Neurotropic Effects of Cortexin on Models of Mental and Physical Developmental Delay
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Characteristics of the Test System
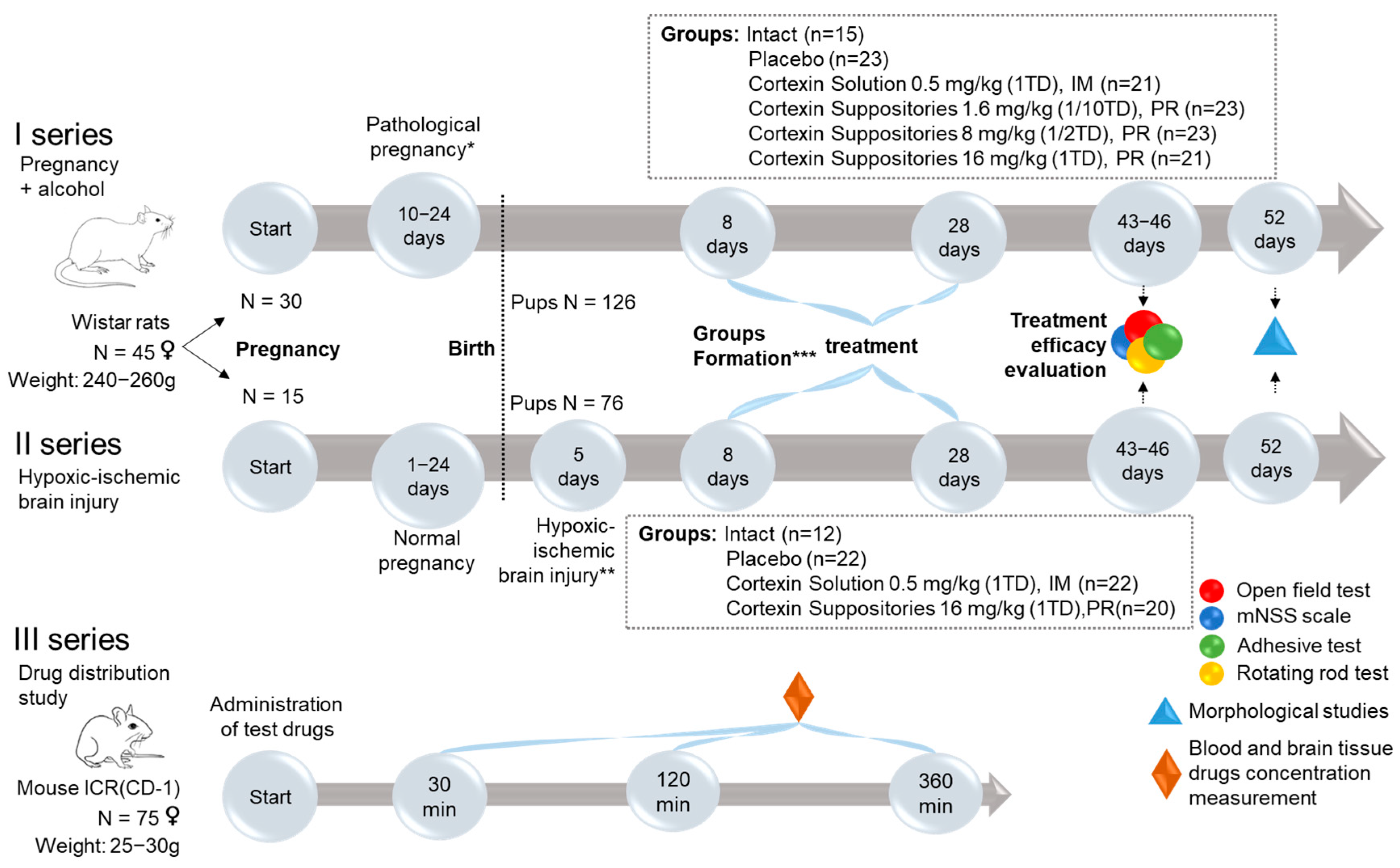
2.3. Study Design
- Pairing of female rats with males for mating for 1 day (30 females for series 1 and 15 females for series 2).
- Pregnancy.
- Modeling of complicated pregnancy (ethanol administration during the last week of pregnancy for series 1, and hypoxic-ischemic brain injury modeling in 5-day-old pups for series 2).
- Monitoring the rats during the development of the pathology.
- Formation of groups.
- Treatment.
- Evaluation of treatment outcomes.
