A Combined Approach Using Strip Grafts and Xenogenic Dermal Matrix for Peri-Implant Keratinized Mucosa Augmentation in the Mandible: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- Age ≥ 18 years;
- Patients with at least one dental implant in the posterior mandible that had been in function for more than three months;
- PIKM-W of less than 2 mm, measured using a UNC-15 periodontal probe;
- Good compliance with follow-up protocols and willingness to participate in long-term maintenance programs;
- Good oral hygiene, with a full-mouth plaque score (FMPS) of less than 20%;
- The absence of uncontrolled or untreated periodontal disease, with a full-mouth bleeding score (FMBS) greater than 20%;
- Active infectious diseases (HBV, HCV, HIV, TB, SARS-CoV-2, etc.);
- Ongoing chemotherapy or radiation therapy;
- Radiation therapy involving the cranial region within the past 2 years;
- Uncontrolled diabetes;
- Clinically significant osteoporosis or other systemic disease involving bone metabolism;
- Clinically significant circulatory disorders including decompensated cardiac failure;
- Haemodynamically significant valvular heart failure or myocardial infarction within the last 3 months;
- Clinically significant coagulopathy;
- Ongoing or previous systemic corticosteroid therapy within the past 2 months;
- Ongoing or previous systemic bisphosphonate therapy;
- Alcohol or drug abuse;
- Smoking;
- Pregnant or lactating women.
2.3. Presurgical Treatment
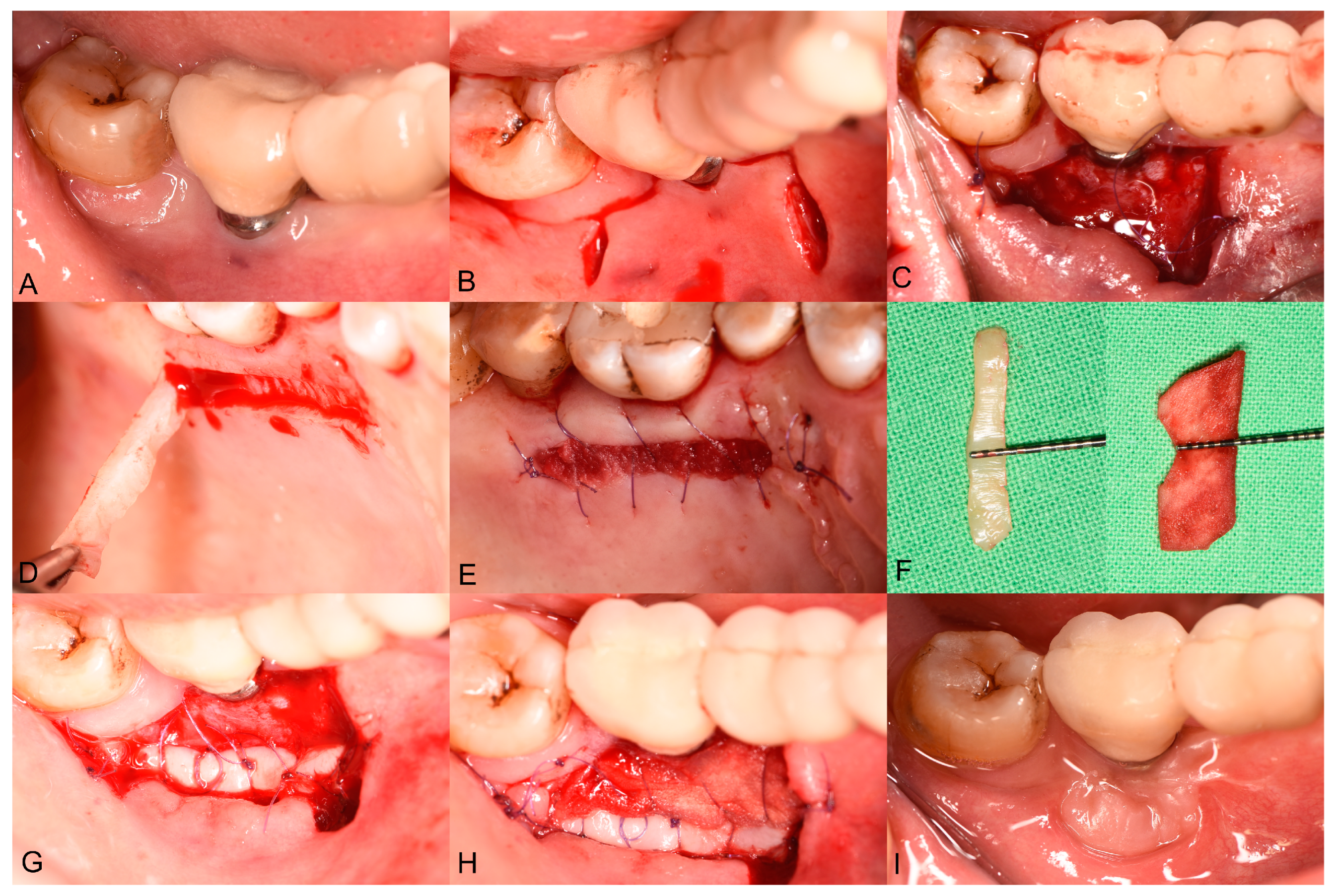
2.4. Surgical Treatment
2.5. Postsurgical Instructions and Infection Control
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Primary Outcome: Changes in PIKM-W
