, L.R.; Woodman, R.J.; Shanahan, E.M.; Mangoni, A.A. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117952. [Google Scholar] [CrossRef]
- Dougados, M.; Soubrier, M.; Antunez, A.; Balint, P.; Balsa, A.; Buch, M.H.; Casado, G.; Detert, J.; El-Zorkany, B.; Emery, P.; et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: Results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 2014, 73, 62–68. [Google Scholar] [CrossRef]
- Steiner, G.; Urowitz, M.B. Lipid profiles in patients with rheumatoid arthritis: Mechanisms and the impact of treatment. Semin. Arthritis. Rheum. 2009, 38, 372–381. [Google Scholar]
- Hurt-Camejo, E.; Paredes, S.; Masana, L.; Camejo, G.; Sartipy, P.; Rosengren, B.; Pedreno, J.; Vallve, J.C.; Benito, P.; Wiklund, O. Elevated levels of small, low-density lipoprotein with high affinity for arterial matrix components in patients with rheumatoid arthritis: Possible contribution of phospholipase A2 to this atherogenic profile. Arthritis Rheum. 2001, 44, 2761–2767. [Google Scholar] [CrossRef]
- van Breukelen-van der Stoep, D.F.; van Zeben, D.; Klop, B.; van de Geijn, G.J.M.; Janssen, H.J.W.; van der Meulen, N.; Njo, T.L.; Birnie, E.; van Zeben, J. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology 2016, 55, 1210–1216. [Google Scholar]
- Burggraaf, B.; van Breukelen-van der Stoep, D.F.; de Vries, M.A.; Klop, B.; van Zeben, J.; van de Geijn, G.J.M.; Van Der Meulen, N.; Birnie, E.; Prinzen, L.; Cabezas, M.C. Progression of subclinical atherosclerosis in subjects with rheumatoid arthritis and the metabolic syndrome. Atherosclerosis 2018, 271, 84–91. [Google Scholar] [PubMed]
- Sheng, X.; Murphy, M.J.; Macdonald, T.M.; Wei, L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J. Rheumatol. 2012, 39, 32–40. [Google Scholar] [CrossRef] [PubMed]
- De Vera, M.A.; Choi, H.; Abrahamowicz, M.; Kopec, J.; Lacaille, D. Impact of statin discontinuation on mortality in patients with rheumatoid arthritis: A population-based study. Arthritis Care Res. 2012, 64, 809–816. [Google Scholar]
- Burggraaf, B.; van Breukelen-van der Stoep, D.F.; de Vries, M.A.; Klop, B.; Liem, A.H.; van de Geijn, G.J.M.; van der Meulen, N.; Birnie, E.; van der Zwan, E.M.; van Zeben, J.; et al. Effect of a treat-to-target intervention of cardiovascular risk factors on subclinical and clinical atherosclerosis in rheumatoid arthritis: A randomised clinical trial. Ann. Rheum. Dis. 2019, 78, 335–341. [Google Scholar]
- Solomon, D.H.; Reed, G.W.; Kremer, J.M.; Curtis, J.R.; Farkouh, M.E.; Harrold, L.R.; Hochberg, M.C.; Tsao, P.; Greenberg, J.D. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455. [Google Scholar]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar]
- Fuertes-Martín, R.; Taverner, D.; Vallvé, J.C.; Paredes, S.; Masana, L.; Correig Blanchar, X.; Grau, N.A. Characterization of 1H NMR Plasma Glycoproteins as a New Strategy To Identify Inflammatory Patterns in Rheumatoid Arthritis. J. Proteome Res. 2018, 17, 3730–3739. [Google Scholar]
- Yang, S.; Ye, Z.M.; Chen, S.; Luo, X.Y.; Chen, S.L.; Mao, L.; Li, Y.; Jin, H.; Yu, C.; Xiang, F.X.; et al. MicroRNA-23a-5p promotes atherosclerotic plaque progression and vulnerability by repressing ATP-binding cassette transporter A1/G1 in macrophages. J. Mol. Cell. Cardiol. 2018, 123, 139–149. [Google Scholar] [CrossRef]
- Ormseth, M.J.; Solus, J.F.; Sheng, Q.; Chen, S.C.; Ye, F.; Wu, Q.; Oeser, A.M.; Allen, R.; Raggi, P.; Vickers, K.C.; et al. Plasma miRNAs improve the prediction of coronary atherosclerosis in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 2211–2219. [Google Scholar] [CrossRef]
- Taverner, D.; Llop, D.; Rosales, R.; Ferré, R.; Masana, L.; Vallvé, J.C.; Paredes, S. Plasma expression of microRNA-425-5p and microRNA-451a as biomarkers of cardiovascular disease in rheumatoid arthritis patients. Sci. Rep. 2021, 11, 15670. [Google Scholar]
- Llop, D.; Ibarretxe, D.; Plana, N.; Rosales, R.; Taverner, D.; Masana, L.; Vallvé, J.C.; Paredes, S. A panel of plasma microRNAs improves the assessment of surrogate markers of cardiovascular disease in rheumatoid arthritis patients. Rheumatology 2023, 62, 1677–1686. [Google Scholar]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar]
- Damen, M.S.M.A.; Popa, C.D.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis 2017, 264, 83–91. [Google Scholar]
- Yang, Z.; Shi, L.; Xue, Y.; Zeng, T.; Shi, Y.; Lin, Y.; Liu, L. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin. Chim. Acta. 2019, 497, 104–109. [Google Scholar]
- Xuan, W.; Huang, W.; Wang, R.; Chen, C.; Chen, Y.; Wang, Y.; Tan, X. Elevated circulating IL-32 presents a poor prognostic outcome in patients with heart failure after myocardial infarction. Int. J. Cardiol. 2017, 243, 367–373. [Google Scholar]
- Li, Y.; Wang, Z. Interleukin 32 participates in cardiomyocyte-induced oxidative stress, inflammation and apoptosis during hypoxia/reoxygenation via the NOD2/NOX2/MAPK signaling pathway. Exp. Ther. Med. 2022, 24, 567. [Google Scholar]