2.4. Modeling Developmental Delay
2.4.1. Modeling Pathological Pregnancy (Administration of Ethanol During the Last Week of Pregnancy)
2.4.2. Modeling Hypoxic-Ischemic Brain Injury
2.5. Test Objects
2.6. Evaluation of Treatment Efficacy
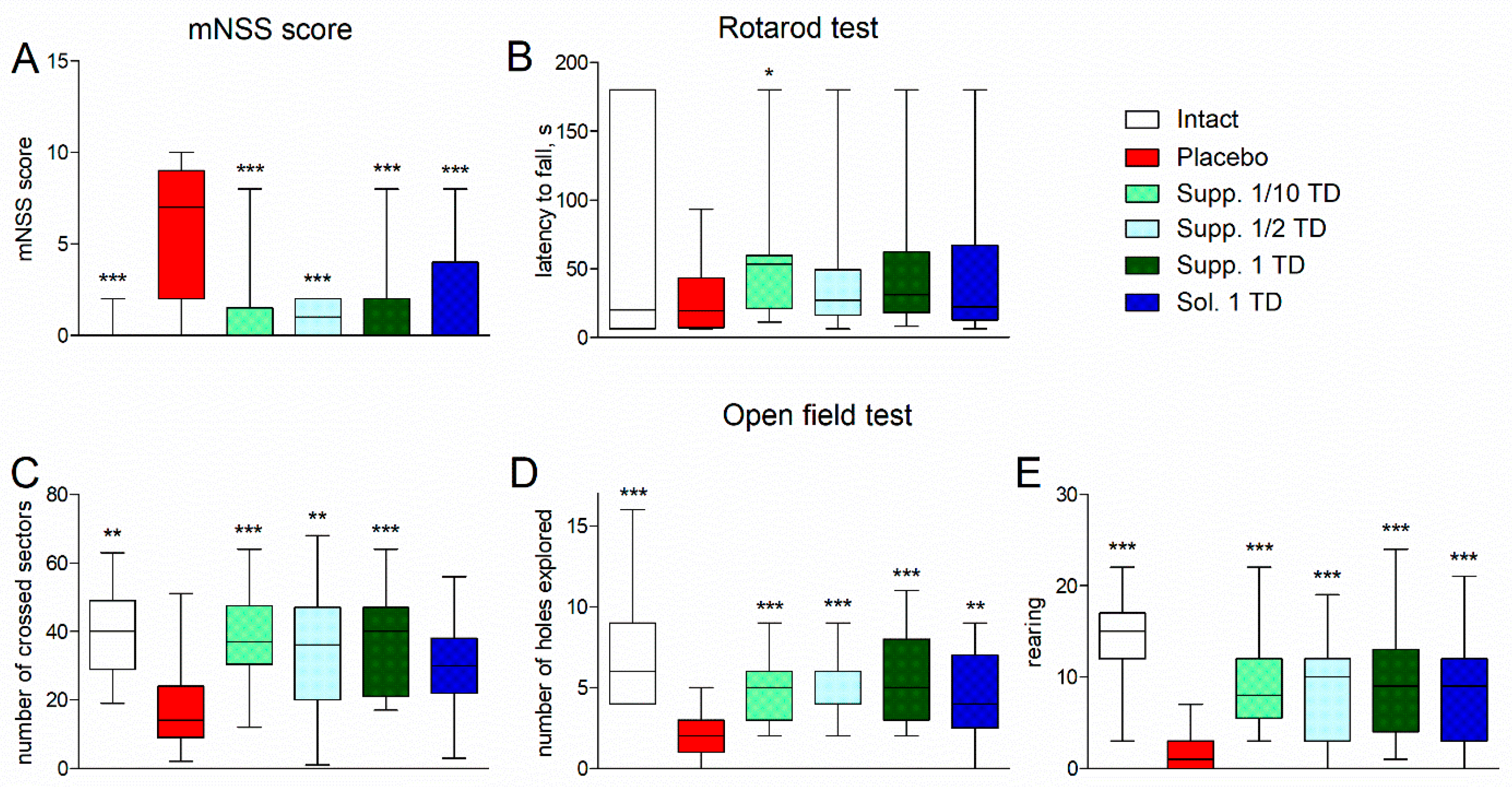
