The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances
Abstract
1. Introduction
2. Mechanisms of NK Cell Activity in Cancer
2.1. Recognition and Signaling Pathways
2.1.1. Recognition of Tumor Cells by Inhibitory and Activating Receptors on NK Cells
2.1.2. Mechanisms of Natural Killer Cell-Mediated Cellular Cytotoxicity
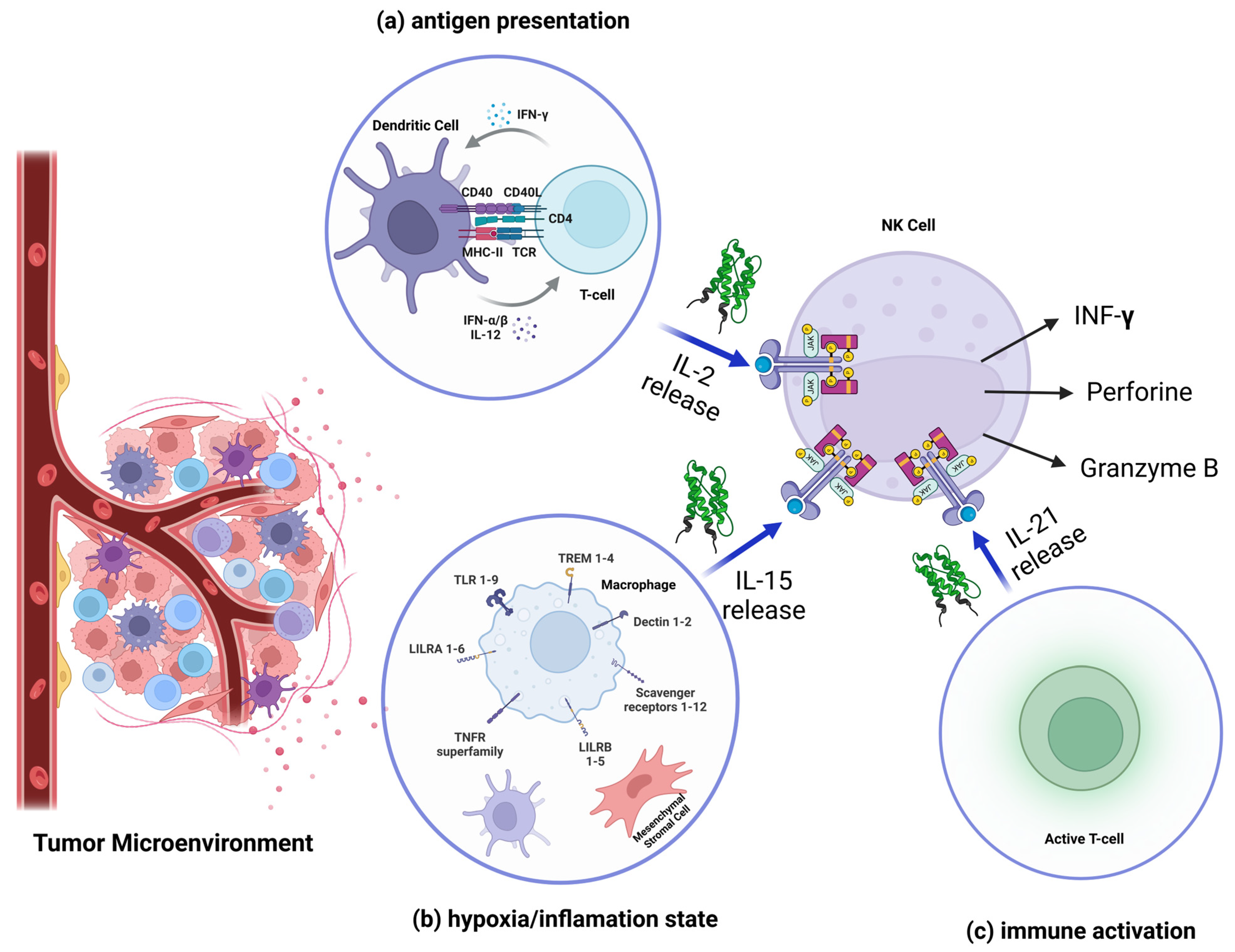
2.1.3. Role of Cytokines in the Activation and Expansion of NK Cells in the Tumor Environment
2.2. Tumor Evasion Mechanisms
2.2.1. Mechanisms of Tumor Escape from Natural Killer Cell-Mediated Immunity
- Proteolytic shedding: Tumor cells release soluble forms of MICA-B into the extracellular milieu through proteolytic cleavage mediated by metalloproteinases such as ADAM10 and ADAM17 [51]. The activation of these metalloproteinases is often driven by oncogenic pathways, including the RAS/RAF/MEK/ERK and PI3K/AKT cascades. These pathways increase the transcription and activity of ADAM family proteases, facilitating ligand shedding [52]. Soluble MIC-A/B acts as a decoy, binding to NKG2D on NK cells and internalizing the receptor, thereby impairing NK cell activation [50]. In many tumors that exhibit this mechanism, it often contributes to poor prognosis and malignancy [53].
- Epigenetic silencing: Tumors downregulate MIC-A/B expression on their surface by modifying their promoters through DNA methylation or histone deacetylation [33]. Hypoxia, a common feature of the tumor microenvironment, induces HIF-1α, which also represses MIC-A/B transcription. HIF-1α directly binds to the promoter regions of MIC-A/B, recruiting co-repressors that inhibit gene transcription [54]. Additionally, the hypoxic environment reduces oxidative stress signals that would otherwise trigger MIC-A/B expression [54].
- Immune suppression by soluble MIC-A/B: Soluble MIC-A/B not only blocks NKG2D signaling but also attracts immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), into the tumor microenvironment, further impairing immune responses [55]. This recruitment is mediated by chemokines and cytokines co-released with soluble MIC-A/B, creating an immunosuppressive niche [56].
- Upregulation of MHC-I to avoid NK cell activation: Some tumors overexpress self-MHC-I molecules to engage inhibitory receptors on NK cells, reducing their cytotoxic response [58]. This upregulation is mediated by interferon signaling, particularly IFN-γ, which activates the JAK/STAT pathway to enhance MHC-I transcription and presentation on the cell surface [59]. Tumors with mutations in the JAK/STAT pathway can evade this regulatory mechanism, creating a heterogeneous immune evasion strategy, either by over-expressing self-MHC protein or by not expressing at all non-self MHC proteins [60].
- Downregulation of MHC-I to escape CTLs: To prevent NK cell activation, tumors also upregulate non-classical MHC-I molecules such as HLA-E, which interact with inhibitory receptors like NKG2A on NK cells. This adaptation prevents NK cell-mediated cytotoxicity, despite the absence of classical MHC-I [18].
- Inhibition of NKG2DL expression: TGF-β downregulates NKG2DL on tumor cells’ surface by activating the SMAD2/3 signaling pathway, which represses the transcription of the ligand’s genes. This mechanism is critical for reducing the recognition of NK cells [64].
- Suppression of effector molecules: TGF-β interferes with the mTOR signaling pathway, reducing the expression of cytotoxic molecules such as granzyme B and perforin. This suppression impairs the ability of NK cells to induce apoptosis in tumor cells [65].
- Induction of NK cell exhaustion: Chronic exposure to TGF-β leads to an exhausted phenotype in NK cells, characterized by reduced cytokine production (e.g., IFN-γ) and diminished cytotoxicity. This effect is mediated by epigenetic modifications that lock NK cells into a hypofunctional state [66].
- Production of immunosuppressive molecules: Tumors or associated immune cells in TME (such as TAMs, tumor-associated macrophages) secrete cytokines such as IL-10. The poor oxygen conditions in the TME and the other cells of the tumor mass (f. e. fibroblasts) induce the production of VEGF, which reduces NK cell activity [67]. IL-10 inhibits antigen-presenting cell (APC) maturation, reducing overall immune activation [68]. VEGF not only promotes angiogenesis but also recruits Regulatory T cells (Tregs) and MDSCs, contributing to an immunosuppressive TME [69].
