Macrophage Proangiogenic VEGF-A Is Required for Inflammatory Arteriogenesis During Vascular Injury
Abstract
1. Introduction
2. Methods
2.1. Resource Availability
2.2. Experimental Model and Subject Details
2.3. Method Details
3. Results
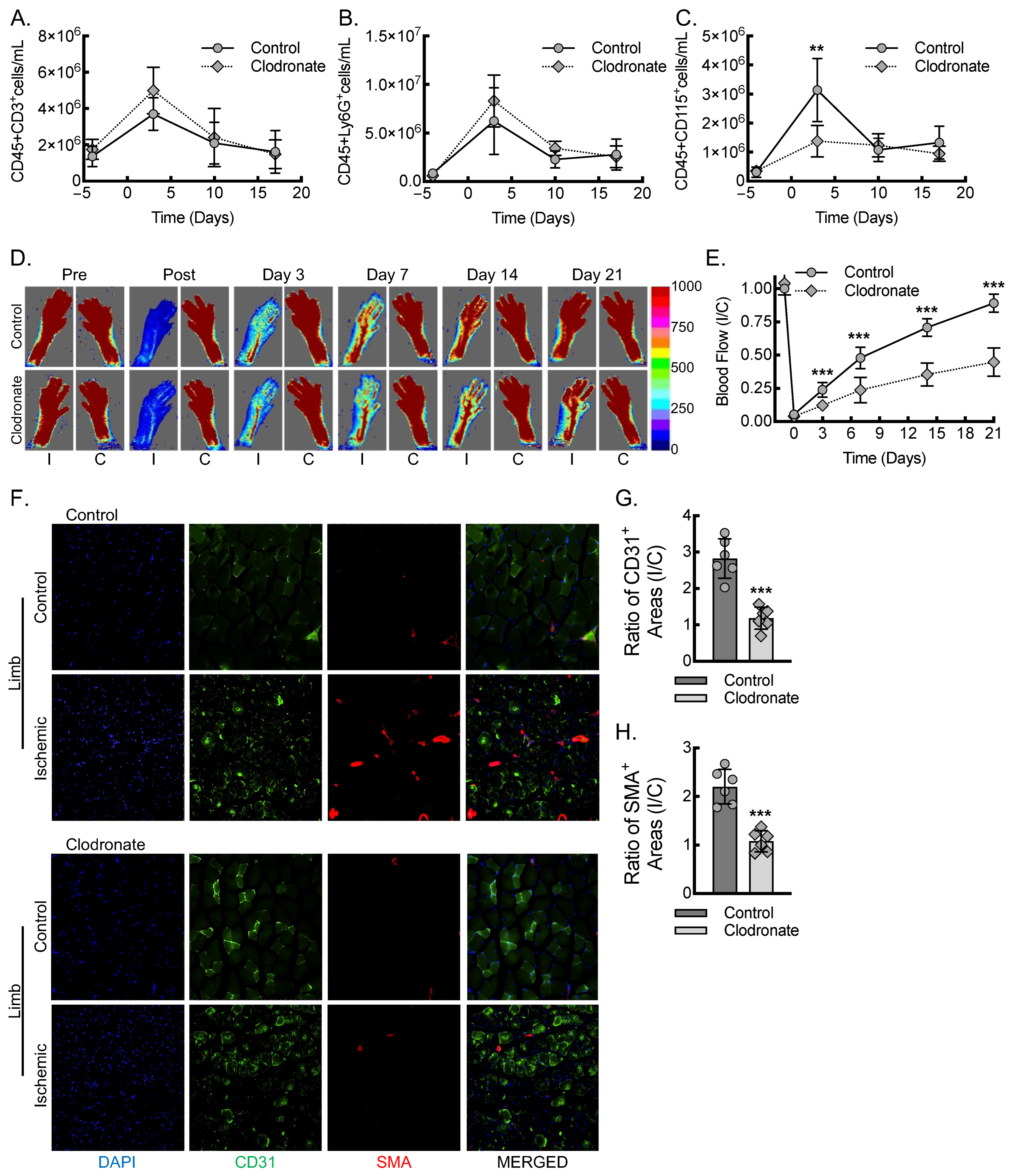
3.1. Macrophages Are Required for Inflammatory Arteriogenesis in Response to Acute Hind Limb Ischemia
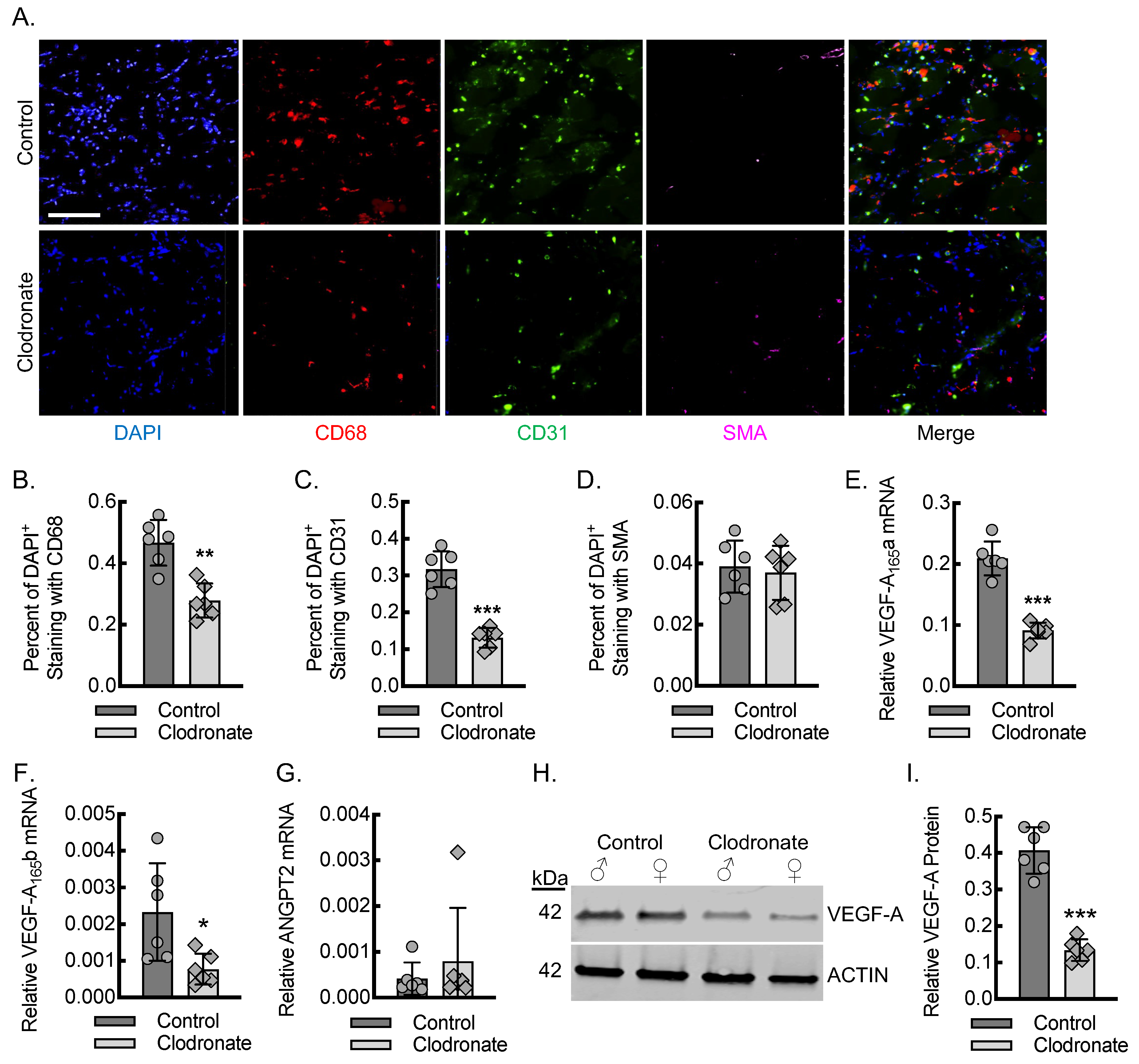
3.2. Macrophages Are Required for Endothelial Cell Recruitment and Adequate Tissue VEGF-A Levels During Early Inflammatory Angiogenesis
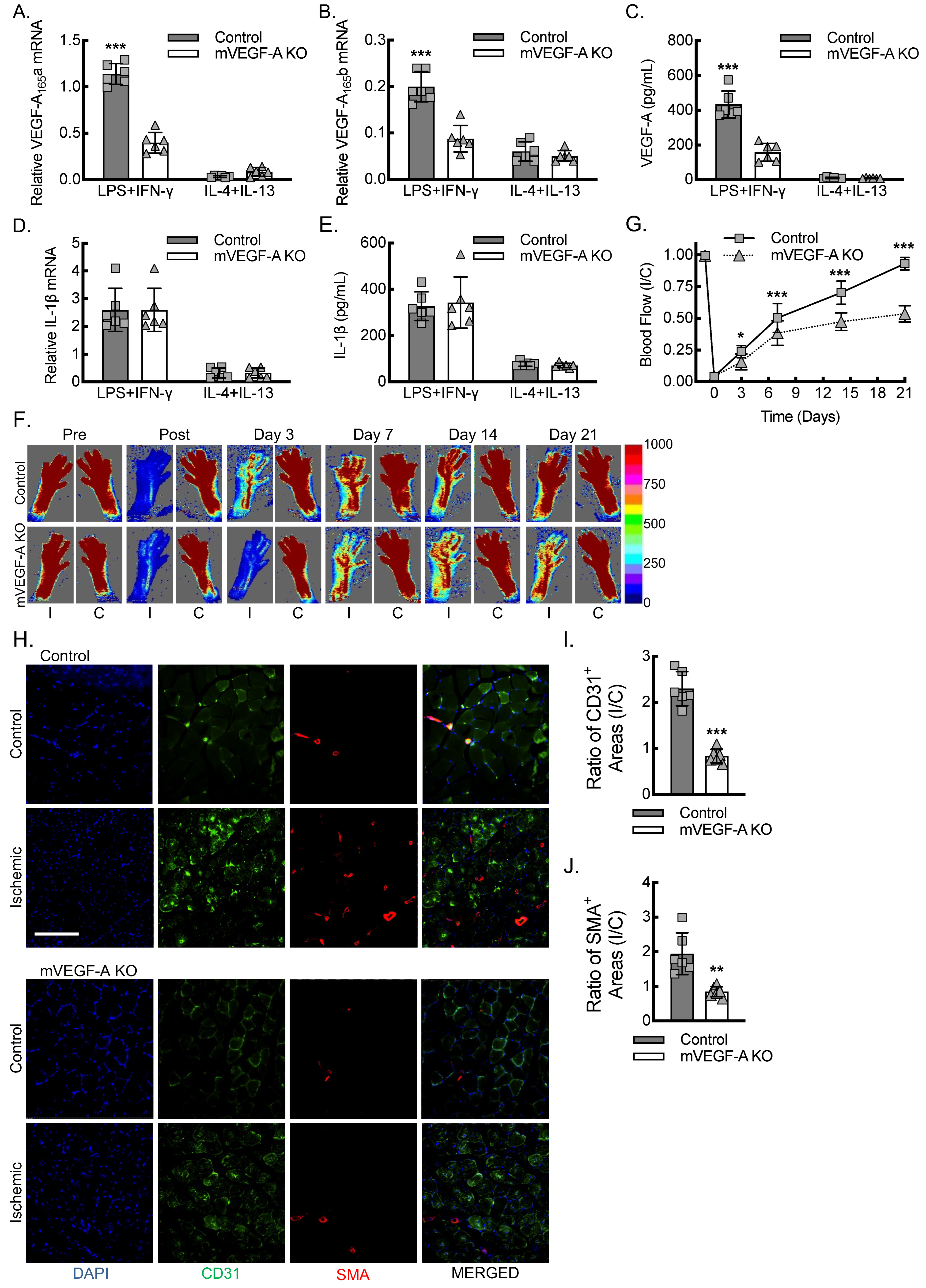
3.3. Myeloid VEGF-A-Deletion Leads to Reduced Macrophage VEGF-A Expression and Impaired Blood Flow Recovery Secondary to Decreased Inflammatory Angiogenesis and Arteriogenesis
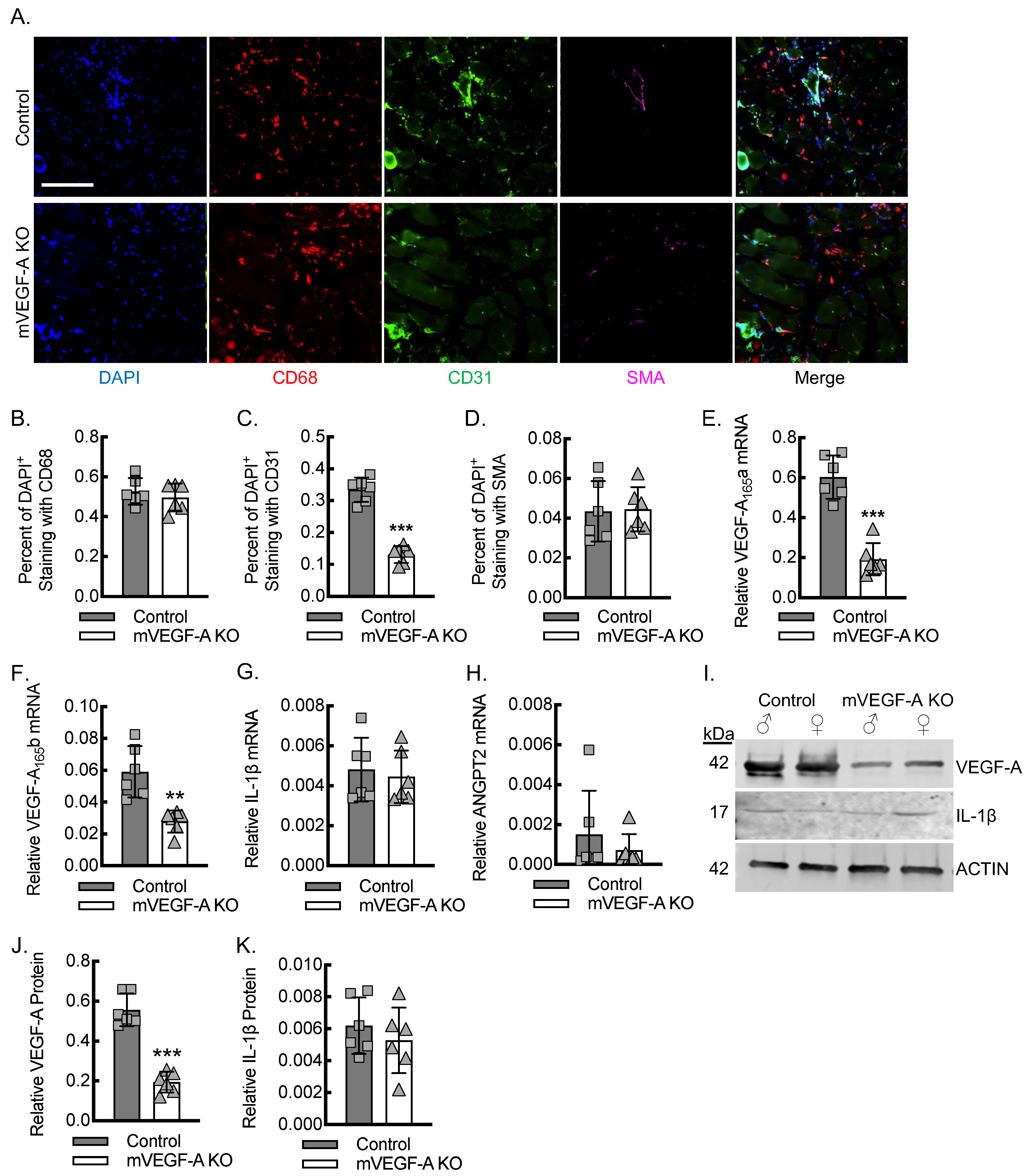
3.4. Myeloid VEGF-A Expression Is Required for Endothelial Cell Recruitment and Sufficient Tissue VEGF-A Levels During Early Inflammatory Angiogenesis
3.5. Adoptive Transfer of Macrophages Polarized Toward an Inflammatory State Is Sufficient to Rescue Blood Flow Recovery in Myeloid VEGF-A-Deleted Mice Undergoing Hind Limb Ischemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farber, A.; Eberhardt, R.T. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg. 2016, 151, 1070–1077. [Google Scholar]
- Shishehbor, M.H.; White, C.J.; Gray, B.H.; Menard, M.T.; Lookstein, R.; Rosenfield, K.; Jaff, M.R. Critical Limb Ischemia: An Expert Statement. J. Am. Coll. Cardiol. 2016, 68, 2002–2015. [Google Scholar]
- Gornik, H.L.; Aronow, H.D.; Goodney, P.P.; Arya, S.; Brewster, L.P.; Byrd, L.; Chandra, V.; Drachman, D.E.; Eaves, J.M.; Ehrman, J.K.; et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the Management of Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1313–e1410. [Google Scholar]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar]