2.6.1. mNSS Scale
2.6.2. Open Field Test
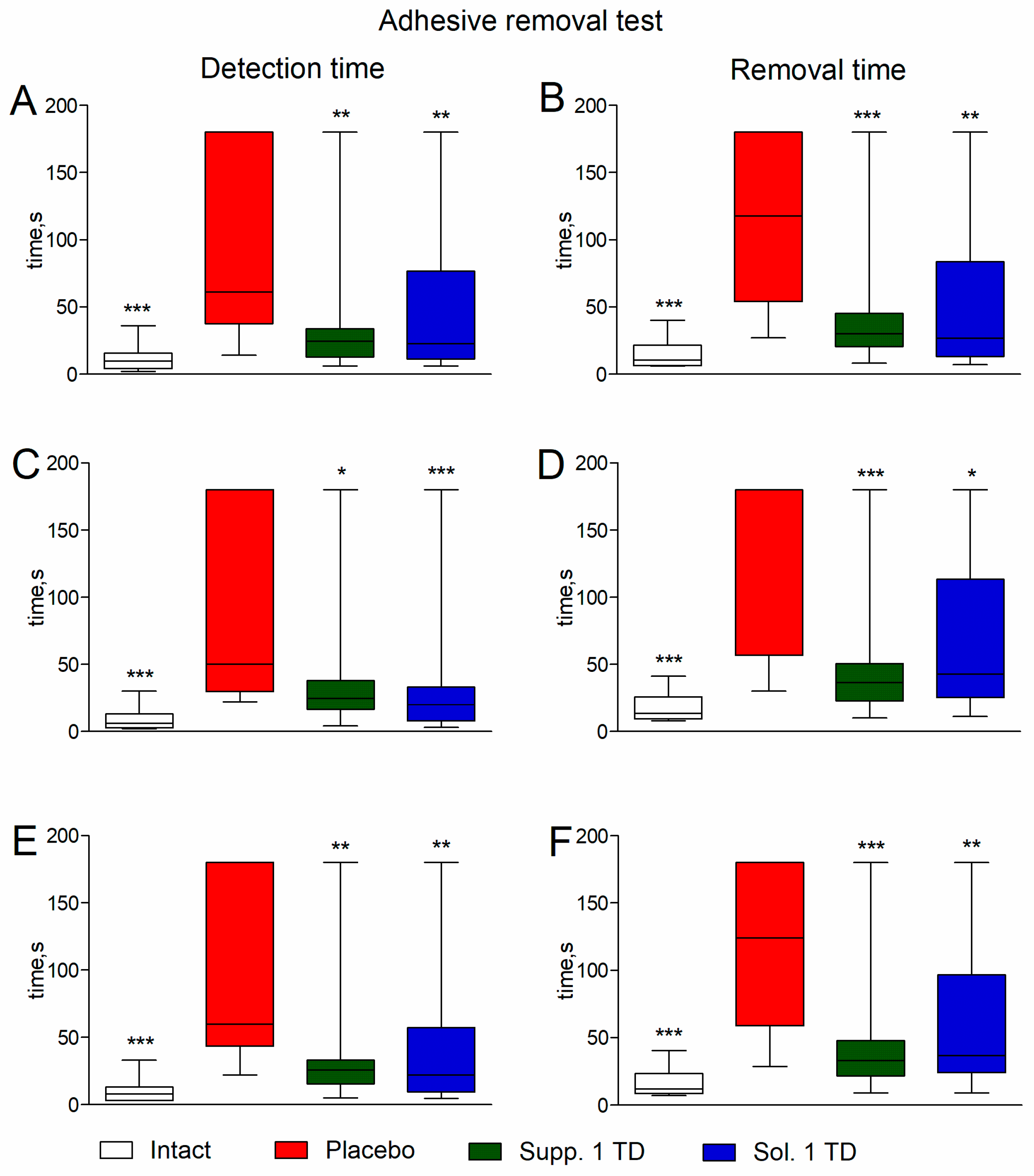
2.6.3. Adhesive Removal Test
2.6.4. Rotarod Test
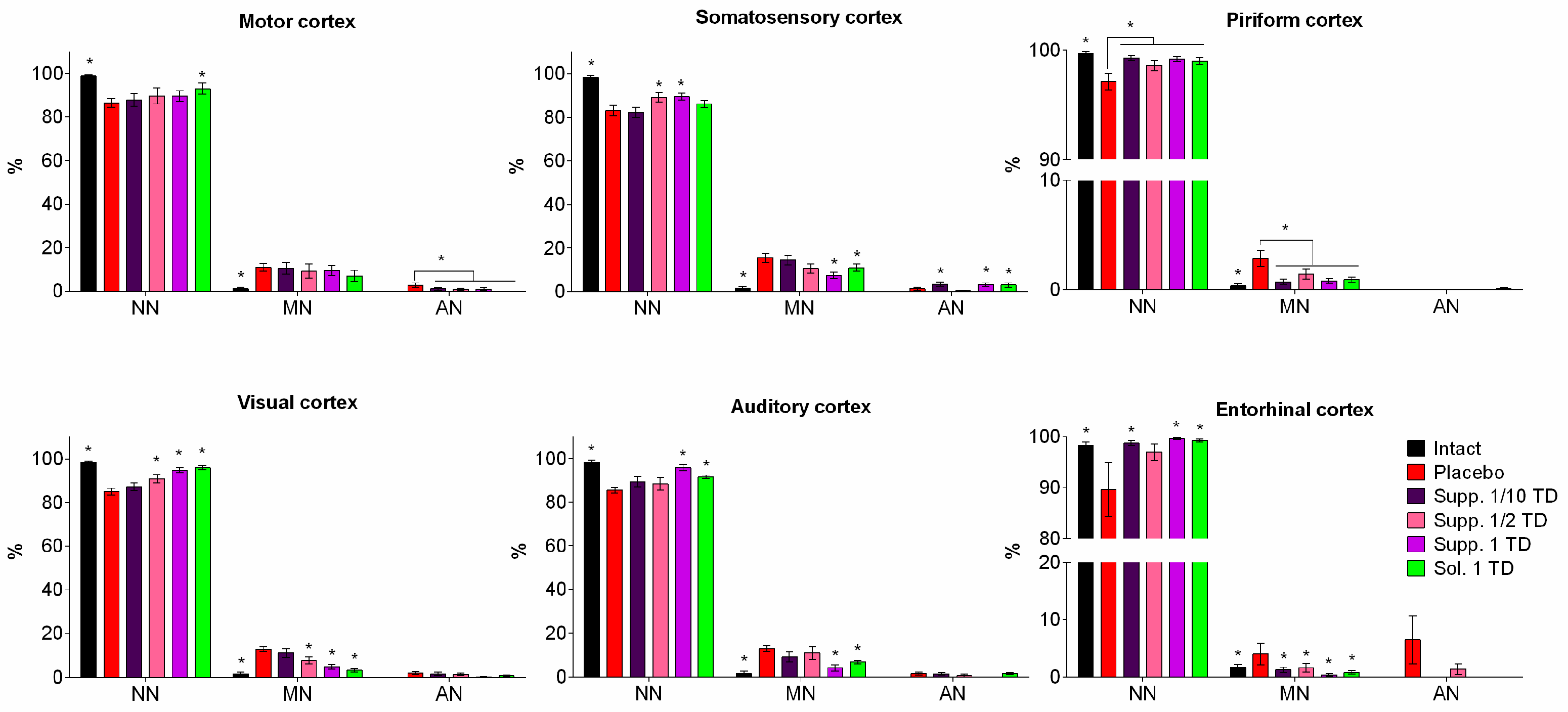
2.6.5. Morphometric Analysis
2.7. Investigation of Drug Distribution
2.8. Data Analysis
3. Results