- Metabolic constraints in the TME: Hypoxia and nutrient depletion in the TME create metabolic stress on NK cells. High levels of lactate (a byproduct of tumor glycolysis) lower the pH and interfere with NK cell signaling and effector functions. The reduced availability of glucose and amino acids further compromises NK cell metabolism and proliferation [70].
2.2.2. Tumor Microenvironment Suppresses NK Cell Activity by Creating an Immunosuppressive Environment
2.2.3. Downregulation of Activating Receptors
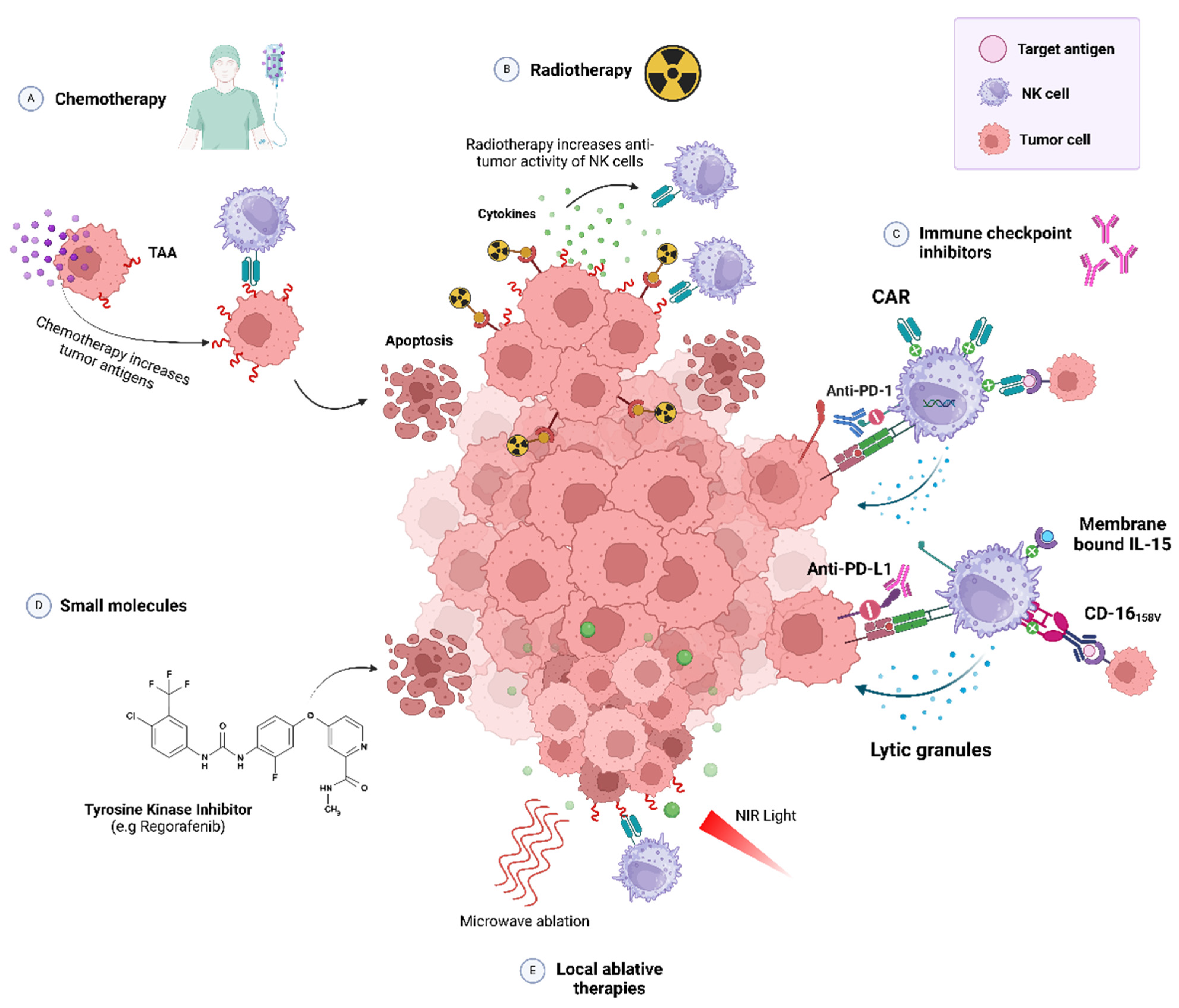
2.3. NK Cell-Based Immunotherapies
2.3.1. NK Cell Expansion
2.3.2. CAR-NK Cells
2.3.3. Combined NK-Based Immunotherapies
2.4. Clinical Trials and Recent Results
2.5. Challenges and Future Directions
2.5.1. Principal Challenges of NK Cell Therapies
Short Lifespan
Expansion and Activation
Mechanisms of Tumor Resistance
Areas of Future Research
2.5.2. Overcoming the Limitations: Strategies
Gene Editing by CRISPR
CAR-NK Therapies
Most Recent Studies
3. Summary and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Nutt, S.L.; Huntington, N.D. Cytotoxic T Lymphocytes and Natural Killer Cells. In Clinical Immunology: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–259.e1. ISBN 9780702068966. [Google Scholar]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural Killer Cell Therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting Natural Killer Cells and Natural Killer T Cells in Cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Guillerey, C. TIGIT as an Emerging Immune Checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Holmes, V.M.; De Motes, C.M.; Richards, P.T.; Roldan, J.; Bhargava, A.K.; Orange, J.S.; Krummenacher, C. Interaction between Nectin-1 and the Human Natural Killer Cell Receptor CD96. PLoS ONE 2019, 14, e0212443. [Google Scholar] [CrossRef]
- Zhu, Y.; Paniccia, A.; Schulick, A.C.; Chen, W.; Koenig, M.R.; Byers, J.T.; Yao, S.; Bevers, S.; Edil, B.H. Identification of CD112R as a Novel Checkpoint for Human T Cells. J. Exp. Med. 2016, 213, 167–176. [Google Scholar] [CrossRef]
- Huntington, N.D.; Cursons, J.; Rautela, J. The Cancer–Natural Killer Cell Immunity Cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar]
- Prager, I.; Watzl, C. Mechanisms of Natural Killer Cell-Mediated Cellular Cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and Granzymes: Function, Dysfunction and Human Pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar]
- Ewen, C.L.; Kane, K.P.; Bleackley, R.C. A Quarter Century of Granzymes. Cell Death Differ. 2012, 19, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Bak, S.H.; Park, T.; Kim, J.W.; Yoon, S.R.; Jung, H.; Noh, J.Y. Understanding NK Cell Biology for Harnessing NK Cell Therapies: Targeting Cancer and Beyond. Front. Immunol. 2023, 14, 1192907. [Google Scholar]
- Berrien-Elliott, M.M.; Jacobs, M.T.; Fehniger, T.A. Allogeneic Natural Killer Cell Therapy. Blood 2023, 141, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as Cellular Cancer Immunotherapy for Solid Tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [PubMed]
- Anderson, D.H.; Sawaya, M.R.; Cascio, D.; Ernst, W.; Modlin, R.; Krensky, A.; Eisenberg, D. Granulysin Crystal Structure and a Structure-Derived Lytic Mechanism. J. Mol. Biol. 2003, 325, 355–365. [Google Scholar] [CrossRef]
- Fischer, U.; Jänicke, R.U.; Schulze-Osthoff, K. Many Cuts to Ruin: A Comprehensive Update of Caspase Substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef]
- Borst, L.; van der Burg, S.H.; van Hall, T. The NKG2A-HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 5549–5556. [Google Scholar] [CrossRef]
- Carrette, F.; Vivier, E. NKG2A Blocks the Anti-Metastatic Functions of Natural Killer Cells. Cancer Cell 2023, 41, 232–234. [Google Scholar] [CrossRef]
- Kaulfuss, M.; Mietz, J.; Fabri, A.; vom Berg, J.; Münz, C.; Chijioke, O. The NK Cell Checkpoint NKG2A Maintains Expansion Capacity of Human NK Cells. Sci. Rep. 2023, 13, 10555. [Google Scholar] [CrossRef]
- Jin, H.S.; Park, Y. Hitting the Complexity of the TIGIT-CD96-CD112R-CD226 Axis for next-Generation Cancer Immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [PubMed]
- O’Donnell, J.S.; Madore, J.; Li, X.Y.; Smyth, M.J. Tumor Intrinsic and Extrinsic Immune Functions of CD155. Semin. Cancer Biol. 2020, 65, 189–196. [Google Scholar] [PubMed]
- Feng, S.; Isayev, O.; Werner, J.; Bazhin, A.V. CD96 as a Potential Immune Regulator in Cancers. Int. J. Mol. Sci. 2023, 24, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Cao, Y.; Jin, T.; Tian, Y.; Dai, C.; Xu, F. The CD112R/CD112 Axis: A Breakthrough in Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 285. [Google Scholar] [PubMed]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct Functions in Immune Regulation. Immunity 2024, 57, 206–222. [Google Scholar]
- Wang, J.; Li, H.; Kulkarni, A.; Anderson, J.L.; Upadhyay, P.; Onyekachi, O.V.; Arantes, L.M.R.B.; Banerjee, H.; Kane, L.P.; Zhang, X.; et al. Differential Impact of TIM-3 Ligands on NK Cell Function. J. Immunother. Cancer 2025, 13, e010618. [Google Scholar] [CrossRef]
- Sauer, N.; Janicka, N.; Szlasa, W.; Skinderowicz, B.; Kołodzińska, K.; Dwernicka, W.; Oślizło, M.; Kulbacka, J.; Novickij, V.; Karłowicz-Bodalska, K. TIM-3 as a Promising Target for Cancer Immunotherapy in a Wide Range of Tumors. Cancer Immunol. Immunother. 2023, 72, 3405–3425. [Google Scholar]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Pesini, C.; Hidalgo, S.; Arias, M.A.; Santiago, L.; Calvo, C.; Ocariz-Díez, M.; Isla, D.; Lanuza, P.M.; Agustín, M.J.; Galvez, E.M.; et al. PD-1 Is Expressed in Cytotoxic Granules of NK Cells and Rapidly Mobilized to the Cell Membrane Following Recognition of Tumor Cells. Oncoimmunology 2022, 11, 2096359. [Google Scholar] [CrossRef]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Human NK Cells, Their Receptors and Function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [PubMed]