- Alehagen, U.; Shamoun, L.; Dimberg, J.I.; Wågsäter, D. Increased mortality in the A/A genotype of the SNP rs28372698 of interleukin 32. Exp. Ther. Med. 2021, 21, 127. [Google Scholar]
- Joosten, L.A.B.; Netea, M.G.; Kim, S.H.; Yoon, D.Y.; Oppers-Walgreen, B.; Radstake, T.R.D.; Barrera, P.; van de Loo, F.A.; Dinarello, C.A.; van den Berg, W.B. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2006, 103, 3298–3303. [Google Scholar]
- Heinhuis, B.; Koenders, M.I.; van de Loo, F.A.; Netea, M.G.; van den Berg, W.B.; Joosten, L.A.B. Inflammation-dependent secretion and splicing of IL-32γ in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 4962–4967. [Google Scholar]
- Cagnard, N.; Letourneur, F.; Essabbani, A.; Devauchelle, V.; Mistou, S.; Rapinat, A.; Decraene, C.; Fournier, C.; Chiocchia, G. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur. Cytokine Netw. 2005, 16, 289–292. [Google Scholar] [PubMed]
- Park, Y.E.; Kim, G.T.; Lee, S.G.; Park, S.H.; Baek, S.H.; Kim, S.I.; Kim, J.I.; Jin, H.S. IL-32 aggravates synovial inflammation and bone destruction and increases synovial natural killer cells in experimental arthritis models. Rheumatol. Int. 2013, 33, 671–679. [Google Scholar] [PubMed]
- Kim, S.H.; Han, S.Y.; Azam, T.; Yoon, D.Y.; Dinarello, C.A. Interleukin-32: A cytokine and inducer of TNFα. Immunity 2005, 22, 131–142. [Google Scholar]
- Shoda, H.; Fujio, K.; Yamaguchi, Y.; Okamoto, A.; Sawada, T.; Kochi, Y.; Kazuhiko, Y. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res. Ther. 2006, 8, R166. [Google Scholar]
- Heinhuis, B.; Koenders, M.I.; van Riel, P.L.; van de Loo, F.A.; Dinarello, C.A.; Netea, M.G.; van den Berg, W.B.; Joosten, L.A. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann. Rheum. Dis. 2011, 70, 660–667. [Google Scholar]
- Mun, S.H.; Kim, J.W.; Nah, S.S.; Ko, N.Y.; Lee, J.H.; Kim, J.D.; Kim, D.K.; Kim, H.S.; Choi, J.D.; Kim, S.H.; et al. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the Syk/protein kinase Cδ/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009, 60, 678–685. [Google Scholar]
- Jung, M.Y.; Son, M.H.; Kim, S.H.; Cho, D.; Kim, T.S. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J. Immunol. 2011, 186, 6848–6859. [Google Scholar]
- Xu, Z.; Dong, A.; Feng, Z.; Li, J. Interleukin-32 promotes lipid accumulation through inhibition of cholesterol efflux. Exp. Ther. Med. 2017, 14, 947–952. [Google Scholar]
- Heinhuis, B.; Popa, C.D.; van Tits, B.L.J.H.; Kim, S.H.; Zeeuwen, P.L.; van den Berg, W.B.; van der Meer, J.W.; van der Vliet, J.A.; Stalenhoef, A.F.; Dinarello, C.A.; et al. Towards a role of interleukin-32 in atherosclerosis. Cytokine 2013, 64, 433–440. [Google Scholar]
- Pontzen, D.L.; Bahls, M.; Albrecht, D.; Felix, S.B.; Dörr, M.; Ittermann, T.; Nauck, M.; Friedrich, N. Low-grade inflammation is associated with a heterogeneous lipoprotein subclass profile in an apparently healthy population sample. Lipids Health Dis. 2023, 22, 100. [Google Scholar]
- Park, J.Y.; Park, H.M.; Kim, S.; Jeon, K.B.; Lim, C.M.; Hong, J.T.; Yoon, D.Y. Human IL-32θA94V mutant attenuates monocyte-endothelial adhesion by suppressing the expression of ICAM-1 and VCAM-1 via binding to cell surface receptor integrin αVβ3 and αVβ6 in TNF-α-stimulated HUVECs. Front. Immunol. 2023, 14, 1160301. [Google Scholar] [CrossRef] [PubMed]
- Damen, M.S.M.A.; Agca, R.; Holewijn, S.; de Graaf, J.; Dos Santos, J.C.; van Riel, P.L.; Fransen, J.; Coenen, M.J.H.; Nurmohamed, M.T.; Netea, M.G.; et al. IL-32 promoter SNP rs4786370 predisposes to modified lipoprotein profiles in patients with rheumatoid arthritis. Sci. Rep. 2017, 7, 41629. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, M.; Cherubini, A.; Pelusi, S.; Margarita, S.; Bianco, C.; Malvestiti, F.; Miano, L.; Romeo, S.; Prati, D.; Valenti, L. Circulating Interlukin-32 and Altered Blood Pressure Control in Individuals with Metabolic Dysfunction. Int. J. Mol. Sci. 2023, 24, 7465. [Google Scholar] [CrossRef]
- El-Far, M.; Durand, M.; Turcotte, I.; Larouche-Anctil, E.; Sylla, M.; Zaidan, S.; Chartrand-Lefebvre, C.; Bunet, R.; Ramani, H.; Sadouni, M.; et al. Upregulated IL-32 Expression and Reduced Gut Short Chain Fatty Acid Caproic Acid in People Living with HIV with Subclinical Atherosclerosis. Front. Immunol. 2021, 12, 664371. [Google Scholar] [CrossRef]
- El-Far, M.; Hanna, D.B.; Durand, M.; Larouche-Anctil, E.; Sylla, M.; Chartrand-Lefebvre, C.; Cloutier, G.; Goulet, J.P.B.; Kassaye, S.; Karim, R.; et al. Brief Report: Subclinical Carotid Artery Atherosclerosis Is Associated with Increased Expression of Peripheral Blood IL-32 Isoforms Among Women Living with, HIV. J. Acquir. Immune Defic. Syndr. 2021, 88, 186–191. [Google Scholar] [CrossRef]
- Bunet, R.; Roy-Cardinal, M.H.; Ramani, H.; Cleret-Buhot, A.; Durand, M.; Chartrand-Lefebvre, C.; Routy, J.-P.; Thomas, R.; Trottier, B.; Ancuta, P.; et al. Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis. Viruses 2023, 15, 700. [Google Scholar] [CrossRef]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins: Emerging New Players in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef]