3.1. Evaluation of Neurological Deficit
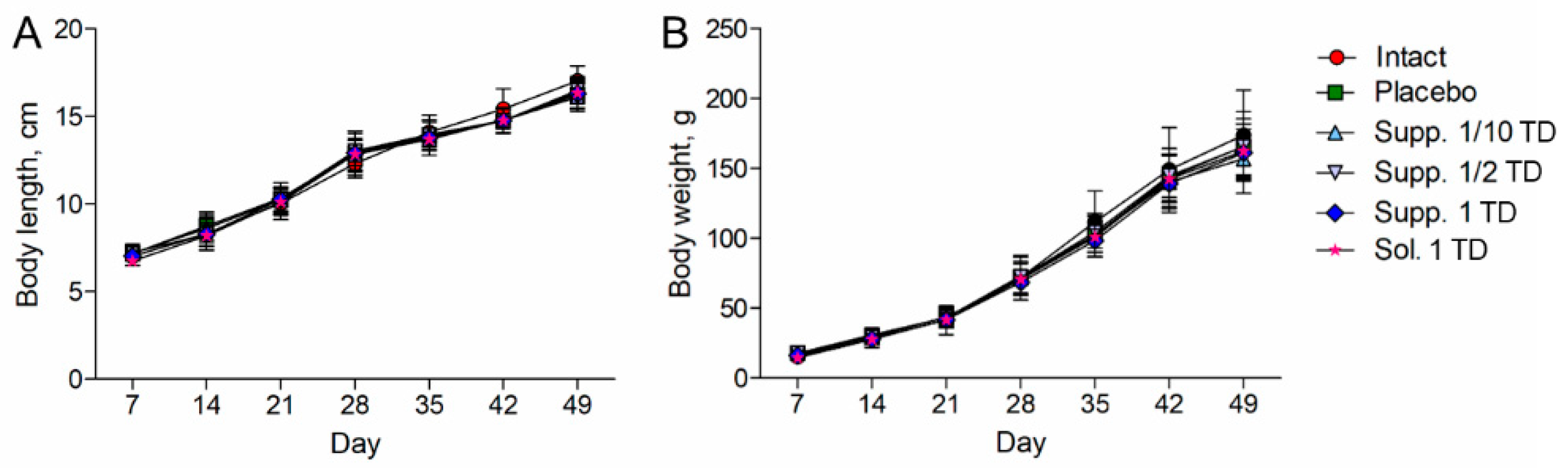
3.1.1. Pathological Pregnancy Model (Ethanol Administration During the Last Week of Pregnancy)
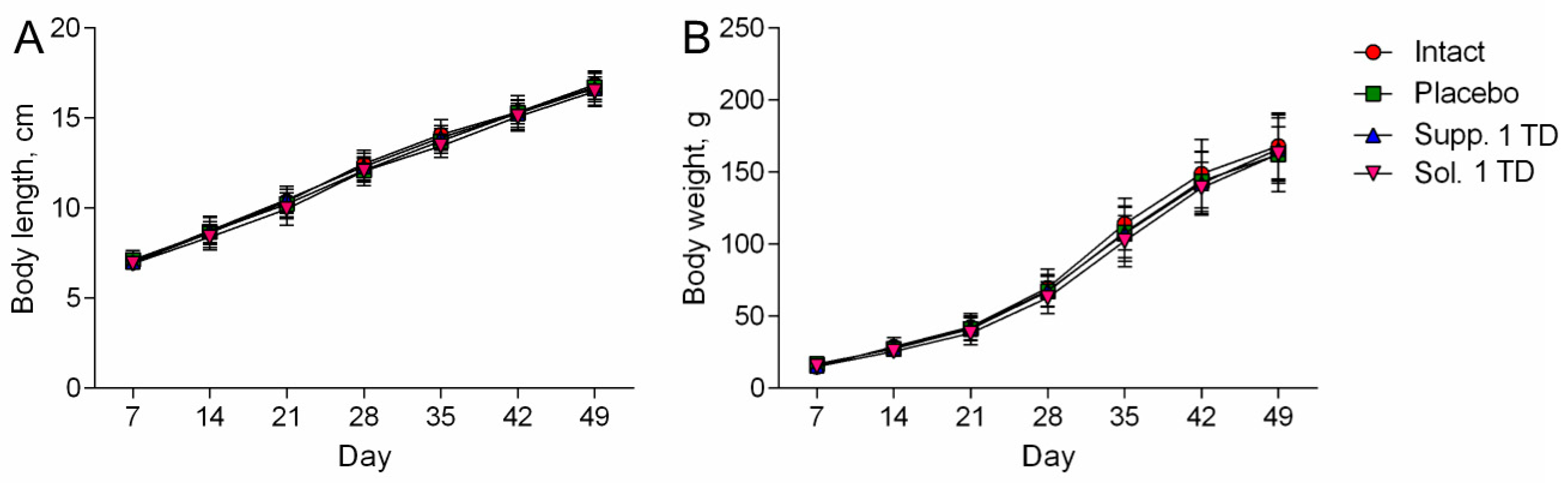
3.1.2. Hypoxic-Ischemic Brain Injury Model
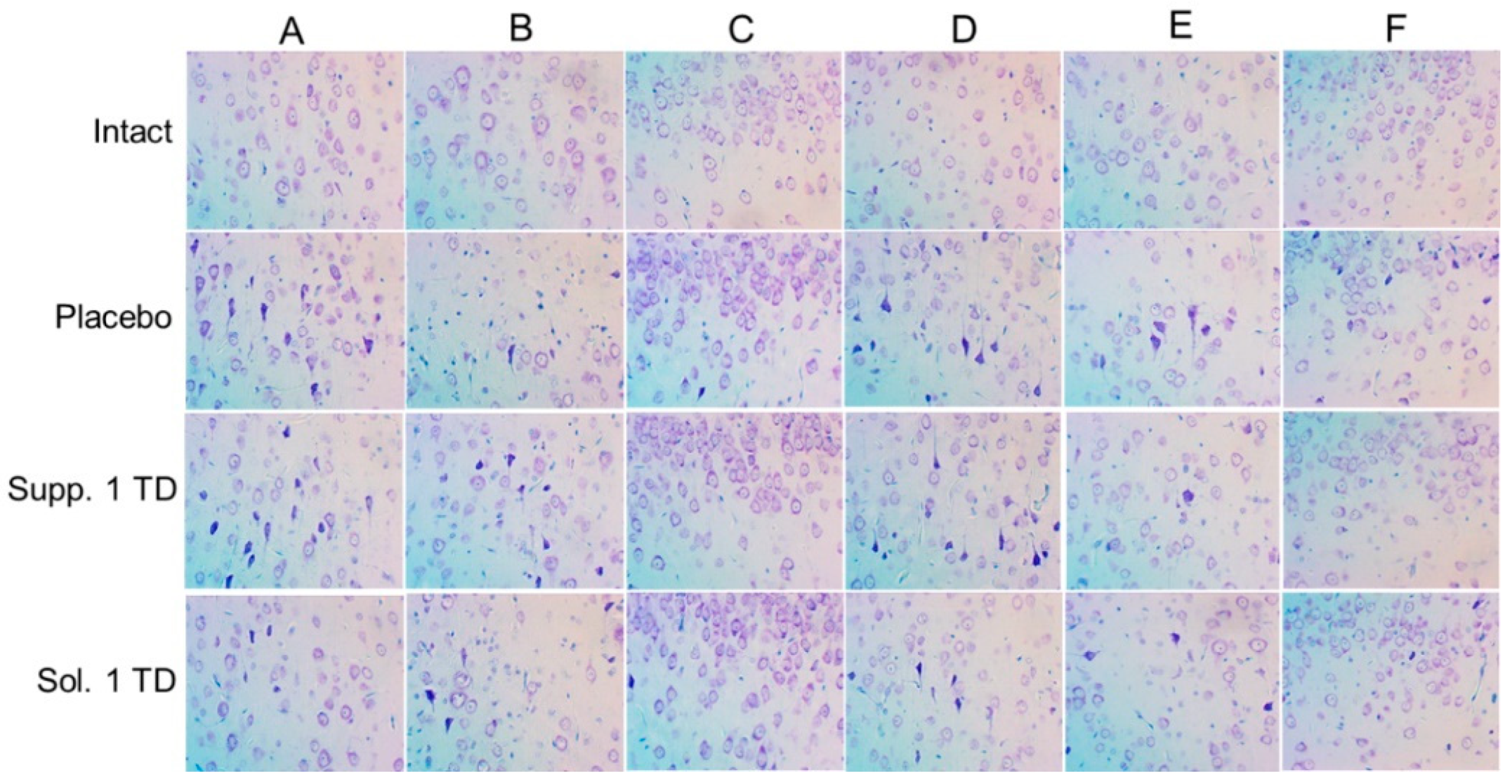
3.2. Results of Histologic Examination
3.2.1. Pathological Pregnancy Model (Ethanol Administration During the Last Week of Pregnancy)
3.2.2. Hypoxic-Ischemic Brain Injury Model
- Chromatolysis of varying degrees is often accompanied by cytoplasmic vacuolization.