- Hinchliffe, R.J.; Brownrigg, J.R.; Andros, G.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 136–144. [Google Scholar]
- Moulik, P.K.; Mtonga, R.; Gill, G.V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003, 26, 491–494. [Google Scholar]
- Iyer, S.R.; Annex, B.H. Therapeutic Angiogenesis for Peripheral Artery Disease: Lessons Learned in Translational Science. JACC Basic Transl. Sci. 2017, 2, 503–512. [Google Scholar]
- Brevetti, G.; Giugliano, G.; Brevetti, L.; Hiatt, W.R. Inflammation in peripheral artery disease. Circulation 2010, 122, 1862–1875. [Google Scholar]
- Hamburg, N.M.; Creager, M.A. Pathophysiology of Intermittent Claudication in Peripheral Artery Disease. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 281–289. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Morrison, A.R.; Yarovinsky, T.O.; Young, B.D.; Moraes, F.; Ross, T.D.; Ceneri, N.; Zhang, J.; Zhuang, Z.W.; Sinusas, A.J.; Pardi, R.; et al. Chemokine-coupled beta2 integrin-induced macrophage Rac2-Myosin IIA interaction regulates VEGF-A mRNA stability and arteriogenesis. J. Exp. Med. 2014, 211, 1957–1968. [Google Scholar] [CrossRef]
- Mantsounga, C.S.; Lee, C.; Neverson, J.; Sharma, S.; Healy, A.; Berus, J.M.; Parry, C.; Ceneri, N.M.; Lopez-Giraldez, F.; Chun, H.J.; et al. Macrophage IL-1beta promotes arteriogenesis by autocrine STAT3- and NF-kappaB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022, 38, 110309. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef]
- Kaikita, K.; Hayasaki, T.; Okuma, T.; Kuziel, W.A.; Ogawa, H.; Takeya, M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 2004, 165, 439–447. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Al Sadoun, H. Macrophage Phenotypes in Normal and Diabetic Wound Healing and Therapeutic Interventions. Cells 2022, 11, 2430. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wei, C.Y.; Wang, Q.; Wang, L.; Lu, L.; Qi, F.Z. M2-polarized macrophages mediate wound healing by regulating connective tissue growth factor via AKT, ERK1/2, and STAT3 signaling pathways. Mol. Biol. Rep. 2021, 48, 6443–6456. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoshida, S.; Nakao, S.; Yoshimura, T.; Kobayashi, Y.; Nakama, T.; Kubo, Y.; Miyawaki, K.; Yamaguchi, M.; Ishikawa, K.; et al. M2 Macrophages Enhance Pathological Neovascularization in the Mouse Model of Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4767–4777. [Google Scholar] [CrossRef]
- Woolard, J.; Wang, W.Y.; Bevan, H.S.; Qiu, Y.; Morbidelli, L.; Pritchard-Jones, R.O.; Cui, T.G.; Sugiono, M.; Waine, E.; Perrin, R.; et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: Mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004, 64, 7822–7835. [Google Scholar] [CrossRef]
- Peiris-Pages, M. The role of VEGF 165b in pathophysiology. Cell Adhes. Migr. 2012, 6, 561–568. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Farber, C.R.; Annex, B.H. Antiangiogenic VEGF165b Regulates Macrophage Polarization via S100A8/S100A9 in Peripheral Artery Disease. Circulation 2019, 139, 226–242. [Google Scholar] [CrossRef]
- Kikuchi, R.; Nakamura, K.; MacLauchlan, S.; Ngo, D.T.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef]
- Ngo, D.T.; Farb, M.G.; Kikuchi, R.; Karki, S.; Tiwari, S.; Bigornia, S.J.; Bates, D.O.; LaValley, M.P.; Hamburg, N.M.; Vita, J.A.; et al. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 2014, 130, 1072–1080. [Google Scholar]
- Amano, K.; Okigaki, M.; Adachi, Y.; Fujiyama, S.; Mori, Y.; Kosaki, A.; Iwasaka, T.; Matsubara, H. Mechanism for IL-1 beta-mediated neovascularization unmasked by IL-1 beta knock-out mice. J. Mol. Cell. Cardiol. 2004, 36, 469–480. [Google Scholar] [CrossRef]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 1993, 15, 532–534, 536–537. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Davies, J.Q.; Gordon, S. Isolation and culture of murine macrophages. Methods Mol. Biol. 2005, 290, 91–103. [Google Scholar]