- Ueland, T.; Otterdal, K.; Lekva, T.; Halvorsen, B.; Gabrielsen, A.; Sandberg, W.J.; Paulsson-Berne, G.; Pedersen, T.M.; Folkersen, L.; Gullestad, L.; et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1228–1234. [Google Scholar] [CrossRef]
- Kim, K.I.; Park, K.U.; Chun, E.J.; Choi, S.I.; Cho, Y.S.; Youn, T.J.; Cho, G.Y.; Chae, I.H.; Song, J.; Choi, D.J.; et al. A novel biomarker of coronary atherosclerosis: Serum DKK1 concentration correlates with coronary artery calcification and atherosclerotic plaques. J. Korean Med. Sci. 2011, 26, 1178–1184. [Google Scholar] [CrossRef]
- Seifert-Held, T.; Pekar, T.; Gattringer, T.; Simmet, N.E.; Scharnagl, H.; Stojakovic, T.; Fazekas, F.; Storch, M.K. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis 2011, 218, 233–237. [Google Scholar] [CrossRef]
- He, X.W.; Wang, E.; Bao, Y.Y.; Wang, F.; Zhu, M.; Hu, X.F.; Jin, X.P. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis 2016, 253, 22–28. [Google Scholar] [PubMed]
- Goliasch, G.; Wiesbauer, F.; Kastl, S.P.; Katsaros, K.M.; Blessberger, H.; Maurer, G.; Schillinger, M.; Huber, K.; Wojta, J.; Speidl, W.S. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012, 222, 251–256. [Google Scholar] [PubMed]
- Lattanzio, S.; Santilli, F.; Liani, R.; Vazzana, N.; Ueland, T.; Di Fulvio, P.; Formoso, G.; Consoli, A.; Aukrust, P.; Davì, G. Circulating dickkopf-1 in diabetes mellitus: Association with platelet activation and effects of improved metabolic control and low-dose aspirin. J. Am. Heart Assoc. 2014, 3, e001000. [Google Scholar]
- Gao, F.; Li, C.; Peng, J.; Lu, W.; Zhu, W.; Zhou, J.; Lu, J.; Ma, X. Decreased Serum Dickkopf-1 Levels After Hypoglycemic Therapy in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 2725–2732. [Google Scholar] [CrossRef]
- Ueland, T.; Åkerblom, A.; Ghukasyan, T.; Michelsen, A.E.; Becker, R.C.; Bertilsson, M.; Himmelmann, A.; James, S.K.; Siegbahn, A.; Storey, R.F.; et al. Admission Levels of DKK1 (Dickkopf-1) Are Associated with Future Cardiovascular Death in Patients with Acute Coronary Syndromes. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 294–302. [Google Scholar]
- Zhu, Z.; Guo, D.; Zhong, C.; Wang, A.; Xie, X.; Xu, T.; Chen, C.-S.; Peng, Y.; Peng, H.; Li, Q.; et al. Serum Dkk-1 (Dickkopf-1) Is a Potential Biomarker in the Prediction of Clinical Outcomes Among Patients with Acute Ischemic Stroke. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 285–293. [Google Scholar]
- Ghardashi-Afousi, A.; Davoodi, M.; Hesamabadi, B.K.; Asvadi-Fard, M.; Bigi, M.A.B.; Izadi, M.R.; Gaeini, A.A. Improved carotid intima-media thickness-induced high-intensity interval training associated with decreased serum levels of Dkk-1 and sclerostin in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107469. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Koumaras, C.; Loutradis, C.; Giamalis, P.; Tziomalos, K.; Karagiannis, A.; Papagianni, A. Increased Sclerostin, but Not Dickkopf-1 Protein, Is Associated with Elevated Pulse Wave Velocity in Hemodialysis Subjects. Kidney Blood Press Res. 2019, 44, 679–689. [Google Scholar] [CrossRef]
- Wu, C.F.; Hou, J.S.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Kuo, C.H.; Liou, H.-H.; Tsai, J.-P.; Hsu, B.-G. Serum Sclerostin But Not DKK-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Loutradis, C.; Memmos, E.; Faitatzidou, D.; Giamalis, P.; Koumaras, C.; Karagiannis, A.; Papagianni, A. Associations of serum sclerostin and Dickkopf-related protein-1 proteins with future cardiovascular events and mortality in haemodialysis patients: A prospective cohort study. Clin. Kidney J. 2021, 14, 1165–1172. [Google Scholar]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Prog. Mol. Biol. Transl. Sci. 2018, 153, 181–207. [Google Scholar] [PubMed]
- Szulc, P.; Schoppet, M.; Rachner, T.D.; Chapurlat, R.; Hofbauer, L.C. Severe abdominal aortic calcification in older men is negatively associated with DKK1 serum levels: The STRAMBO study. J. Clin. Endocrinol. Metab. 2014, 99, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hampson, G.; Edwards, S.; Conroy, S.; Blake, G.M.; Fogelman, I.; Frost, M.L. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 2013, 56, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Younis, D.; Bahie, A.; Elzehery, R.; El-Kannishy, G.; Wahab, A.M. Association between Serum Dickkopf-1 (DKK-1) Glycoprotein and Calcific Deposits on Cardiac Valves and Carotid Intimal-Medial Thickness in Hemodialysis Patients. Cardiorenal Med. 2020, 10, 313–322. [Google Scholar] [CrossRef]
- Zheng, P.F.; Rong, J.J.; Zheng, Z.F.; Liu, Z.Y.; Pan, H.W.; Liu, P. Investigating the causal effect of Dickkopf-1 on coronary artery disease and ischemic stroke: A Mendelian randomization study. Aging 2023, 15, 9797–9808. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Wang, M.; Xia, Q.; Yang, J.; Wu, M.; Han, R.; Chen, M.; Hu, X.; Yuan, Y.; et al. The serum level of Dickkopf-1 in patients with rheumatoid arthritis: A systematic review and meta-analysis. Int. Immunopharmacol. 2018, 59, 227–232. [Google Scholar] [CrossRef]