3.3. Secondary Outcome: PIKM-T, Graft Remodeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adibrad, M.; Shahabuei, M.; Sahabi, M. Significance of the Width of Keratinized Mucosa on the Health Status of the Supporting Tissue around Implants Supporting Overdentures. J. Oral Implantol. 2009, 35, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Grasso, G.; Dalmasso, P. Keratinized Mucosa around Implants in Partially Edentulous Posterior Mandible: 10-Year Results of a Prospective Comparative Study. Clin. Oral Implants Res. 2016, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Löe, H. The Relationship between the Width of Keratinized Gingiva and Gingival Health. J. Periodontol. 1972, 43, 623–627. [Google Scholar]
- Kabir, L.; Stiesch, M.; Grischke, J. The Effect of Keratinized Mucosa on the Severity of Peri-Implant Mucositis Differs between Periodontally Healthy Subjects and the General Population: A Cross-Sectional Study. Clin. Oral Investig. 2021, 25, 1183–1193. [Google Scholar] [PubMed]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of Soft Tissue Augmentation Procedures on Peri-Implant Health or Disease: A Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. 15), 32–49. [Google Scholar]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A Systematic Review of the 5-Year Survival and Complication Rates of Implant-Supported Single Crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar]
- Wilson, V. An Insight into Peri-Implantitis: A Systematic Literature Review. Prim. Dent. J. 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S246–S266. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar]
- Bassetti, R.G.; Stähli, A.; Bassetti, M.A.; Sculean, A. Soft Tissue Augmentation around Osseointegrated and Uncovered Dental Implants: A Systematic Review. Clin. Oral Investig. 2017, 21, 53–70. [Google Scholar] [CrossRef]