- Tan, G.; Spillane, K.M.; Maher, J. The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer. Biology 2023, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Horton, N.C.; Mathew, S.O.; Mathew, P.A. Novel Interaction between Proliferating Cell Nuclear Antigen and HLA I on the Surface of Tumor Cells Inhibits NK Cell Function through NKp44. PLoS ONE 2013, 8, e59552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutton, V.R.; Davis, J.E.; Cancilla, M.; Johnstone, R.W.; Ruefli, A.A.; Sedelies, K.; Browne, K.A.; Trapani, J.A. Initiation of Apoptosis by Granzyme B Requires Direct Cleavage of Bid, but Not Direct Granzyme B-Mediated Caspase Activation; Rockefeller University Press: New York, NY, USA, 2000; Volume 192. [Google Scholar]
- Peter, M.E.; Krammer, P.H. The CD95(APO-1/Fas) DISC and Beyond. Cell Death Differ. 2003, 10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs Less Travelled: TRAIL in Cancer Biology and Therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The Role of Cytokines in the Regulation of NK Cells in the Tumor Environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Gotthardt, D.; Trifinopoulos, J.; Sexl, V.; Putz, E.M. JAK/STAT Cytokine Signaling at the Crossroad of NK Cell Development and Maturation. Front. Immunol. 2019, 10, 2590. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Ghosh, S.; Badar, S.M.; Nazir, A.; Bamigbade, G.B.; Aji, N.; Roy, P.; Kachani, H.; Garg, N.; Lawal, L.; et al. The Paradoxical Role of Cytokines and Chemokines at the Tumor Microenvironment: A Comprehensive Review. Eur. J. Med. Res. 2024, 29, 124. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-Inducible Factors: Master Regulators of Hypoxic Tumor Immune Escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- McCall, K.D.; Muccioli, M.; Benencia, F. Toll-Like Receptors Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1223, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Bai, X.; Handley, M.; Shan, F. Biological Effects of IL-15 on Immune Cells and Its Potential for the Treatment of Cancer. Int. Immunopharmacol. 2021, 91, 107318. [Google Scholar] [CrossRef] [PubMed]
- Batchu, R.B.; Gruzdyn, O.V.; Kolli, B.K.; Dachepalli, R.; Umar, P.S.; Rai, S.K.; Singh, N.; Tavva, P.S.; Weaver, D.W.; Gruber, S.A. IL-10 Signaling in the Tumor Microenvironment of Ovarian Cancer. Adv. Exp. Med. Biol. 2021, 1290, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Chabab, G.; Bonnefoy, N.; Lafont, V. IL-21 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 73–82. [Google Scholar] [CrossRef]
- Alves, C.L.; Ditzel, H.J. Drugging the PI3K/AKT/MTOR Pathway in ER+ Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4522. [Google Scholar] [CrossRef]
- Malarkannan, S. Molecular Mechanisms of FasL-Mediated “Reverse-Signaling”. Mol. Immunol. 2020, 127, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shiota, G. Immune Evasion by Cancer Stem Cells. Regen. Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Ferrari de Andrade, L. NKG2D and MICA/B Shedding: A ‘Tag Game’ between NK Cells and Malignant Cells. Clin. Transl. Immunol. 2020, 9, e1230. [Google Scholar] [CrossRef]
- Zingoni, A.; Vulpis, E.; Loconte, L.; Santoni, A. NKG2D Ligand Shedding in Response to Stress: Role of ADAM10. Front. Immunol. 2020, 11, 447. [Google Scholar] [CrossRef]
- Saha, N.; Robev, D.; Himanen, J.P.; Nikolov, D.B. ADAM Proteases: Emerging Role and Targeting of the Non-Catalytic Domains. Cancer Lett. 2019, 467, 50–57. [Google Scholar] [CrossRef]
- Di Donato, M.; Cristiani, C.M.; Capone, M.; Garofalo, C.; Madonna, G.; Passacatini, L.C.; Ottaviano, M.; Ascierto, P.A.; Auricchio, F.; Carbone, E.; et al. Role of the Androgen Receptor in Melanoma Aggressiveness. Cell Death Dis. 2025, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-T.; Chen, Y.-H.; Li, Z.-Y.; Hsiao, A.-C.; Huang, Y.-L.; Hao, R.-X.; Tai, S.-K.; Chu, P.-Y.; Shih, J.-W.; Kung, H.-J.; et al. Hypoxia-Induced Long Noncoding RNA HIF1A-AS2 Regulates Stability of MHC Class I Protein in Head and Neck Cancer. Cancer Immunol. Res. 2024, 12, 1468–1484. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Wang, X.; Sheng, J.; Lu, S.; Yu, X.; Wu, J.D. Soluble NKG2D Ligand Promotes MDSC Expansion and Skews Macrophage to the Alternatively Activated Phenotype. J. Hematol. Oncol. 2015, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-Derived Suppressor Cells in Cancer and Cancer Therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Arosa, F.A.; Esgalhado, A.J.; Reste-Ferreira, D.; Cardoso, E.M. Open MHC Class I Conformers: A Look through the Looking Glass. Int. J. Mol. Sci. 2021, 22, 9738. [Google Scholar] [CrossRef]
- Hofman, T.; Ng, S.W.; Garcés-Lázaro, I.; Heigwer, F.; Boutros, M.; Cerwenka, A. IFNγ Mediates the Resistance of Tumor Cells to Distinct NK Cell Subsets. J. Immunother. Cancer 2024, 12, e009410. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Lu, T.; Bi, G.; Li, M.; Liang, J.; Hu, Z.; Zheng, Y.; Yin, J.; Xi, J.; et al. HIF-1α Switches the Functionality of TGF-β Signaling via Changing the Partners of Smads to Drive Glucose Metabolic Reprogramming in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-L.; Su, F.; Yang, J.-R.; Xiao, R.-W.; Wu, R.-Y.; Cao, M.-Y.; Ling, X.-L.; Zhang, T. TP53 to Mediate Immune Escape in Tumor Microenvironment: An Overview of the Research Progress. Mol. Biol. Rep. 2024, 51, 205. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, H.; Cho, H.-R.; Son, W.-C.; Park, Y.-S.; Kang, C.-D.; Bae, J. Downregulation of NKG2DLs by TGF-β in Human Lung Cancer Cells. BMC Immunol. 2021, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Marçais, A.; Guimaraes, F.S.-F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-β Inhibits the Activation and Functions of NK Cells by Repressing the MTOR Pathway. Sci. Signal 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chung, C.-L.; Hu, T.-H.; Chen, J.-J.; Liu, P.-F.; Chen, C.-L. Recent Progress in TGF-β Inhibitors for Cancer Therapy. Biomed. Pharmacother. 2021, 134, 111046. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The Cancer-Immunity Cycle: Indication, Genotype, and Immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Lu, T.; Liu, Y.; Hong, W.; He, X.; Cheng, Y.; Liu, J.; Wei, Y.; Wei, X. JMJD6 in Tumor-Associated Macrophage Regulates Macrophage Polarization and Cancer Progression via STAT3/IL-10 Axis. Oncogene 2023, 42, 2737–2750. [Google Scholar] [CrossRef]
- Zhang, Y.; Brekken, R.A. Direct and Indirect Regulation of the Tumor Immune Microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Truffi, M.; Sorrentino, L.; Corsi, F. Fibroblasts in the Tumor Microenvironment. In Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 15–29. [Google Scholar]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar]