- Choe, J.Y.; Hun Kim, J.; Park, K.Y.; Choi, C.H.; Kim, S.K. Activation of dickkopf-1 and focal adhesion kinase pathway by tumour necrosis factor α induces enhanced migration of fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatology 2016, 55, 928–938. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, F.; Bian, W.; Li, Y.; Zhang, L.; Shi, L.; Ma, X.; Liu, Y.; Zhang, X.; Li, Z. Dickkopf-1 perpetuated synovial fibroblast activation and synovial angiogenesis in rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 4279–4288. [Google Scholar] [CrossRef]
- Gómez-Vaquero, C.; Martín, I.; Loza, E.; Carmona, L.; Ivorra, J.; Narváez, J.A.; Hernández-Gañán, J.; Alía, P.; Narváez, J. Effect of Osteoprotegerin and Dickkopf-Related Protein 1 on Radiological Progression in Tightly Controlled Rheumatoid Arthritis. PLoS ONE 2016, 11, e0166691. [Google Scholar] [CrossRef]
- Seror, R.; Boudaoud, S.; Pavy, S.; Nocturne, G.; Schaeverbeke, T.; Saraux, A.; Chanson, P.; Gottenberg, J.-E.; Devauchelle-Pensec, V.; Tobón, G.J.; et al. Increased Dickkopf-1 in Recent-onset Rheumatoid Arthritis is a New Biomarker of Structural Severity. Data from the ESPOIR Cohort. Sci. Rep. 2016, 6, 18421. [Google Scholar] [CrossRef]
- de Rooy, D.P.C.; Yeremenko, N.G.; Wilson, A.G.; Knevel, R.; Lindqvist, E.; Saxne, T.; Krabben, A.; Leijsma, M.K.; Daha, N.A.; Tsonaka, S.; et al. Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 769–775. [Google Scholar] [PubMed]
- Miceli-Richard, C.; Taylor, K.E.; Nititham, J.; Seror, R.; Nocturne, G.; Boudaoud, S.; Dieude, P.; Constantin, A.; Devauchelle-Pensec, V.; Tobón, G.J.; et al. Genetic contribution of DKK-1 polymorphisms to RA structural severity and DKK-1 level of expression. Ann. Rheum. Dis. 2015, 74, 1480–1481. [Google Scholar] [PubMed]
- Cardona-Rincón, A.D.; Acevedo-Godoy, M.A.; Bello-Gualtero, J.M.; Valle-Oñate, R.; Chalem-Choueka, P.; Perdomo, S.J.; Arias-Arias, A.M.; Chila-Moreno, L.; Bautista-Molano, W.; Romero-Sánchez, C. Association of Dickkopf-1 Polymorphisms with Radiological Damage and Periodontal Disease in Patients with Early Rheumatoid Arthritis: A Cross-Sectional Study. J. Clin. Rheumatol. 2020, 26, S187–S194. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Zhang, Y.; Di, M.; Wang, H.; Wang, L.; Chen, Y.; Liu, X.; Cao, X.; Zeng, R.; et al. Upregulation of Dickkopf1 by oscillatory shear stress accelerates atherogenesis. J. Mol. Med. Berl. Ger. 2016, 94, 431–441. [Google Scholar]
- Bernardes, M.; Vieira, T.S.; Martins, M.J.; Lucas, R.; Costa, L.; Pereira, J.G.; Ventura, F.; Martins, E. Myocardial Perfusion in Rheumatoid Arthritis Patients: Associations with Traditional Risk Factors and Novel Biomarkers. BioMed Res. Int. 2017, 2017, 6509754. [Google Scholar]
- Biță, C.E.; Dinescu, S.C.; Riza, A.L.; Ciurea, P.L.; Mușetescu, A.E.; Marinescu, D.; Dumitrașcu, R.M.; Șuiu, L.I.; Ionescu, R.A.; Popoviciu, H.V.; et al. Dickkopf-Related Protein 1 (DKK-1) as a Possible Link between Bone Erosions and Increased Carotid Intima-Media Thickness in Psoriatic Arthritis: An Ultrasound Study. Int. J. Mol. Sci. 2023, 24, 14970. [Google Scholar] [CrossRef]
- Pàmies, A.; Llop, D.; Ibarretxe, D.; Rosales, R.; Girona, J.; Masana, L.; Vallvé, J.-C.; Paredes, S. Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2024, 25, 9910. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the role of galectin-3 in cardiac pathology and physiology. Front. Physiol. 2023, 14, 1304735. [Google Scholar]
- Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 5284728. [Google Scholar]
- Mendez-Huergo, S.P.; Hockl, P.F.; Stupirski, J.C.; Maller, S.M.; Morosi, L.G.; Pinto, N.A.; Berón, A.M.; Musuruana, J.L.; Nasswetter, G.G.; Cavallasca, J.A.; et al. Clinical Relevance of Galectin-1 and Galectin-3 in Rheumatoid Arthritis Patients: Differential Regulation and Correlation with Disease Activity. Front. Immunol. 2018, 9, 3057. [Google Scholar]
- Zhong, X.; Qian, X.; Chen, G.; Song, X. The role of galectin-3 in heart failure and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.R.; Meijers, W.C.; Ho, J.E.; Brouwers, F.P.; Rienstra, M.; Bakker, S.J.L.; Kobold, A.C.M.; van Veldhuisen, D.J.; van Gilst, W.H.; van der Harst, P.; et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart 2016, 102, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Zhang, S.; Florido, R.; Pankow, J.S.; Michos, E.D.; Goldberg, R.B.; Nambi, V.; Gerstenblith, G.; Post, W.S.; Blumenthal, R.S.; et al. Galectin-3, Metabolic Risk, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2024, 13, e031607. [Google Scholar] [CrossRef]
- Wang, X.; Gaur, M.; Mounzih, K.; Rodriguez, H.J.; Qiu, H.; Chen, M.; Yan, L.; Cooper, B.A.; Narayan, S.; Derakhshandeh, R.; et al. Inhibition of galectin-3 post-infarction impedes progressive fibrosis by regulating inflammatory profibrotic cascades. Cardiovasc. Res. 2023, 119, 2536–2549. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Fuster, V.; Muntendam, P.; Mehran, R.; Baber, U.; Sartori, S.; Falk, E. Negative Risk Markers for Cardiovascular Events in the Elderly. J. Am. Coll. Cardiol. 2019, 74, 1–11. [Google Scholar] [CrossRef]