- Bassetti, M.; Kaufmann, R.; Salvi, G.E.; Sculean, A.; Bassetti, R. Soft Tissue Grafting to Improve the Attached Mucosa at Dental Implants: A Review of the Literature and Proposal of a Decision Tree. Quintessence Int. 1985 2015, 46, 499–510. [Google Scholar]
- Wu, Q.; Qu, Y.; Gong, P.; Wang, T.; Gong, T.; Man, Y. Evaluation of the Efficacy of Keratinized Mucosa Augmentation Techniques around Dental Implants: A Systematic Review. J. Prosthet. Dent. 2015, 113, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Agudio, G.; Nieri, M.; Rotundo, R.; Cortellini, P.; Prato, G. Free Gingival Grafts to Increase Keratinized Tissue: A Retrospective Long-Term Evaluation (10 to 25 Years) of Outcomes. J. Periodontol. 2008, 79, 587–594. [Google Scholar] [CrossRef]
- Hutton, C.G.; Johnson, G.K.; Barwacz, C.A.; Allareddy, V.; Avila-Ortiz, G. Comparison of Two Different Surgical Approaches to Increase Peri-Implant Mucosal Thickness: A Randomized Controlled Clinical Trial. J. Periodontol. 2018, 89, 807–814. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H.-L. Peri-Implant Soft Tissue Phenotype Modification and Its Impact on Peri-Implant Health: A Systematic Review and Network Meta-Analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Basma, H.S.; Saleh, M.H.A.; Abou-Arraj, R.V.; Imbrogno, M.; Ravida, A.; Wang, H.-L.; Li, P.; Geurs, N. Patient-Reported Outcomes of Palatal Donor Site Healing Using Four Different Wound Dressing Modalities Following Free Epithelialized Mucosal Grafts: A Four-Arm Randomized Controlled Clinical Trial. J. Periodontol. 2023, 94, 88–97. [Google Scholar] [CrossRef]