- Crusio, W.E.; Radeke, H.H. Tumor Microenvironment; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1270, ISBN 978-3-030-47188-0. [Google Scholar]
- Vitale, M.; Cantoni, C.; Pietra, G.; Mingari, M.C.; Moretta, L. Effect of Tumor Cells and Tumor Microenvironment on NK-Cell Function. Eur. J. Immunol. 2014, 44, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Nie, Y.; Lv, M.; Shen, S.; Tang, R.; Xu, Y.; Hou, Y.; Zhao, S.; Wang, T. Estrogen Upregulates MICA/B Expression in Human Non-Small Cell Lung Cancer through the Regulation of ADAM17. Cell. Mol. Immunol. 2015, 12, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; Wen, J.; Moutsopoulos, N.M. The Kiss of Death: Interrupted by NK-Cell Close Encounters of Another Kind. Trends Immunol. 2006, 27, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ Regulatory T Cells Inhibit Natural Killer Cell Functions in a Transforming Growth Factor-Beta-Dependent Manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Martin, F.; Zitvogel, L. The Role of Regulatory T Cells in the Control of Natural Killer Cells: Relevance during Tumor Progression. Immunol. Rev. 2006, 214, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK Cells Are Never Alone: Crosstalk and Communication in Tumour Microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [PubMed]
- Hong, D.S.; Rixe, O.; Chiu, V.K.; Forde, P.M.; Dragovich, T.; Lou, Y.; Nayak-Kapoor, A.; Leidner, R.; Atkins, J.N.; Collaku, A.; et al. Mogamulizumab in Combination with Nivolumab in a Phase I/II Study of Patients with Locally Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2022, 28, 479–488. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-Β1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef]
- Nuñez, S.Y.; Ziblat, A.; Secchiari, F.; Torres, N.I.; Sierra, J.M.; Raffo Iraolagoitia, X.L.; Araya, R.E.; Domaica, C.I.; Fuertes, M.B.; Zwirner, N.W. Human M2 Macrophages Limit NK Cell Effector Functions through Secretion of TGF-β and Engagement of CD85j. J. Immunol. 2018, 200, 1008–1015. [Google Scholar] [CrossRef]
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-Associated Neutrophils Suppress Antitumor Immunity of NK Cells through the PD-L1/PD-1 Axis. Transl. Oncol. 2020, 13, 100825. [Google Scholar] [CrossRef]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.-M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.M.; Lupo, K.B.; Wang, J.; Cao, J.; Utturkar, S.; Lanman, N.; Bernal-Crespo, V.; Jalal, S.; Pine, S.R.; Torregrosa-Allen, S.; et al. Engineered Natural Killer Cells Impede the Immunometabolic CD73-Adenosine Axis in Solid Tumors. Elife 2022, 11, e73699. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.H.; Manguso, R.T. Tumor-Derived PGE2 Gives NK Cells a Headache. Immunity 2020, 53, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal Stem Cells Inhibit Natural Killer–Cell Proliferation, Cytotoxicity, and Cytokine Production: Role of Indoleamine 2,3-Dioxygenase and Prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E2 (PGE2) Suppresses Natural Killer Cell Function Primarily through the PGE2 Receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Wang, X.; Liu, K. The Role of Indoleamine 2, 3-Dioxygenase 1 in Regulating Tumor Microenvironment. Cancers 2022, 14, 2756. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The Tryptophan Catabolite L-Kynurenine Inhibits the Surface Expression of NKp46- and NKG2D-Activating Receptors and Regulates NK-Cell Function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, F.; Wang, Y. Hypoxic Tumor Microenvironment: Destroyer of Natural Killer Cell Function. Chin. J. Cancer Res. 2024, 36, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Germeraad, W.T.V.; Rouschop, K.M.A.; Steeghs, E.M.P.; van Gelder, M.; Bos, G.M.J.; Wieten, L. Hypoxia Induced Impairment of NK Cell Cytotoxicity against Multiple Myeloma Can Be Overcome by IL-2 Activation of the NK Cells. PLoS ONE 2013, 8, e64835. [Google Scholar] [CrossRef]
- Vitale, M.; Parodi, M. Blocking HIF to Enhance NK Cells: Hints for New Anti-Tumor Therapeutic Strategies? Vaccines 2021, 9, 1144. [Google Scholar] [CrossRef]
- ZHENG, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.-P. Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions. Front. Immunol. 2020, 11, 202. [Google Scholar] [CrossRef]
- Miao, L.; Lu, C.; Zhang, B.; Li, H.; Zhao, X.; Chen, H.; Liu, Y.; Cui, X. Advances in Metabolic Reprogramming of NK Cells in the Tumor Microenvironment on the Impact of NK Therapy. J. Transl. Med. 2024, 22, 229. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Zheng, W.; Ling, S.; Cao, Y.; Shao, C.; Sun, X. Combined Use of NK Cells and Radiotherapy in the Treatment of Solid Tumors. Front. Immunol. 2023, 14, 1306534. [Google Scholar]
- Martin-Iglesias, S.; Herrera, L.; Santos, S.; Vesga, M.Á.; Eguizabal, C.; Lanceros-Mendez, S.; Silvan, U. Analysis of the Impact of Handling and Culture on the Expansion and Functionality of NK Cells. Front. Immunol. 2023, 14, 1225549. [Google Scholar] [CrossRef]
- Becker, P.S.A.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and Expansion of Natural Killer Cells for NK Cell-Based Immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Koh, S.K.; Park, J.; Kim, S.E.; Lim, Y.; Phan, M.T.T.; Kim, J.; Hwang, I.; Ahn, Y.O.; Shin, S.; Doh, J.; et al. Natural Killer Cell Expansion and Cytotoxicity Differ Depending on the Culture Medium Used. Ann. Lab. Med. 2022, 42, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xie, S.; Chen, M.; Li, Y.; Yue, J.; Ma, J.; Shu, X.; He, Y.; Xiao, W.; Tian, Z. Advances in NK Cell Production. Cell. Mol. Immunol. 2022, 19, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Goldenson, B.H.; Hor, P.; Kaufman, D.S. IPSC-Derived Natural Killer Cell Therapies—Expansion and Targeting. Front. Immunol. 2022, 13, 841107. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Y.; Xie, Z.; Huang, H.; Tan, W.S.; Cai, H. Vitamin Combination Promotes Ex Vivo Expansion of NK-92 Cells by Reprogramming Glucose Metabolism. Bioresour. Bioprocess. 2022, 9, 87. [Google Scholar] [CrossRef]