- Imran, T.F.; Shin, H.J.; Mathenge, N.; Wang, F.; Kim, B.; Joseph, J.; Gaziano, J.M.; Djoussé, L. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients with Heart Failure and in the General Population. Am. J. Cardiol. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Fang, J.; Wang, R.; Nie, W. Circulating galectin-3 on admission and prognosis in acute heart failure patients: A meta-analysis. Heart Fail. Rev. 2020, 25, 331–341. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.H.; Hulthe, J.; Muntendam, P.; McMurray, J.J.; Wikstrand, J.; Aukrust, P. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am. Heart J. 2012, 164, 878–883. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, F.; Yuan, Y.; Li, K.; Zhang, P.; Wei, X.; Liu, D.; Zhang, H. Circulating galectin-3 levels are inversely associated with subclinical cardiovascular disease in obese adults. Heart Vessels 2023, 38, 671–679. [Google Scholar]
- Baccouche, B.M.; Mahmoud, M.A.; Nief, C.; Patel, K.; Natterson-Horowitz, B. Galectin-3 is Associated with Heart Failure Incidence: A Meta-Analysis. Curr. Cardiol. Rev. 2023, 19, e171122211004. [Google Scholar] [PubMed]
- Wang, A.; Zhong, C.; Zhu, Z.; Xu, T.; Peng, Y.; Xu, T.; Peng, H.; Chen, C.-S.; Wang, J.; Ju, Z.; et al. Serum Galectin-3 and Poor Outcomes Among Patients with Acute Ischemic Stroke. Stroke 2018, 49, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, M.D.; Sonkawade, S.D.; Jonnala, V.; Pokharel, S.; Khazaeli, M.; Yatsynovich, Y.; Kalot, M.A.; Weil, B.R.; Canty, J.M.; Sharma, U.C. Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death. Cells 2023, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Noh, H.M.; Song, H.J.; Lee, S.; Kim, S.G.; Kim, J.K. Mediating effect of vascular calcification in galectin-3-related mortality in hemodialysis patients. Sci. Rep. 2024, 14, 939. [Google Scholar]
- Pusuroglu, H.; Somuncu, U.; Bolat, I.; Akgul, O.; Ornek, V.; Yıldırım, H.A.; Akkaya, E.; Karakurt, H.; Yıldırım, A.; Savaş, A.U. Galectin-3 is associated with coronary plaque burden and obstructive sleep apnoea syndrome severity. Kardiol. Pol. 2017, 75, 351–359. [Google Scholar]
- Ozturk, D.; Celik, O.; Satilmis, S.; Aslan, S.; Erturk, M.; Cakmak, H.A.; Kalkan, A.K.; Ozyilmaz, S.; Diker, V.; Gul, M. Association between serum galectin-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron. Artery Dis. 2015, 26, 396. [Google Scholar]
- Papaspyridonos, M.; McNeill, E.; de Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [Google Scholar]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J. Am. Heart Assoc. 2014, 3, e000785. [Google Scholar]
- Ohshima, S.; Kuchen, S.; Seemayer, C.A.; Kyburz, D.; Hirt, A.; Klinzing, S.; Michel, B.A.; Gay, R.E.; Liu, F.T.; Gay, S.; et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2788–2795. [Google Scholar]
- Neidhart, M.; Zaucke, F.; von Knoch, R.; Jüngel, A.; Michel, B.A.; Gay, R.E.; Gay, S. Galectin-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric matrix protein. Ann. Rheum. Dis. 2005, 64, 419–424. [Google Scholar] [CrossRef]
- Issa, S.F.; Christensen, A.F.; Lindegaard, H.M.; Hetland, M.L.; Hørslev-Petersen, K.; Stengaard-Pedersen, K.; Ejbjerg, B.J.; Lottenburger, T.; Ellingsen, T.; Pedersen, J.K.; et al. Galectin-3 is Persistently Increased in Early Rheumatoid Arthritis (RA) and Associates with Anti-CCP Seropositivity and MRI Bone Lesions, While Early Fibrosis Markers Correlate with Disease Activity. Scand. J. Immunol. 2017, 86, 471–478. [Google Scholar] [PubMed]
- Issa, S.F.; Duer, A.; Østergaard, M.; Hørslev-Petersen, K.; Hetland, M.L.; Hansen, M.S.; Junker, K.; Lindegaard, H.M.; Møller, J.M.; Junker, P. Increased galectin-3 may serve as a serologic signature of pre-rheumatoid arthritis while markers of synovitis and cartilage do not differ between early undifferentiated arthritis subsets. Arthritis Res. Ther. 2017, 19, 80. [Google Scholar] [PubMed]
- Kaur, T.; Thakur, K.; Singh, J.; Arora, S.; Kaur, M. Genotypic-Phenotypic Screening of Galectin-3 in Relation to Risk Towards Rheumatoid Arthritis. Arch. Med. Res. 2019, 50, 214–224. [Google Scholar] [PubMed]
- Xu, W.D.; Wu, Q.; He, Y.W.; Huang, A.F.; Lan, Y.Y.; Fu, L.; Zhou, J.; Liu, X.-Y. Gene polymorphisms of LGALS2, LGALS3 and LGALS9 in patients with rheumatoid arthritis. Cell. Immunol. 2021, 368, 104419. [Google Scholar]
- Gruszewska, E.; Cylwik, B.; Gińdzieńska-Sieśkiewicz, E.; Kowal-Bielecka, O.; Mroczko, B.; Chrostek, L. Diagnostic Power of Galectin-3 in Rheumatic Diseases. J. Clin. Med. 2020, 9, 3312. [Google Scholar] [CrossRef]
- Nielsen, M.A.; Køster, D.; Greisen, S.; Troldborg, A.; Stengaard-Pedersen, K.; Junker, P.; Hørslev-Petersen, K.; Hetland, M.; Østergaard, M.; Hvid, M.; et al. Increased synovial galectin-3 induce inflammatory fibroblast activation and osteoclastogenesis in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2023, 52, 33–41. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.C.; et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 2009, 60, 1604–1614. [Google Scholar]