- Wessel, J.R.; Tatakis, D.N. Patient Outcomes Following Subepithelial Connective Tissue Graft and Free Gingival Graft Procedures. J. Periodontol. 2008, 79, 425–430. [Google Scholar] [CrossRef]
- Cairo, F.; Barbato, L.; Tonelli, P.; Batalocco, G.; Pagavino, G.; Nieri, M. Xenogeneic Collagen Matrix versus Connective Tissue Graft for Buccal Soft Tissue Augmentation at Implant Site. A Randomized, Controlled Clinical Trial. J. Clin. Periodontol. 2017, 44, 769–776. [Google Scholar] [CrossRef]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical Evaluation of a New Collagen Matrix (Mucograft Prototype) to Enhance the Width of Keratinized Tissue in Patients with Fixed Prosthetic Restorations: A Randomized Prospective Clinical Trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef]
- Rokn, A.; Zare, H.; Haddadi, P. Use of Mucograft Collagen Matrix® versus Free Gingival Graft to Augment Keratinized Tissue around Teeth: A Randomized Controlled Clinical Trial. Front. Dent. 2020, 17, 5. [Google Scholar] [CrossRef]
- Lorenzo, R.; García, V.; Orsini, M.; Martin, C.; Sanz, M. Clinical Efficacy of a Xenogeneic Collagen Matrix in Augmenting Keratinized Mucosa around Implants: A Randomized Controlled Prospective Clinical Trial. Clin. Oral Implants Res. 2012, 23, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Puisys, A.; Zukauskas, S.; Kubilius, R.; Barbeck, M.; Razukevičius, D.; Linkevičiene, L.; Linkevičius, T. Clinical and Histologic Evaluations of Porcine-Derived Collagen Matrix Membrane Used for Vertical Soft Tissue Augmentation: A Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 341–347. [Google Scholar]