- Masuyama, J.I.; Murakami, T.; Iwamoto, S.; Fujita, S. Ex Vivo Expansion of Natural Killer Cells from Human Peripheral Blood Mononuclear Cells Co-Stimulated with Anti-CD3 and Anti-CD52 Monoclonal Antibodies. Cytotherapy 2016, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Choi, H.; Her, J.H.; Jung, M.Y.; Kim, H.J.; Jung, M.Y.; Lee, E.K.; Cho, S.Y.; Hwang, Y.K.; Shin, E.C. Optimization of Large-Scale Expansion and Cryopreservation of Human Natural Killer Cells for Anti-Tumor Therapy. Immune Netw. 2018, 18, e31. [Google Scholar] [CrossRef]
- Kim, S. Ex Vivo Expansion of Highly Cytotoxic Natural Killer Cells Using Optimal Culture Medium. Ann. Lab. Med. 2022, 42, 619–620. [Google Scholar] [CrossRef]
- Pierson, B.A.; Mcglave, P.B.; Hu, W.S.; Miller, J.S. Natural Killer Cell Proliferation Is Dependent on Human Serum and Markedly Increased Utilizing an Enriched Supplemented Basal Medium. J. Hematother. 1995, 4, 149–158. [Google Scholar] [CrossRef]
- Liu, M.; Meng, Y.; Zhang, L.; Han, Z.; Feng, X. High-Efficient Generation of Natural Killer Cells from Peripheral Blood with Preferable Cell Vitality and Enhanced Cytotoxicity by Combination of IL-2, IL-15 and IL-18. Biochem. Biophys. Res. Commun. 2021, 534, 149–156. [Google Scholar] [CrossRef]
- Granzin, M.; Wagner, J.; Köhl, U.; Cerwenka, A.; Huppert, V.; Ullrich, E. Shaping of Natural Killer Cell Antitumor Activity by Ex Vivo Cultivation. Front. Immunol. 2017, 8, 458. [Google Scholar]
- Sivonen, M.; Sirviö, K.A.; Wojciechowski, S.; Kailaanmäki, A.; Kaipainen, S.; Bailey, A.; Villalba, M.; Kekarainen, T. Cytokines Impact Natural Killer Cell Phenotype and Functionality against Glioblastoma In Vitro. Front. Immunol. 2023, 14, 1227064. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Cadavid, J.V.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z.; et al. Expansion, Persistence, and Efficacy of Donor Memory-like NK Cells Infused for Posttransplant Relapse. J. Clin. Investig. 2022, 132, e154334. [Google Scholar] [CrossRef] [PubMed]
- Saultz, J.N.; Otegbeye, F. Optimizing the Cryopreservation and Post-Thaw Recovery of Natural Killer Cells Is Critical for the Success of off-the-Shelf Platforms. Front. Immunol. 2023, 14, 1304689. [Google Scholar] [CrossRef]
- Berjis, A.; Muthumani, D.; Aguilar, O.A.; Pomp, O.; Johnson, O.; Finck, A.V.; Engel, N.W.; Chen, L.; Plachta, N.; Scholler, J.; et al. Pretreatment with IL-15 and IL-18 Rescues Natural Killer Cells from Granzyme B-Mediated Apoptosis after Cryopreservation. Nat. Commun. 2024, 15, 3937. [Google Scholar] [CrossRef]
- Wang, X.; Byrne, M.E.; Liu, C.; Ma, M.T.; Liu, D. Scalable Process Development of NK and CAR-NK Expansion in a Closed Bioreactor. Front. Immunol. 2024, 15, 1412378. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Hao, M.; Tan, W.S.; Cai, H. A Novel Magnetically Controlled Bioreactor for Ex Vivo Expansion of NK-92 Cells. Bioresour. Bioprocess. 2022, 9, 50. [Google Scholar] [CrossRef]
- Gu, R.; Liu, F.; Zou, D.; Xu, Y.; Lu, Y.; Liu, B.; Liu, W.; Chen, X.; Liu, K.; Guo, Y.; et al. Efficacy and Safety of CD19 CAR T Constructed with a New Anti-CD19 Chimeric Antigen Receptor in Relapsed or Refractory Acute Lymphoblastic Leukemia. J. Hematol. Oncol. 2020, 13, 122. [Google Scholar] [CrossRef]
- Jaklevic, M.C. CAR-T Therapy Is Approved for Non-Hodgkin Lymphoma. JAMA 2021, 325, 1032. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-Cell Therapy in the Era of Solid Tumor Treatment: Current Challenges and Emerging Therapeutic Advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maus, M.V.; Hinrichs, C.S. CAR T Cells and T-Cell Therapies for Cancer. JAMA 2024, 332, 1924. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; He, Z.; Li, L.; Liu, S.; Jiang, M.; Zhao, B.; Deng, M.; Wang, W.; Mi, X.; et al. Breakthrough of Solid Tumor Treatment: CAR-NK Immunotherapy. Cell Death Discov. 2024, 10, 40. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, M.; Zhang, W.; Liu, N.; Wang, D.; Jing, L.; Xu, N.; Yang, N.; Ren, T. Chimeric Antigen Receptor-Based Natural Killer Cell Immunotherapy in Cancer: From Bench to Bedside. Cell Death Dis. 2024, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-S.; Matsushita, M.; Plotkin, J.; Riviere, I.; Sadelain, M. Chimeric Antigen Receptors Combining 4-1BB and CD28 Signaling Domains Augment PI3kinase/AKT/Bcl-XL Activation and CD8+ T Cell–Mediated Tumor Eradication. Mol. Ther. 2010, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Esser, R.; Priesner, C.; Suerth, J.D.; Schambach, A.; Wels, W.S.; Grez, M.; Kloess, S.; Arseniev, L.; Koehl, U. Advantages and Applications of CAR-Expressing Natural Killer Cells. Front. Pharmacol. 2015, 6, 21. [Google Scholar] [CrossRef]
- Marofi, F.; Rahman, H.S.; Thangavelu, L.; Dorofeev, A.; Bayas-Morejón, F.; Shirafkan, N.; Shomali, N.; Chartrand, M.S.; Jarahian, M.; Vahedi, G.; et al. Renaissance of Armored Immune Effector Cells, CAR-NK Cells, Brings the Higher Hope for Successful Cancer Therapy. Stem Cell Res. Ther. 2021, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Lupo, K.B.; Matosevic, S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef]
- Chou, C.K.; Turtle, C.J. Insight into Mechanisms Associated with Cytokine Release Syndrome and Neurotoxicity after CD19 CAR-T Cell Immunotherapy. Bone Marrow Transplant. 2019, 54, 780–784. [Google Scholar] [CrossRef]
- Hay, K.A. Cytokine Release Syndrome and Neurotoxicity after CD19 Chimeric Antigen Receptor-modified (CAR-) T Cell Therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Hunter, B.D.; Jacobson, C.A. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. JNCI J. Natl. Cancer Inst. 2019, 111, 646–654. [Google Scholar] [CrossRef]
- Cifaldi, L.; Melaiu, O.; Giovannoni, R.; Benvenuto, M.; Focaccetti, C.; Nardozi, D.; Barillari, G.; Bei, R. DNAM-1 Chimeric Receptor-Engineered NK Cells: A New Frontier for CAR-NK Cell-Based Immunotherapy. Front. Immunol. 2023, 14, 1197053. [Google Scholar] [CrossRef]
- Lamers-Kok, N.; Panella, D.; Georgoudaki, A.M.; Liu, H.; Özkazanc, D.; Kučerová, L.; Duru, A.D.; Spanholtz, J.; Raimo, M. Natural Killer Cells in Clinical Development as Non-Engineered, Engineered, and Combination Therapies. J. Hematol. Oncol. 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural Killer Cells in Antitumour Adoptive Cell Immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Fabian, K.P.; Padget, M.R.; Donahue, R.N.; Solocinski, K.; Robbins, Y.; Allen, C.T.; Lee, J.H.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. PD-L1 Targeting High-Affinity NK (t-HaNK) Cells Induce Direct Antitumor Effects and Target Suppressive MDSC Populations. J. Immunother. Cancer 2020, 8, e000450. [Google Scholar] [CrossRef] [PubMed]