- Jetten, N.; Donners, M.M.; Wagenaar, A.; Cleutjens, J.P.; van Rooijen, N.; de Winther, M.P.; Post, M.J. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PloS ONE 2013, 8, e68811. [Google Scholar]
- Moreno, S.G. Depleting Macrophages In Vivo with Clodronate-Liposomes. Methods Mol. Biol. 2018, 1784, 259–262. [Google Scholar]
- Pilny, E.; Smolarczyk, R.; Jarosz-Biej, M.; Hadyk, A.; Skorupa, A.; Ciszek, M.; Krakowczyk, L.; Kulach, N.; Gillner, D.; Sokol, M.; et al. Human ADSC xenograft through IL-6 secretion activates M2 macrophages responsible for the repair of damaged muscle tissue. Stem Cell Res. Ther. 2019, 10, 93. [Google Scholar]
- Giordano, F.J.; Gerber, H.P.; Williams, S.P.; VanBruggen, N.; Bunting, S.; Ruiz-Lozano, P.; Gu, Y.; Nath, A.K.; Huang, Y.; Hickey, R.; et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc. Natl. Acad. Sci. USA 2001, 98, 5780–5785. [Google Scholar] [CrossRef]
- McCubbrey, A.L.; Allison, K.C.; Lee-Sherick, A.B.; Jakubzick, C.V.; Janssen, W.J. Promoter Specificity and Efficacy in Conditional and Inducible Transgenic Targeting of Lung Macrophages. Front. Immunol. 2017, 8, 1618. [Google Scholar]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar]
- Huang, M.; Nguyen, P.; Jia, F.; Hu, S.; Gong, Y.; de Almeida, P.E.; Wang, L.; Nag, D.; Kay, M.A.; Giaccia, A.J.; et al. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation 2011, 124 (Suppl. S11), S46–S54. [Google Scholar]
- Wragg, J.W.; Durant, S.; McGettrick, H.M.; Sample, K.M.; Egginton, S.; Bicknell, R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 2014, 21, 290–300. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar]