- Forsman, H.; Islander, U.; Andréasson, E.; Andersson, A.; Onnheim, K.; Karlström, A.; Sävman, K.; Magnusson, M.; Brown, K.L.; Karlsson, A. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011, 63, 445–454. [Google Scholar]
- Jiménez-González, S.; Delgado-Valero, B.; Islas, F.; Romero-Miranda, A.; Luaces, M.; Ramchandani, B.; Cuesta-Corral, M.; Montoro-Garrido, A.; Martínez-Martínez, E.; Cachofeiro, V. The detrimental role of galectin-3 and endoplasmic reticulum stress in the cardiac consequences of myocardial ischemia in the context of obesity. FASEB J. 2024, 38, e23818. [Google Scholar]
- Zhu, N.; Zhu, L.; Huang, B.; Xiang, W.; Zhao, X. Galectin-3 Inhibition Ameliorates Streptozotocin-Induced Diabetic Cardiomyopathy in Mice. Front. Cardiovasc. Med. 2022, 9, 868372. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Ho, L.C.; Chiou, J.Y.; Meng, T.C. Abstract Tu130: Intracellular Galectin-3 Interacts with Cytosolically Exposed Glycans to Promote the Injury of Cardiomyocytes Under Oxidative Stress. Circ. Res. 2024, 135, ATu130. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Zheng, Y.; Yang, J.; Liu, Y.; Qi, Z.; Wu, M.; Fan, Z.; Yin, K.; Chen, Y.; et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: A translational study. Eur. Heart J. 2022, 43, 3556–3574. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gkaliagkousi, E.; Gavriilaki, E.; Triantafyllou, A.; Dolgyras, P.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Association of galectin-3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin. Cardiol. 2019, 42, 62–68. [Google Scholar] [CrossRef]
- Anyfanti, P.; Dimitriadou, A.; Dara, A.; Angeloudi, E.; Gavriilaki, E.; Nikolaidou, B.; Triantafyllou, A.; Dimitroulas, T.; Gkaliagkousi, E. Circulating levels of galectin-3 and coronary microvascular perfusion in rheumatoid arthritis patients with suppressed inflammation. Clin. Rheumatol. 2023, 42, 2881–2887. [Google Scholar] [CrossRef]
- Nussdorf, A.; Park, E.; Amigues, I.; Geraldino-Pardilla, L.; Bokhari, S.; Giles, J.T.; Bathon, J.M. Associations of galectin-3 levels with measures of vascular disease in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2024, 65, 152357. [Google Scholar] [CrossRef]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Urlic, H.; Martinovic, D.; Vilovic, M.; Mrcela, N.T.; Borovac, J.A. Catestatin as a Biomarker of Cardiovascular Diseases: A Clinical Perspective. Biomedicines 2021, 9, 1757. [Google Scholar] [CrossRef]
- Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; Stanisic, L.; D’Amario, D.; Kwok, C.S.; Bozic, J. Circulating sST2 and catestatin levels in patients with acute worsening of heart failure: A report from the CATSTAT-HF study. ESC Heart Fail. 2020, 7, 2818–2828. [Google Scholar] [CrossRef]
- Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; D’Amario, D.; Bozic, J. Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study. J. Clin. Med. 2019, 8, 1132. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Banach, J.; Budzyński, J.; Wołowiec, A.; Kozakiewicz, M.; Bieliński, M.; Jaśniak, A.; Olejarczyk, A.; Grześk, G. Prognostic Value of Plasma Catestatin Concentration in Patients with Heart Failure with Reduced Ejection Fraction in Two-Year Follow-Up. J. Clin. Med. 2023, 12, 4208. [Google Scholar] [CrossRef]
- Qiu, Z.; Fan, Y.; Wang, Z.; Huang, F.; Li, Z.; Sun, Z.; Hua, S.; Jin, W.; Chen, Y. Catestatin Protects Against Diastolic Dysfunction by Attenuating Mitochondrial Reactive Oxygen Species Generation. J. Am. Heart Assoc. 2023, 12, e029470. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, J.; Ding, W.H.; Han, P.; Yang, Y.; Qi, L.T.; Zhang, B.W. Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgrad. Med. J. 2013, 89, 193–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Correlation of plasma catestatin level and the prognosis of patients with acute myocardial infarction. PLoS ONE 2015, 10, e0122993. [Google Scholar] [CrossRef][Green Version]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Catestatin-A Novel Predictor of Left Ventricular Remodeling After Acute Myocardial Infarction. Sci. Rep. 2017, 7, 44168. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, D.; Ji, L.; Zhang, J.; Su, J.; Xue, W.; Chen, X. Usefulness of catestatin to predict malignant arrhythmia in patients with acute myocardial infarction. Peptides 2014, 55, 131–135. [Google Scholar] [CrossRef]
- Katic, J.; Jurisic, Z.; Kumric, M.; Borovac, J.A.; Anic, A.; Breskovic, T.; Supe-Domic, D.; Bozic, J. Serum Catestatin Concentrations Are Increased in Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2023, 10, 85. [Google Scholar] [CrossRef]
- Yan, M.; Liu, T.; Zhong, P.; Xiong, F.; Cui, B.; Wu, J.; Wu, G. Chronic catestatin treatment reduces atrial fibrillation susceptibility via improving calcium handling in post-infarction heart failure rats. Peptides 2023, 159, 170904. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Xu, W.; Yu, H.; Wu, H.; Li, S.; Chen, B.; Gao, W. Plasma Catestatin in Patients with Acute Coronary Syndrome. Cardiology 2017, 136, 164–169. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Rogowicz, D.; Banach, J.; Gilewski, W.; Sinkiewicz, W.; Grześk, G. Catestatin as a New Prognostic Marker in Stable Patients with Heart Failure with Reduced Ejection Fraction in Two-Year Follow-Up. Dis. Markers 2020, 2020, 8847211. [Google Scholar] [CrossRef]