- Hyperchromatosis, in its extreme form, where nerve cells appear as shrunken, dark homogeneous formations with poorly defined nuclei and nucleoli.
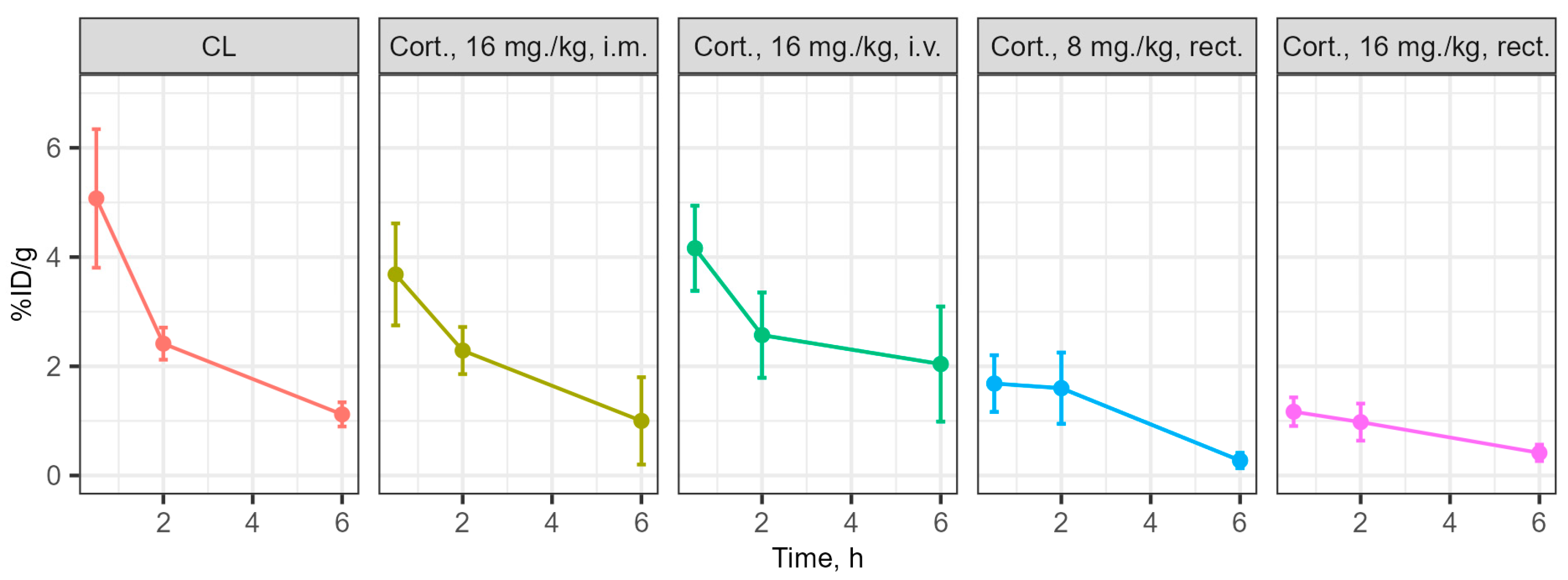
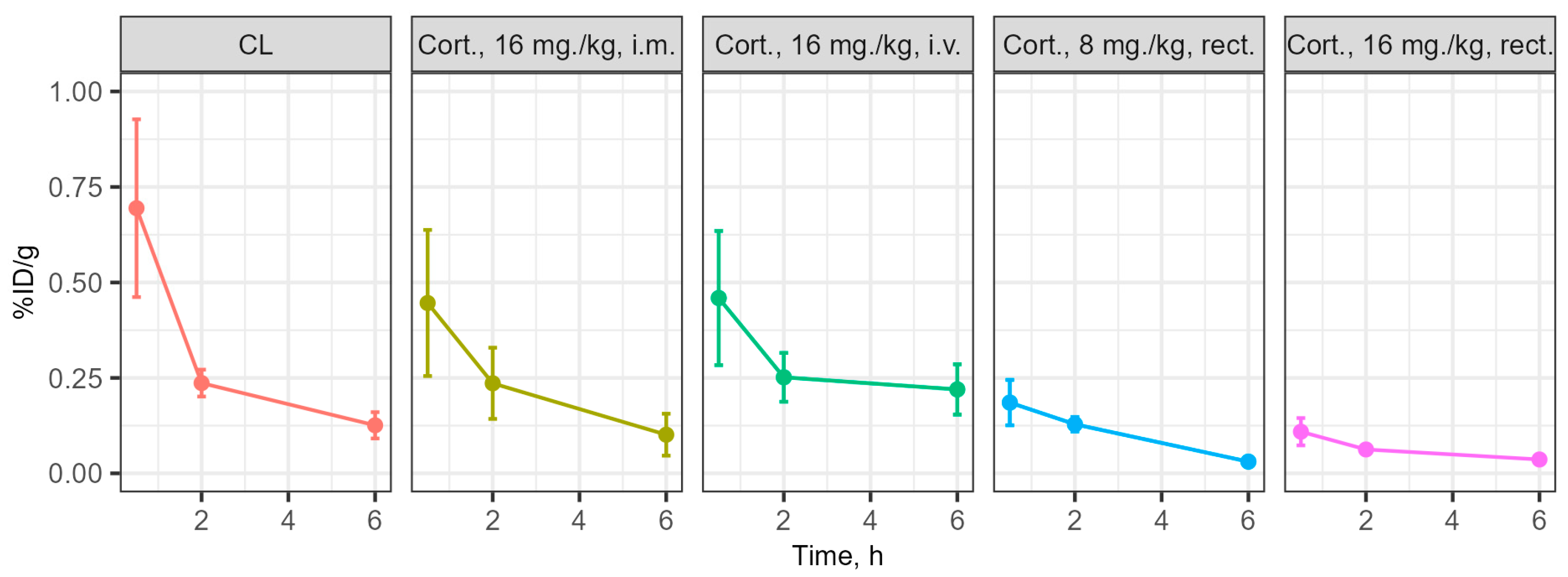
3.3. Determination of the Concentration of the Substances Under Study in Blood and Brain During Intravenous and Rectal Administration
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Roughly altered neurons |
| CNS | Central nervous system |
| MN | Slightly modified neurons |
| mNSS | Modified Neurological Severity Scores |
| NN | Unchanged (normal) neurons |
| PR | Per rectum (rectal administration) |
| SD | Standard deviation |
| TD | Therapeutic dose |
| TLC | Thin-layer chromatography |
References
- Lan, X.B.; Ni, Y.S.; Liu, N.; Wei, W.; Liu, Y.; Yang, J.M.; Ma, L.; Bai, R.; Zhang, J.; Yu, J.Q. Neuroprotective effects of oxymatrine on hypoxic-ischemic brain damage in neonatal rats by activating the Wnt/β-catenin pathway. Biomed. Pharmacother. 2023, 159, 114266. [Google Scholar] [CrossRef]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef]
- Bornstein, N.M.; Guekht, A.; Vester, J.; Heiss, W.D.; Gusev, E.; Hömberg, V.; Rahlfs, V.W.; Bajenaru, O.; Popescu, B.O.; Muresanu, D. Safety and efficacy of Cerebrolysin in early post-stroke recovery: A meta-analysis of nine randomized clinical trials. Neurol. Sci. 2018, 39, 629–640. [Google Scholar] [CrossRef]
- Kurkin, D.V.; Bakulin, D.A.; Morkovin, E.I.; Kalatanova, A.V.; Makarenko, I.E.; Dorotenko, A.R.; Kovalev, N.S.; Dubrovina, M.A.; Verkholyak, D.V.; Abrosimova, E.E.; et al. Neuroprotective action of Cortexin, Cerebrolysin and Actovegin in acute or chronic brain ischemia in rats. PLoS ONE 2021, 16, e0254493. [Google Scholar] [CrossRef]
- Zykov, V.P.; Serebrennikova, E.B.; Panchenko, T.N.; Sycheva, Y.B.; Presnyakova, S.N.; Mazur, E.L.; Salova, M.N.; Golubeva, E.S.; Khromova, S.K. Rezul’taty mul’titsentrovogo issledovaniia éffektivnosti primeneniia korteksina pri kognitivnykh disfunktsiiakh u deteĭ [Results of a multicenter study on the efficacy of cortexin in treatment of cognitive dysfunction in children]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2018, 118, 27–31. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Nasef, N.; Fathy, K.; El-Gilany, A.H.; Yahia, S. Effect of cerebrolysin on neurodevelopmental outcome of high risk preterm infants: A randomized controlled trial. J. Neonatal Perinat. Med. 2022, 15, 37–45. [Google Scholar] [CrossRef]