- Foubert, P.; Silvestre, J.S.; Souttou, B.; Barateau, V.; Martin, C.; Ebrahimian, T.G.; Lere-Dean, C.; Contreres, J.O.; Sulpice, E.; Levy, B.I.; et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J. Clin. Investig. 2007, 117, 1527–1537. [Google Scholar] [PubMed]
- Silvestre, J.S.; Mallat, Z.; Tamarat, R.; Duriez, M.; Tedgui, A.; Levy, B.I. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: Role in ischemia-induced angiogenesis. Circ. Res. 2001, 89, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tritsaris, K.; Myren, M.; Ditlev, S.B.; Hubschmann, M.V.; van der Blom, I.; Hansen, A.J.; Olsen, U.B.; Cao, R.; Zhang, J.; Jia, T.; et al. IL-20 is an arteriogenic cytokine that remodels collateral networks and improves functions of ischemic hind limbs. Proc. Natl. Acad. Sci. USA 2007, 104, 15364–15369. [Google Scholar]
- Barbay, V.; Houssari, M.; Mekki, M.; Banquet, S.; Edwards-Levy, F.; Henry, J.P.; Dumesnil, A.; Adriouch, S.; Thuillez, C.; Richard, V.; et al. Role of M2-like macrophage recruitment during angiogenic growth factor therapy. Angiogenesis 2015, 18, 191–200. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.J.; Chen, H.Y.; Chen, H.W.; Ho, C.C.; Chen, Y.Y.; Liu, Y.C.; Hong, T.H.; Yu, S.L.; Chen, J.J.; et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 2020, 115, 22. [Google Scholar]
- Yang, H.; Lan, W.; Liu, W.; Chen, T.; Tang, Y. Dapagliflozin promotes angiogenesis in hindlimb ischemia mice by inducing M2 macrophage polarization. Front. Pharmacol. 2023, 14, 1255904. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar]
- Andreeva, E.R.; Pugach, I.M.; Orekhov, A.N. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 1997, 135, 19–27. [Google Scholar] [CrossRef]