- Papi, P.; Pompa, G. The Use of a Novel Porcine Derived Acellular Dermal Matrix (Mucoderm) in Peri-Implant Soft Tissue Augmentation: Preliminary Results of a Prospective Pilot Cohort Study. BioMed Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Horváth, A.; Windisch, P.; Palkovics, D.; Li, X. Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series. Dent. J. 2024, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Zafiropoulos, G.-G.; Al-Asfour, A.A.; Abuzayeda, M.; Kačarević, Z.P.; Murray, C.A.; Trajkovski, B. Peri-Implant Mucosa Augmentation with an Acellular Collagen Matrix. Membranes 2021, 11, 698. [Google Scholar] [CrossRef]
- Han, T.J.; Klokkevold, P.R.; Takei, H.H. Strip Gingival Autograft Used to Correct Mucogingival Problems around Implants. Int. J. Periodontics Restor. Dent. 1995, 15, 404–411. [Google Scholar]
- Urban, I.A.; Nagy, K.; Werner, S.; Meyer, M. Evaluation of the Combination of Strip Gingival Grafts and a Xenogeneic Collagen Matrix for the Treatment of Severe Mucogingival Defects: A Human Histologic Study. Int. J. Periodontics Restor. Dent. 2019, 39, 9–14. [Google Scholar]
- Urban, I.A.; Lozada, J.L.; Nagy, K.; Sanz, M. Treatment of Severe Mucogingival Defects with a Combination of Strip Gingival Grafts and a Xenogeneic Collagen Matrix: A Prospective Case Series Study. Int. J. Periodontics Restor. Dent. 2015, 35, 345–353. [Google Scholar] [CrossRef]
- Urban, I.A.; Tavelli, L.; Barootchi, S.; Wang, H.-L.; Barath, Z. Labial Strip Gingival Graft for the Reconstruction of Severely Distorted Mucogingival Defects: A Prospective Case Series. Int. J. Periodontics Restor. Dent. 2020, 40, 845–852. [Google Scholar]
- Emanuel, E.J. Reconsidering the Declaration of Helsinki. Lancet 2013, 381, 1532–1533. [Google Scholar] [CrossRef]