- Wrona, E.; Borowiec, M.; Potemski, P. Car-Nk Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899. [Google Scholar] [CrossRef] [PubMed]
- Strecker, M.I.; Wlotzka, K.; Strassheimer, F.; Roller, B.; Ludmirski, G.; König, S.; Röder, J.; Opitz, C.; Alekseeva, T.; Reul, J.; et al. AAV-Mediated Gene Transfer of a Checkpoint Inhibitor in Combination with HER2-Targeted CAR-NK Cells as Experimental Therapy for Glioblastoma. Oncoimmunology 2022, 11, 2127508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, H.; Lian, K.; Zhang, D.; Hu, P.; He, Z.; Zhang, Z.; Wang, Y. CAR-NK Cells in Combination Therapy against Cancer: A Potential Paradigm. Heliyon 2024, 10, e27196. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Ding, J.; Liu, H.; Li, H.; Li, H.; Lu, M.; Miao, Y.; Li, L.; Zheng, J. Combination Therapy with EpCAM-CAR-NK-92 Cells and Regorafenib against Human Colorectal Cancer Models. J. Immunol. Res. 2018, 2018, 4263520. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, K.; Xu, J.; Zhang, H.; Li, L.; Fu, Q.; Chai, D.; Li, H.; Zheng, J. Synergistic Effects of Cabozantinib and EGFR-Specific CAR-NK-92 Cells in Renal Cell Carcinoma. J. Immunol. Res. 2017, 2017, 6915912. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Z.; Dong, X.; Wang, K.; Sun, Y.; Zhang, H.; Wang, F.; Chen, Y.; Ling, J.; Guo, Y.; et al. Radiotherapy Enhances the Anti-Tumor Effect of CAR-NK Cells for Hepatocellular Carcinoma. J. Transl. Med. 2024, 22, 929. [Google Scholar] [CrossRef]
- Makowska, A.; Lelabi, N.; Nothbaum, C.; Shen, L.; Busson, P.; Tran, T.T.B.; Eble, M.; Kontny, U. Radiotherapy Combined with PD-1 Inhibition Increases NK Cell Cytotoxicity towards Nasopharyngeal Carcinoma Cells. Cells 2021, 10, 2458. [Google Scholar] [CrossRef]
- Klapdor, R.; Wang, S.; Morgan, M.A.; Zimmermann, K.; Hachenberg, J.; Büning, H.; Dörk, T.; Hillemanns, P.; Schambach, A. NK Cell-Mediated Eradication of Ovarian Cancer Cells with a Novel Chimeric Antigen Receptor Directed against CD44. Biomedicines 2021, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Wang, S.; Hacker, U.; Büning, H.; Morgan, M.; Dörk, T.; Hillemanns, P.; Schambach, A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor–Based Immunotherapy and Chemotherapy. Hum. Gene Ther. 2017, 28, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.K.; Lee, B.C.; Kim, H.J.; Lee, J.J.; Chung, I.J.; Cho, S.B.; Koh, Y.S. A Phase I Study of Locoregional High-Dose Autologous Natural Killer Cell Therapy With Hepatic Arterial Infusion Chemotherapy in Patients With Locally Advanced Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 879452. [Google Scholar] [CrossRef]
- Nguyen, H.P.Q.; Bae, W.K.; Park, M.S.; Chung, I.J.; Nam, T.K.; Jeong, J.U.; Uong, T.N.T.; Cho, D.; Kim, S.K.; Yoon, M. Intensified NK Cell Therapy in Combination with Low-Dose Chemoradiotherapy against Human Colorectal Cancer. Cancer Immunol. Immunother. 2023, 72, 4089–4102. [Google Scholar] [CrossRef]
- Kokowski, K.; Stangl, S.; Seier, S.; Hildebrandt, M.; Vaupel, P.; Multhoff, G. Radiochemotherapy Combined with NK Cell Transfer Followed by Second-Line PD-1 Inhibition in a Patient with NSCLC Stage IIIb Inducing Long-Term Tumor Control: A Case Study. Strahlenther. Onkol. 2019, 195, 352–361. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yan, Y.; Zhang, J.; Wei, Z.; Li, H.; Xing, L. Synergistic Treatment Strategy: Combining CAR-NK Cell Therapy and Radiotherapy to Combat Solid Tumors. Front. Immunol. 2023, 14, 1298683. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell-Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, B.; Wang, Y.; Wang, D.; Gao, D.; Yang, S.; Zhao, Q.; Zhou, C.; Ruan, S.; Yuan, Z. Temperature-Feedback Nanoplatform for NIR-II Penta-Modal Imaging-Guided Synergistic Photothermal Therapy and CAR-NK Immunotherapy of Lung Cancer. Small 2021, 17, 2101397. [Google Scholar] [CrossRef]
- Baker, D.J.; Arany, Z.; Baur, J.A.; Epstein, J.A.; June, C.H. CAR T Therapy beyond Cancer: The Evolution of a Living Drug. Nature 2023, 619, 707–715. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune Checkpoint Therapy—Current Perspectives and Future Directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Li, T.; Niu, M.; Zhang, W.; Qin, S.; Zhou, J.; Yi, M. CAR-NK Cells for Cancer Immunotherapy: Recent Advances and Future Directions. Front. Immunol. 2024, 15, 1361194. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. Nk Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef] [PubMed]
- MacKay, M.; Afshinnekoo, E.; Rub, J.; Hassan, C.; Khunte, M.; Baskaran, N.; Owens, B.; Liu, L.; Roboz, G.J.; Guzman, M.L.; et al. The Therapeutic Landscape for Cells Engineered with Chimeric Antigen Receptors. Nat. Biotechnol. 2020, 38, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, J. Emerging Roles of CAR-NK Cell Therapies in Tumor Immunotherapy: Current Status and Future Directions. Cell Death Discov. 2024, 10, 318. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Li, H.; Song, W.; Li, Z.; Zhang, M. Preclinical and Clinical Studies of CAR-NK-Cell Therapies for Malignancies. Front. Immunol. 2022, 13, 992232. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-Man Clinical Trial of CAR NK-92 Cells: Safety Test. of CD33-CAR NK-92 Cells in Patients with Relapsed and Refractory Acute Myeloid Leukemia. Am. J. Cancer Res. 2018, 8, 1083. [Google Scholar]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK Cell–Cancer Cycle: Advances and New Challenges in NK Cell–Based Immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [PubMed]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.J.; Lee, K.M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [PubMed]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J.; Guedan, S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front. Immunol. 2020, 11, 1109. [Google Scholar]