- Pankova, O.; Korzh, O. Plasma catestatin levels are related to metabolic parameters in patients with essential hypertension and type 2 diabetes mellitus. Heart Vessels 2024, 39, 144–159. [Google Scholar] [PubMed]
- Bourebaba, Y.; Mularczyk, M.; Marycz, K.; Bourebaba, L. Catestatin peptide of chromogranin A as a potential new target for several risk factors management in the course of metabolic syndrome. Biomed. Pharmacother. 2021, 134, 111113. [Google Scholar] [CrossRef]
- Meng, L.; Ye, X.; Ding, W.; Yang, Y.; Di, B.; Liu, L.; Huo, Y. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J. Cardiovasc. Med. 2011, 12, 643–647. [Google Scholar]
- Kumric, M.; Vrdoljak, J.; Dujic, G.; Supe-Domic, D.; Ticinovic Kurir, T.; Dujic, Z.; Bozic, J. Serum Catestatin Levels Correlate with Ambulatory Blood Pressure and Indices of Arterial Stiffness in Patients with Primary Hypertension. Biomolecules 2022, 12, 1204. [Google Scholar] [CrossRef]
- O’Connor, D.T.; Kailasam, M.T.; Kennedy, B.P.; Ziegler, M.G.; Yanaihara, N.; Parmer, R.J. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J. Hypertens. 2002, 20, 1335–1345. [Google Scholar]
- Durakoğlugil, M.E.; Ayaz, T.; Kocaman, S.A.; Kırbaş, A.; Durakoğlugil, T.; Erdoğan, T.; Cetin, M.; Sahin, O.Z.; Cicek, Y. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: Influence on high-density lipoprotein cholesterol. Anatol. J. Cardiol. 2015, 15, 577–585. [Google Scholar] [PubMed]
- Simac, P.; Perkovic, D.; Bozic, I.; Matijas, M.; Gugo, K.; Martinovic, D.; Bozic, J. Serum catestatin levels in patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 3812. [Google Scholar]
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.-A.; Ishibashi-Ueda, H.; et al. Catestatin Prevents Macrophage-Driven Atherosclerosis but Not Arterial Injury-Induced Neointimal Hyperplasia. Thromb. Haemost. 2018, 118, 182–194. [Google Scholar]
- Lener, D.; Noflatscher, M.; Kirchmair, E.; Bauer, A.; Holfeld, J.; Gollmann-Tepeköylü, C.; Kirchmair, R.; Theurl, M. The angiogenic neuropeptide catestatin exerts beneficial effects on human coronary vascular cells and cardiomyocytes. Peptides 2023, 168, 171077. [Google Scholar]
- Zivkovic, P.M.; Matetic, A.; Tadin Hadjina, I.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Borovac, J.A.; Mudnic, I.; Tonkic, A.; Bozic, J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 628. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Tilahun Muche, Z.; Behaile, T.; Mariam, A.; Mengie Ayele, T.; Mekonnen Agidew, M.; Teshome Azezew, M.; Zewde, E.A.; Dejenie, T.A.; Mengstie, M.A. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front. Cell. Dev. Biol. 2022, 10, 945287. [Google Scholar]
- Bourebaba, L.; Marycz, K. Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review. J. Clin. Med. 2019, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Moutereau, S.; Simon, T.; Gallet, R.; Probst, V.; Ferrieres, J.; Gueret, P.; Danchin, N. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am. J. Cardiol. 2013, 111, 31–37. [Google Scholar]
- Xie, W.M.; Ran, L.S.; Jiang, J.; Chen, Y.S.; Ji, H.Y.; Quan, X.Q. Association between fetuin-A and prognosis of CAD: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13091. [Google Scholar]
- Laughlin, G.A.; Cummins, K.M.; Wassel, C.L.; Daniels, L.B.; Ix, J.H. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: The Rancho Bernardo study. J. Am. Coll. Cardiol. 2012, 59, 1688–1696. [Google Scholar]
- Jensen, M.K.; Bartz, T.M.; Mukamal, K.J.; Djoussé, L.; Kizer, J.R.; Tracy, R.P.; Zieman, S.J.; Rimm, E.B.; Siscovick, D.S.; Shlipak, M.; et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: The cardiovascular health study. Diabetes Care 2013, 36, 1222–1228. [Google Scholar]
- Aroner, S.A.; St-Jules, D.E.; Mukamal, K.J.; Katz, R.; Shlipak, M.G.; Criqui, M.H.; Kestenbaum, B.; Siscovick, D.S.; de Boer, I.H.; Jenny, N.S.; et al. Fetuin-A, glycemic status, and risk of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2016, 248, 224–229. [Google Scholar]
- Ix, J.H.; Barrett-Connor, E.; Wassel, C.L.; Cummins, K.; Bergstrom, J.; Daniels, L.B.; Laughlin, G.A. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2011, 58, 2372–2379. [Google Scholar]
- Guo, V.Y.; Cao, B.; Cai, C.; Cheng, K.K.Y.; Cheung, B.M.Y. Fetuin-A levels and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2018, 55, 87–98. [Google Scholar]
- Pan, X.; Wen, S.W.; Bestman, P.L.; Kaminga, A.C.; Acheampong, K.; Liu, A. Fetuin-A in Metabolic syndrome: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229776. [Google Scholar]
- Brix, J.M.; Stingl, H.; Höllerl, F.; Schernthaner, G.H.; Kopp, H.P.; Schernthaner, G. Elevated Fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J. Clin. Endocrino.l Metab. 2010, 95, 4877–4881. [Google Scholar]