- Ravidà, A.; Arena, C.; Tattan, M.; Caponio, V.C.A.; Saleh, M.H.A.; Wang, H.; Troiano, G. The Role of Keratinized Mucosa Width as a Risk Factor for Peri-implant Disease: A Systematic Review, Meta-analysis, and Trial Sequential Analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Schwarz, F.; Sader, R. Influence of Width of Keratinized Tissue on the Prevalence of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2022, 33 (Suppl. 23), 8–31. [Google Scholar] [PubMed]
- Gharpure, A.S.; Latimer, J.M.; Aljofi, F.E.; Kahng, J.H.; Daubert, D.M. Role of Thin Gingival Phenotype and Inadequate Keratinized Mucosa Width (<2 Mm) as Risk Indicators for Peri-Implantitis and Peri-Implant Mucositis. J. Periodontol. 2021, 92, 1687–1696. [Google Scholar] [PubMed]
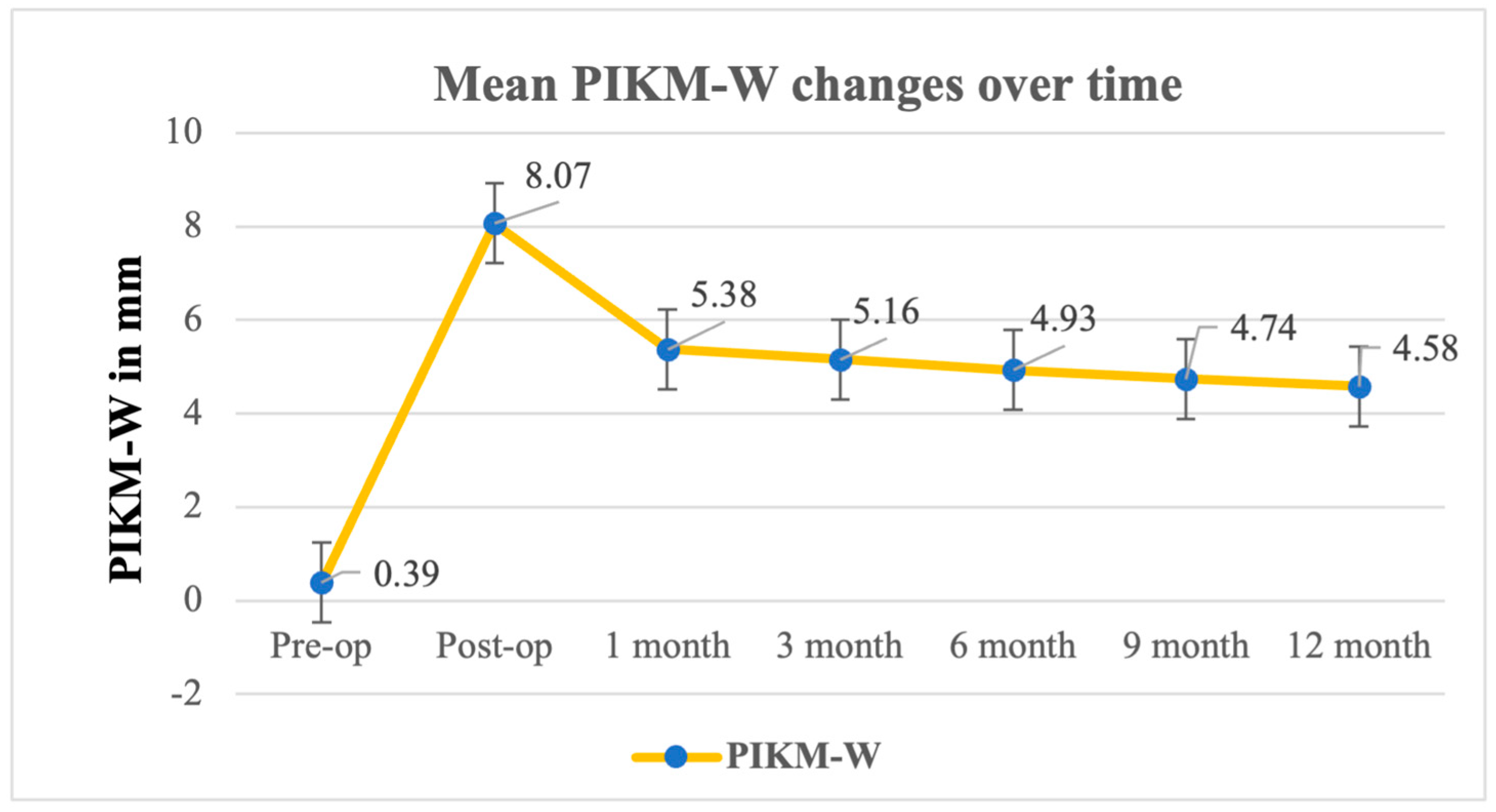
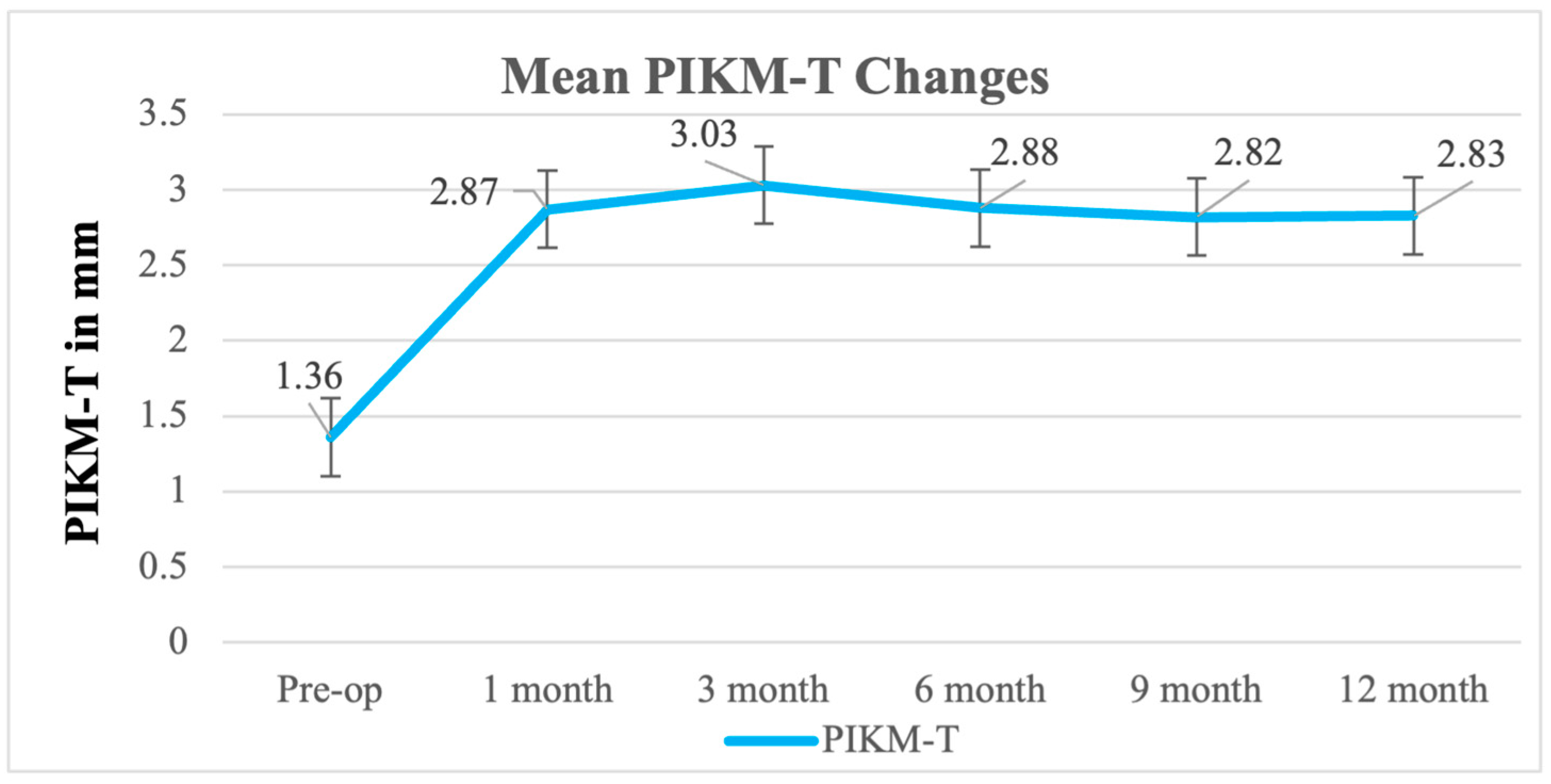
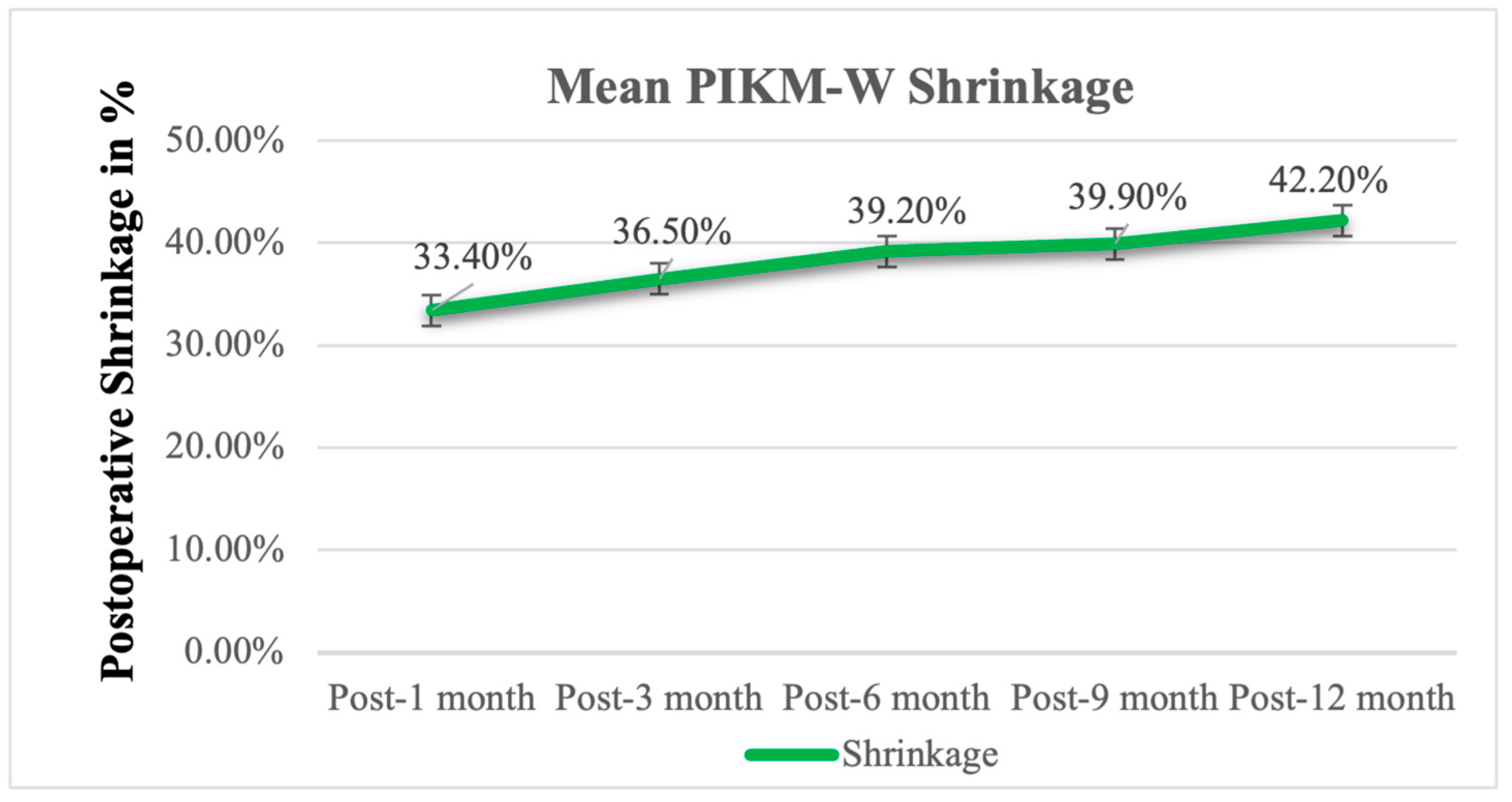
| Baseline | Postop | 1 Month | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|---|---|
| Mean | 0.39 | 8.07 | 5.38 | 5.16 | 4.93 | 4.74 | 4.58 |
| St. Dev. 1 | 0.40 | 1.43 | 0.93 | 0.95 | 0.98 | 1.11 | 1.28 |
| Min. | 0.00 | 5.00 | 4.00 | 3.70 | 3.50 | 3.00 | 6.70 |
| Max. | 1.00 | 10.00 | 7.00 | 6.70 | 6.70 | 3.00 | 7.00 |
| Median | 0.40 | 8.15 | 5.00 | 5.00 | 4.85 | 5.00 | 4.50 |
| p-value 2 | F (6, 66) = 96.86; p < 0.0001; partial η2 = 0.90 (95% CI: [0.76, 0.94] | ||||||
| Primary Outcome: Peri-Implant Keratinized Mucosa Width (PIKM-W) | |||
|---|---|---|---|
| Difference (Δ) | Patients (n = 12) | ||
| Δ | SD | p-Value | |
| Preop-1m | 4.98 | 1.05 | <0.0001 |
| Preop-3m | 4.77 | 1.03 | <0.0001 |
| Preop-6m | 4.53 | 1.11 | <0.0001 |
| Preop-9m | 4.35 | 1.33 | <0.0001 |
| Preop-12m | 4.19 | 1.45 | <0.0001 |
| Baseline | 1 Month | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|---|
| Mean | 1.36 | 2.87 | 3.03 | 2.88 | 2.82 | 2.83 |
| St. Dev. 1 | 0.43 | 0.82 | 0.74 | 0.80 | 0.59 | 0.65 |