- Shin, H.E.; Han, J.H.; Park, J.D.; Park, M.; Han, J.; Kang, M.H.; Lee, J.S.; Park, C.G.; Park, J.; Kim, H.Y.; et al. Enhancing CAR-NK Cells Against Solid Tumors Through Chemical and Genetic Fortification with DOTAP-Functionalized Lipid Nanoparticles. Adv. Funct. Mater. 2024, 34, 2315721. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, X.; Liu, C.; Huang, Y.; Li, L.; Zhu, Y. Peptide-Based CAR-NK Cells: A Novel Strategy for the Treatment of Solid Tumors. Biochem. Pharmacol. 2025, 232, 116741. [Google Scholar] [CrossRef]
- Shin, M.H.; Oh, E.; Kim, Y.; Nam, D.H.; Jeon, S.Y.; Yu, J.H.; Minn, D. Recent Advances in CAR-Based Solid Tumor Immunotherapy. Cells 2023, 12, 1606. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Wang, X.; Wang, X.; Ren, S.; He, X.; Su, J.; Zheng, A.; Guo, S.; Chen, Y.; et al. The Investigation of Oncolytic Viruses in the Field of Cancer Therapy. Front. Oncol. 2024, 14, 1423143. [Google Scholar] [CrossRef]
- Lynch, C.; Pitroda, S.P.; Weichselbaum, R.R. Radiotherapy, Immunity, and Immune Checkpoint Inhibitors. Lancet Oncol. 2024, 25, e352–e362. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Z.; Chen, Y.; Xu, J.; Wang, J.; Wang, Z. Enhancing Cancer Therapy: The Integration of Oncolytic Virus Therapy with Diverse Treatments. Cancer Cell Int. 2024, 24, 242. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, Z.; Jiang, Z.; Gan, L.; Zhang, Z.; Xie, Z. Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5490. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.M.; Romee, R. Autologous Cellular Therapy for Myeloma: Giving Ex Vivo Expanded NK Cells Their Due. Cell Rep. Med. 2022, 3, 100537. [Google Scholar] [CrossRef] [PubMed]
- Sordo-Bahamonde, C.; Vitale, M.; Lorenzo-Herrero, S.; López-Soto, A.; Gonzalez, S. Mechanisms of Resistance to NK Cell Immunotherapy. Cancers 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, M.; Atilla, P.A.; Atilla, E. New Orders to an Old Soldier: Optimizing NK Cells for Adoptive Immunotherapy in Hematology. Biomedicines 2021, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Valeri, A.; García-Ortiz, A.; Castellano, E.; Córdoba, L.; Maroto-Martín, E.; Encinas, J.; Leivas, A.; Río, P.; Martínez-López, J. Overcoming Tumor Resistance Mechanisms in CAR-NK Cell Therapy. Front. Immunol. 2022, 13, 953849. [Google Scholar] [CrossRef]
- Tarannum, M.; Romee, R.; Shapiro, R.M. Innovative Strategies to Improve the Clinical Application of NK Cell-Based Immunotherapy. Front. Immunol. 2022, 13, 859177. [Google Scholar]
- Marofi, F.; Saleh, M.M.; Rahman, H.S.; Suksatan, W.; Al-Gazally, M.E.; Abdelbasset, W.K.; Thangavelu, L.; Yumashev, A.V.; Hassanzadeh, A.; Yazdanifar, M.; et al. CAR-Engineered NK Cells; a Promising Therapeutic Option for Treatment of Hematological Malignancies. Stem Cell Res. Ther. 2021, 12, 374. [Google Scholar] [CrossRef]
- Mendoza-Valderrey, A.; Alvarez, M.; De Maria, A.; Margolin, K.; Melero, I.; Ascierto, M.L. Next Generation Immuno-Oncology Strategies: Unleashing NK Cells Activity. Cells 2022, 11, 3147. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Felices, M.; Miller, J.S. Challenges to the Broad Application of Allogeneic Natural Killer Cell Immunotherapy of Cancer. Stem Cell Res. Ther. 2022, 13, 165. [Google Scholar]
- Kilgour, M.K.; Bastin, D.J.; Lee, S.H.; Ardolino, M.; McComb, S.; Visram, A. Advancements in CAR-NK Therapy: Lessons to Be Learned from CAR-T Therapy. Front. Immunol. 2023, 14, 1166038. [Google Scholar]
- Dash, C.P.; Sonowal, D.; Dhaka, P.; Yadav, R.; Chettri, D.; Satapathy, B.P.; Sheoran, P.; Uttam, V.; Jain, M.; Jain, A. Antitumor Activity of Genetically Engineered NK-Cells in Non-Hematological Solid Tumor: A Comprehensive Review. Front. Immunol. 2024, 15, 1390498. [Google Scholar]
- Park, J.D.; Shin, H.E.; An, Y.S.; Jang, H.J.; Park, J.; Kim, S.-N.; Park, C.G.; Park, W. Advancing Natural Killer Cell Therapy: Genetic Engineering Strategies for Enhanced Cancer Immunotherapy. Ann. Lab. Med. 2025, 45, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK Cell-Based Cancer Immunotherapies through Receptor Engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [PubMed]
- Tao, R.; Han, X.; Bai, X.; Yu, J.; Ma, Y.; Chen, W.; Zhang, D.; Li, Z. Revolutionizing Cancer Treatment: Enhancing CAR-T Cell Therapy with CRISPR/Cas9 Gene Editing Technology. Front. Immunol. 2024, 15, 1354825. [Google Scholar]
- Nakazawa, T.; Morimoto, T.; Maeoka, R.; Matsuda, R.; Nakamura, M.; Nishimura, F.; Ouji, N.; Yamada, S.; Nakagawa, I.; Park, Y.S.; et al. CIS Deletion by CRISPR/Cas9 Enhances Human Primary Natural Killer Cell Functions against Allogeneic Glioblastoma. J. Exp. Clin. Cancer Res. 2023, 42, 205. [Google Scholar] [CrossRef]
- Clara, J.A.; Levy, E.R.; Reger, R.; Barisic, S.; Chen, L.; Cherkasova, E.; Chakraborty, M.; Allan, D.S.J.; Childs, R. High-Affinity CD16 Integration into a CRISPR/Cas9-Edited CD38 Locus Augments CD38-Directed Antitumor Activity of Primary Human Natural Killer Cells. J. Immunother. Cancer 2022, 10, e003804. [Google Scholar] [CrossRef]
- Pituch-Noworolska, A.M. NK Cells in SARS-CoV-2 Infection. Cent. Eur. J. Immunol. 2022, 47, 95–101. [Google Scholar]
- Alrubayyi, A.; Rowland-Jones, S.; Peppa, D. Natural Killer Cells during Acute HIV-1 Infection: Clues for HIV-1 Prevention and Therapy. AIDS 2022, 36, 1903–1915. [Google Scholar]
- Wendel, P.; Reindl, L.M.; Bexte, T.; Künnemeyer, L.; Särchen, V.; Albinger, N.; Mackensen, A.; Rettinger, E.; Bopp, T.; Ullrich, E. Arming Immune Cells for Battle: A Brief Journey through the Advancements of t and Nk Cell Immunotherapy. Cancers 2021, 13, 1481. [Google Scholar] [CrossRef]
- Klaihmon, P.; Kang, X.; Issaragrisil, S.; Luanpitpong, S. Generation and Functional Characterization of Anti-CD19 Chimeric Antigen Receptor-Natural Killer Cells from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2023, 24, 10508. [Google Scholar] [CrossRef]
- Lam, P.Y.; Omer, N.; Wong, J.K.M.; Tu, C.; Alim, L.; Rossi, G.R.; Victorova, M.; Tompkins, H.; Lin, C.; Mehdi, A.M.; et al. Enhancement of Anti-sarcoma Immunity by NK Cells Engineered with MRNA for Expression of a EphA2-targeted CAR. Clin. Transl. Med. 2025, 15, e70140. [Google Scholar] [CrossRef]
- Bröker, K.; Sinelnikov, E.; Gustavus, D.; Schumacher, U.; Pörtner, R.; Hoffmeister, H.; Lüth, S.; Dammermann, W. Mass Production of Highly Active Nk Cells for Cancer Immunotherapy in a Gmp Conform Perfusion Bioreactor. Front. Bioeng. Biotechnol. 2019, 7, 194. [Google Scholar] [CrossRef]