- Gromova, O.A.; Torshin, I.Y.; Zgoda, V.G.; Tikhonova, O.V. Kompleksnyĭ proteomnyĭ analiz ‘legkoĭ’ peptidnoĭ fraktsii preparata Tserebrolizin [An analysis of the peptide composition of a ‘light’ peptide fraction of cerebrolysin]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2019, 119, 75–83. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Molekuliarnye mekhanizmy deĭstviia preparatov, soderzhashchikh peptidy mozga: Korteksin [Molecular mechanisms of brain peptide-containing drugs: Cortexin]. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2018, 118, 93–96. [Google Scholar] [CrossRef]
- Mironov, A.N. (Ed.) Guidelines for Preclinical Trials of Medicinal Products; Part 1; Grif i K: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Pueta, M.; Abate, P.; Haymal, O.B.; Spear, N.E.; Molina, J.C. Ethanol exposure during late gestation and nursing in the rat: Effects upon maternal care, ethanol metabolism and infantile milk intake. Pharmacol. Biochem. Behav. 2008, 91, 21–31. [Google Scholar] [CrossRef]
- Almeida, L.; Andreu-Fernández, V.; Navarro-Tapia, E.; Aras-López, R.; Serra-Delgado, M.; Martínez, L.; García-Algar, O.; Gómez-Roig, M.D. Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatr. 2020, 8, 359. [Google Scholar] [CrossRef]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.; Eide, S.; Sun, H.S.; Feng, Z.P. Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp. Neurol. 2020, 334, 113457. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Bouet, V.; Boulouard, M.; Toutain, J.; Divoux, D.; Bernaudin, M.; Schumann-Bard, P.; Freret, T. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat. Protoc. 2009, 4, 1560–1564. [Google Scholar] [CrossRef]
- Monville, C.; Torres, E.M.; Dunnett, S.B. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods. 2006, 158, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.C. A low cost gamma-vial for counting 125I with a liquid scintillation counter. Clin. Chim. Acta. 1976, 66, 141–144. [Google Scholar] [CrossRef]
- Hunter, W.M.; Greenwood, F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962, 194, 495–496. [Google Scholar] [CrossRef]