- Culemann, S.; Knab, K.; Euler, M.; Wegner, A.; Garibagaoglu, H.; Ackermann, J.; Fischer, K.; Kienhofer, D.; Crainiciuc, G.; Hahn, J.; et al. Stunning of neutrophils accounts for the anti-inflammatory effects of clodronate liposomes. J. Exp. Med. 2023, 220, e20220525. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Uddin, F.J.; Burke, D. Angiopoietins in malignancy. Eur. J. Surg. Oncol. 2007, 33, 7–15. [Google Scholar] [PubMed]
- Fiedler, U.; Scharpfenecker, M.; Koidl, S.; Hegen, A.; Grunow, V.; Schmidt, J.M.; Kriz, W.; Thurston, G.; Augustin, H.G. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004, 103, 4150–4156. [Google Scholar]
- Tan, X.; Yan, K.; Ren, M.; Chen, N.; Li, Y.; Deng, X.; Wang, L.; Li, R.; Luo, M.; Liu, Y.; et al. Angiopoietin-2 impairs collateral artery growth associated with the suppression of the infiltration of macrophages in mouse hindlimb ischaemia. J. Transl. Med. 2016, 14, 306. [Google Scholar] [CrossRef]
- Adeyemo, A.; Johnson, C.; Stiene, A.; LaSance, K.; Qi, Z.; Lemen, L.; Schultz, J.E.J. Limb functional recovery is impaired in fibroblast growth factor-2 (FGF2) deficient mice despite chronic ischaemia-induced vascular growth. Growth Factors 2020, 38, 75–93. [Google Scholar] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar]
- Varey, A.H.; Rennel, E.S.; Qiu, Y.; Bevan, H.S.; Perrin, R.M.; Raffy, S.; Dixon, A.R.; Paraskeva, C.; Zaccheo, O.; Hassan, A.B.; et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: Balance of pro-and antiangiogenic VEGF-A isoforms has implications for therapy. Br. J. Cancer 2008, 98, 1366–1379. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Jesmin, S.; Mowa, C.N.; Sultana, S.N.; Mia, S.; Islam, R.; Zaedi, S.; Sakuma, I.; Hattori, Y.; Hiroe, M.; Yamaguchi, N. Estrogen receptor alpha and beta are both involved in the cerebral VEGF/Akt/NO pathway and cerebral angiogenesis in female mice. Biomed. Res. 2010, 31, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Elkin, M.; Orgel, A.; Kleinman, H.K. An