- Maia, A.; Tarannum, M.; Romee, R. Genetic Manipulation Approaches to Enhance the Clinical Application of NK Cell-Based Immunotherapy. Stem Cells Transl. Med. 2024, 13, 230–242. [Google Scholar] [PubMed]
- Huang, R.; Wang, X.; Yan, H.; Tan, X.; Ma, Y.; Wang, M.; Han, X.; Liu, J.; Gao, L.; Gao, L.; et al. Safety and Efficacy of CD33-Targeted CAR-NK Cell Therapy for Relapsed/Refractory AML: Preclinical Evaluation and Phase I Trial. Exp. Hematol. Oncol. 2025, 14, 1. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Yoo, W.; Choi, H.; Jung, D.; Choi, E.; Jo, S.G.; Gong, E.Y.; Jeoung, Y.H.; Park, Y.S.; et al. Therapeutic Potential of Anti-ErbB3 Chimeric Antigen Receptor Natural Killer Cells against Breast Cancer. Cancer Immunol. Immunother. 2025, 74, 73. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Nakazawa, T.; Matsuda, R.; Nishimura, F.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Park, Y.S.; Tsujimura, T.; Nakase, H. CRISPR-Cas9–Mediated TIM3 Knockout in Human Natural Killer Cells Enhances Growth Inhibitory Effects on Human Glioma Cells. Int. J. Mol. Sci. 2021, 22, 3489. [Google Scholar] [CrossRef]


| Receptor | Type | Ligand | Signaling Pathway | Function |
|---|---|---|---|---|
| NKG2D | Activating | MICA/B, ULBPs | DAP10 | Cytotoxicity, cytokine production |
| NKp30 | Activating | B7-H6 | ITAM | Tumor cell recognition |
| NKp44 | Activating | Unknown/Stress ligands | ITAM | Enhances NK activation |
| NKp46 | Activating | Viral hemagglutinins | ITAM | Viral defense |
| CD226 | Activating | CD155 (PVR) | ITAM | Co-stimulation of NK response |
| CD16 | Activating | Fc region of antibodies | FcγRIIIa (CD16) | ADCC |
| NKG2A | Inhibitory | HLA-E | ITIM | Inhibition of NK activity |
| KIR | Inhibitory | HLA-A/B/C | ITIM | Self-tolerance, inhibition |
| TIGIT | Inhibitory | CD155 (PVR) | ITIM | Suppresses NK function |
| CD94/NKG2A | Inhibitory | HLA-E | ITIM | Inhibits cytotoxicity |
| PD-1 | Inhibitory | PD-L1 | ITIM | Suppresses NK response |
| CD96 | Inhibitory | CD155 | ITIM | Inhibits IFN-γ production |
| TIM-3 | Inhibitory | Galectin-9 | ITIM | Induces exhaustion |
| CAR Target | NK Cell Source | Targeting Tumor | National Clinical Trial Identifier |
|---|---|---|---|
| CD19 | Umbilical cord blood (UCB) | Hematological malignancies | NCT03056339 |
| Nonreferred | B cell hematologic malignancies | NCT05570188 | |
| Hematopoietic progenitor cells (HPCs) | B-cell lymphoma | NCT05654038 | |
| CD70 | Umbilical cord blood (UCB) | Hematological malignancies | NCT05092451 |
| Umbilical cord blood (UCB) | Solid tumors | NCT05703854 | |
| CD19/CD70 | Umbilical cord blood (UCB) | B-cell non-Hodgkin lymphoma | NCT05842707 |
| CD19/CD28 | Umbilical cord blood (UCB) | B-cell non-Hodgkin lymphoma | NCT03579927 |
| CD5 | Umbilical cord blood (UCB) | Hematological malignancies | NCT05110742 |
| CD7 | Peripheral blood mononuclear cells (PBMCs) | Leukemia and lymphoma | NCT02742727 |
| CD123 | Peripheral blood mononuclear cells (PBMCs) | Acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm | NCT06006403 |
| PD-L1 | NK92 | Gastroesophageal junction cancers or head and neck squamous cell carcinoma | NCT04847466 |
| Cladin6 | Peripheral blood mononuclear cells (PBMCs) | Reproductive system tumors | NCT05410717 |
| BCMA | NK92 | Multiple myeloma | NCT03940833 |
| CD33 | NK92 | Acute myeloid leukemia | NCT02944162 |
| MUC1 | Peripheral blood mononuclear cells (PBMCs) | Solid tumors | NCT02839954 |
| Robo1 | NK92 | Pancreatic cancer | NCT03941457 |
| TROP2 | Umbilical cord blood (UCB) | Ovarian cancer, mesonephric-like adenocarcinoma, and pancreatic cancer | NCT05922930 |
| Characteristic/Therapy | NK Cells | CAR-T Cells | CAR-NK Cells |
|---|---|---|---|
| Mechanism of action | Recognition and direct lysis of tumor cells | CAR receptor-mediated antigen recognition and cell lysis | CAR receptor-mediated antigen recognition and cell lysis |
| Activation time | Fast, can act immediately after infusion | Requires weeks due to necessary ex vivo expansion and activation | Requires ex vivo expansion and activation, like CAR-T cells |
| Shelf life | Short, limited to days or weeks | Prolonged, can last for months or even years | Variable, although shorter than that of CAR-T cells |
| Side effects | Minor compared to CAR-T | Significant, including cytokine release syndrome (CRS) and neurotoxicity | Minor compared to CAR-T, although there is risk of CRS |
| Applicability | Hematological and some solid tumors | Leukemias and lymphomas, with limited efficacy in solid tumors | Promising for solid and hematologic tumors (in development) |
| Response rate | Good, although it varies according to the type of tumor | High in certain types of leukemias and lymphomas | Promising, although still in development |
| Disadvantages | Short shelf life and resistance of the tumor microenvironment | High cost and complexity in production and handling | Need for optimization in production and efficacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcillo-Martín-Romo, P.; Valverde-Pozo, J.; Ortiz-Bueno, M.; Arnone, M.; Espinar-Barranco, L.; Espinar-Barranco, C.; García-Rubiño, M.E. The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines 2025, 13, 857. https://doi.org/10.3390/biomedicines13040857
Morcillo-Martín-Romo P, Valverde-Pozo J, Ortiz-Bueno M, Arnone M, Espinar-Barranco L, Espinar-Barranco C, García-Rubiño ME. The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines. 2025; 13(4):857. https://doi.org/10.3390/biomedicines13040857
Chicago/Turabian StyleMorcillo-Martín-Romo, Paula, Javier Valverde-Pozo, María Ortiz-Bueno, Maurizio Arnone, Laura Espinar-Barranco, Celia Espinar-Barranco, and María Eugenia García-Rubiño. 2025. "The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances" Biomedicines 13, no. 4: 857. https://doi.org/10.3390/biomedicines13040857
APA StyleMorcillo-Martín-Romo, P., Valverde-Pozo, J., Ortiz-Bueno, M., Arnone, M., Espinar-Barranco, L., Espinar-Barranco, C., & García-Rubiño, M. E. (2025). The Role of NK Cells in Cancer Immunotherapy: Mechanisms, Evasion Strategies, and Therapeutic Advances. Biomedicines, 13(4), 857. https://doi.org/10.3390/biomedicines13040857