- Perret, P.; Ahmadi, M.; Riou, L.; Bacot, S.; Pecher, J.; Poillot, C.; Broisat, A.; Ghezzi, C.; De Waard, M. Biodistribution, Stability, and Blood Distribution of the Cell Penetrating Peptide Maurocalcine in Mice. Int. J. Mol. Sci. 2015, 16, 27730–27740. [Google Scholar] [CrossRef]
- Bulić, M.; Tuleu, C. Rectal drug delivery to paediatric population. Hrvat. Časopis Zdr. Znan. 2021, 1, 76–80. [Google Scholar] [CrossRef]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st Century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Rathi, R.; Sanshita; Kumar, A.; Vishvakarma, V.; Huanbutta, K.; Singh, I.; Sangnim, T. Advancements in Rectal Drug Delivery Systems: Clinical Trials, and Patents Perspective. Pharmaceutics 2022, 14, 2210. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkin, D.V.; Bakulin, D.A.; Morkovin, E.I.; Petrov, V.I.; Strygin, A.V.; Smirnov, A.V.; Shmidt, M.V.; Gorbunova, J.V.; Kolosov, Y.A.; Ivanova, O.V.; et al. Neurotropic Effects of Cortexin on Models of Mental and Physical Developmental Delay. Biomedicines 2025, 13, 860. https://doi.org/10.3390/biomedicines13040860
Kurkin DV, Bakulin DA, Morkovin EI, Petrov VI, Strygin AV, Smirnov AV, Shmidt MV, Gorbunova JV, Kolosov YA, Ivanova OV, et al. Neurotropic Effects of Cortexin on Models of Mental and Physical Developmental Delay. Biomedicines. 2025; 13(4):860. https://doi.org/10.3390/biomedicines13040860
Chicago/Turabian StyleKurkin, Denis V., Dmitry A. Bakulin, Evgeny I. Morkovin, Vladimir I. Petrov, Andrei V. Strygin, Alexey V. Smirnov, Maksim V. Shmidt, Julia V. Gorbunova, Yury A. Kolosov, Olga V. Ivanova, and et al. 2025. "Neurotropic Effects of Cortexin on Models of Mental and Physical Developmental Delay" Biomedicines 13, no. 4: 860. https://doi.org/10.3390/biomedicines13040860
APA StyleKurkin, D. V., Bakulin, D. A., Morkovin, E. I., Petrov, V. I., Strygin, A. V., Smirnov, A. V., Shmidt, M. V., Gorbunova, J. V., Kolosov, Y. A., Ivanova, O. V., Krysanov, I. S., Dzhavakhyan, M. A., Zaborovsky, A. V., Saparova, V. B., Makarenko, I. E., Drai, R. I., Lugovik, I. A., Verlov, N. A., & Burdakov, V. S. (2025). Neurotropic Effects of Cortexin on Models of Mental and Physical Developmental Delay. Biomedicines, 13(4), 860. https://doi.org/10.3390/biomedicines13040860