- Ramírez-Vélez, R.; García-Hermoso, A.; Hackney, A.C.; Izquierdo, M. Effects of exercise training on Fetuin-a in obese, type 2 diabetes and cardiovascular disease in adults and elderly: A systematic review and Meta-analysis. Lipids Health Dis. 2019, 18, 23. [Google Scholar] [PubMed]
- Sato, H.; Kazama, J.J.; Wada, Y.; Kuroda, T.; Narita, I.; Gejyo, F.; Gao, P.; Yamashita, H. Decreased levels of circulating α2-Heremans-Schmid glycoprotein/Fetuin-A (AHSG) in patients with rheumatoid arthritis. Intern. Med. 2007, 46, 1685–1691. [Google Scholar]
- Saroha, A.; Kumar, S.; Chatterjee, B.P.; Das, H.R. Jacalin bound plasma O-glycoproteome and reduced sialylation of alpha 2-HS glycoprotein (A2HSG) in rheumatoid arthritis patients. PLoS ONE 2012, 7, e46374. [Google Scholar]
- Sinem, S.A.Ğ.; Güzel, D.; Sağ, M.S.; Tekeoğlu, İ.; Kamanli, A.; Kemal, N.A.S.; Doğanay, S. The Evaluation of Serum Tumor Necrosis Factor-Like Weak Inducer of Apoptosis, Interleukin-6, Fetuin-A, Homeostatic Model Assessment-Insulin Resistance, and Insulin Levels in Rheumatoid Arthritis Patients in Clinical Remission. Arch. Rheumatol. 2018, 34, 71–78. [Google Scholar]
- Harman, H.; Tekeoğlu, İ.; Gürol, G.; Sağ, M.S.; Karakeçe, E.; Çİftçİ, İ.H.; Kamanlı, A.; Nas, K. Comparison of fetuin-A and transforming growth factor beta 1 levels in patients with spondyloarthropathies and rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 2020–2027. [Google Scholar]
- Tekeoğlu, İ.; Harman, H.; Sağ, S.; Altındiş, M.; Kamanlı, A.; Nas, K. Levels of serum pentraxin 3, IL-6, fetuin A and insulin in patients with rheumatoid arthritis. Cytokine 2016, 83, 171–175. [Google Scholar]
- Nguyen, M.V.C.; Courtier, A.; Adrait, A.; Defendi, F.; Couté, Y.; Baillet, A.; Guigue, L.; Gottenberg, J.-E.; Dumestre-Pérard, C.; Brun, V.; et al. Fetuin-A and thyroxin binding globulin predict rituximab response in rheumatoid arthritis patients with insufficient response to anti-TNFα. Clin. Rheumatol. 2020, 39, 2553–2562. [Google Scholar]
- Jäger, E.; Murthy, S.; Schmidt, C.; Hahn, M.; Strobel, S.; Peters, A.; Stäubert, C.; Sungur, P.; Venus, T.; Geisler, M.; et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat. Commun. 2020, 11, 4243. [Google Scholar]
- Murthy, S.; Karkossa, I.; Schmidt, C.; Hoffmann, A.; Hagemann, T.; Rothe, K.; Seifert, O.; Anderegg, U.; von Bergen, M.; Schubert, K.; et al. Danger signal extracellular calcium initiates differentiation of monocytes into SPP1/osteopontin-producing macrophages. Cell Death Dis. 2022, 13, 53. [Google Scholar]
- Wang, H.; Sama, A.E. Anti-inflammatory role of fetuin-A in injury and infection. Curr. Mol. Med. 2012, 12, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Jersmann, H.P.A.; Dransfield, I.; Hart, S.P. Fetuin/α2-HS glycoprotein enhances phagocytosis of apoptotic cells and macropinocytosis by human macrophages. Clin. Sci. 1979 2003, 105, 273–278. [Google Scholar] [CrossRef]
- Mathews, S.T.; Chellam, N.; Srinivas, P.R.; Cintron, V.J.; Leon, M.A.; Goustin, A.S.; Grunberger, G. α2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol. Cell. Endocrinol. 2000, 164, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Merx, M.W.; Schäfer, C.; Westenfeld, R.; Brandenburg, V.; Hidajat, S.; Weber, C.; Ketteler, M.; Jahnen-Dechent, W. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J. Am. Soc. Nephrol. 2005, 16, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef]
- Smith, E.R.; Hanssen, E.; McMahon, L.P.; Holt, S.G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS ONE 2013, 8, e60904. [Google Scholar] [CrossRef]
- Theofilatos, K.; Stojkovic, S.; Hasman, M.; van der Laan, S.W.; Baig, F.; Barallobre-Barreiro, J.; Schmidt, L.E.; Yin, S.; Yin, X.; Burnap, S.; et al. Proteomic Atlas of Atherosclerosis: The Contribution of Proteoglycans to Sex Differences, Plaque Phenotypes, and Outcomes. Circ. Res. 2023, 133, 542–558. [Google Scholar] [CrossRef]

| Biomarker | ccKey Role | Evidence in RA | Clinical Evidence Strength | Limitations Current Clinical Use |
|---|---|---|---|---|
| IL-32 |
|
|
| |
| DKK-1 |
|
|
| |
| Gal-3 |
|
|
|
|
| CST |
|
| ||
| Fet-A |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamies, A.; Vallvé, J.-C.; Paredes, S. New Cardiovascular Risk Biomarkers in Rheumatoid Arthritis: Implications and Clinical Utility—A Narrative Review. Biomedicines 2025, 13, 870. https://doi.org/10.3390/biomedicines13040870
Pamies A, Vallvé J-C, Paredes S. New Cardiovascular Risk Biomarkers in Rheumatoid Arthritis: Implications and Clinical Utility—A Narrative Review. Biomedicines. 2025; 13(4):870. https://doi.org/10.3390/biomedicines13040870
Chicago/Turabian StylePamies, Anna, Joan-Carles Vallvé, and Silvia Paredes. 2025. "New Cardiovascular Risk Biomarkers in Rheumatoid Arthritis: Implications and Clinical Utility—A Narrative Review" Biomedicines 13, no. 4: 870. https://doi.org/10.3390/biomedicines13040870
APA StylePamies, A., Vallvé, J.-C., & Paredes, S. (2025). New Cardiovascular Risk Biomarkers in Rheumatoid Arthritis: Implications and Clinical Utility—A Narrative Review. Biomedicines, 13(4), 870. https://doi.org/10.3390/biomedicines13040870