angiogenic switch in breast cancer involves estrogen and soluble vascular endothelial growth factor receptor 1. J. Natl. Cancer Inst. 2004, 96, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C.; Morfoisse, F.; Tatin, F.; Zamora, A.; Zahreddine, R.; Henrion, D.; Arnal, J.F.; Lenfant, F.; Garmy-Susini, B. The Impact of Estrogen Receptor in Arterial and Lymphatic Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3244. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Pierce, J.; Neverson, J.C.; Khan, R.; Lee, C.F.; Uppuluri, S.; Parry, C.; Amelotte, E.; Butler, C.A.; Sellke, F.W.; et al. Macrophage Proangiogenic VEGF-A Is Required for Inflammatory Arteriogenesis During Vascular Injury. Biomedicines 2025, 13, 828. https://doi.org/10.3390/biomedicines13040828
Sharma S, Pierce J, Neverson JC, Khan R, Lee CF, Uppuluri S, Parry C, Amelotte E, Butler CA, Sellke FW, et al. Macrophage Proangiogenic VEGF-A Is Required for Inflammatory Arteriogenesis During Vascular Injury. Biomedicines. 2025; 13(4):828. https://doi.org/10.3390/biomedicines13040828
Chicago/Turabian StyleSharma, Sheila, Julia Pierce, Jade C. Neverson, Rachel Khan, Cadence F. Lee, Saketh Uppuluri, Crystal Parry, Elizabeth Amelotte, Celia A. Butler, Frank W. Sellke, and et al. 2025. "Macrophage Proangiogenic VEGF-A Is Required for Inflammatory Arteriogenesis During Vascular Injury" Biomedicines 13, no. 4: 828. https://doi.org/10.3390/biomedicines13040828
APA StyleSharma, S., Pierce, J., Neverson, J. C., Khan, R., Lee, C. F., Uppuluri, S., Parry, C., Amelotte, E., Butler, C. A., Sellke, F. W., Harrington, E. O., Choudhary, G., Morrison, A. R., & Mantsounga, C. S. (2025). Macrophage Proangiogenic VEGF-A Is Required for Inflammatory Arteriogenesis During Vascular Injury. Biomedicines, 13(4), 828. https://doi.org/10.3390/biomedicines13040828