| Min. | 1.00 | 2.00 | 2.00 | 1.50 | 2.00 | 2.00 |
| Max. | 2.00 | 5.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Median | 1.15 | 2.85 | 3.00 | 3.00 | 3.00 | 2.75 |
| p-value 2 | F (5, 54) = 12.83, p < 0.0001; partial η2 = 0.44 (95% CI: [0.34, 0.54] | |||||
| PIKM-W | Postop-1M | Postop-3M | Postop-6M | Postop-9M | Postop-12M |
|---|---|---|---|---|---|
| Mean | 33.4% | 36.5% | 39.2% | 39.9% | 42.2% |
| SD | 13.3% | 14.3% | 14.1% | 16.0% | 16.8% |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Palkovics, D.; Windisch, P.; Perić Kačarević, Ž.; Horváth, A. A Combined Approach Using Strip Grafts and Xenogenic Dermal Matrix for Peri-Implant Keratinized Mucosa Augmentation in the Mandible: A Case Series. Biomedicines 2025, 13, 806. https://doi.org/10.3390/biomedicines13040806
Li X, Palkovics D, Windisch P, Perić Kačarević Ž, Horváth A. A Combined Approach Using Strip Grafts and Xenogenic Dermal Matrix for Peri-Implant Keratinized Mucosa Augmentation in the Mandible: A Case Series. Biomedicines. 2025; 13(4):806. https://doi.org/10.3390/biomedicines13040806
Chicago/Turabian StyleLi, Xinda, Dániel Palkovics, Péter Windisch, Željka Perić Kačarević, and Attila Horváth. 2025. "A Combined Approach Using Strip Grafts and Xenogenic Dermal Matrix for Peri-Implant Keratinized Mucosa Augmentation in the Mandible: A Case Series" Biomedicines 13, no. 4: 806. https://doi.org/10.3390/biomedicines13040806
APA StyleLi, X., Palkovics, D., Windisch, P., Perić Kačarević, Ž., & Horváth, A. (2025). A Combined Approach Using Strip Grafts and Xenogenic Dermal Matrix for Peri-Implant Keratinized Mucosa Augmentation in the Mandible: A Case Series. Biomedicines, 13(4), 806. https://doi.org/10.3390/biomedicines13040806






