Abstract
Background/Objectives: Duchenne muscular dystrophy (DMD) is an X-linked inherited muscle disease. Patients with DMD demonstrate improved prognosis with angiotensin-converting enzyme inhibitors and beta-blockers at the time of cardiac dysfunction. However, most deaths due to DMD are due to cardiac dysfunction. Fragmented QRS (fQRS) is an abnormal finding that forms a notch in the QRS wave on electrocardiography (ECG) and is associated with fibrosis and scarring of the myocardium. Methods: Patients with DMD were examined for the number of leads of fQRS, their sites of appearance, changes in cardiac dysfunction, and age using the chest leads of a synthesized 18-ECG. A retrospective analysis of 184 patients under 20 years of age with DMD and known genetic mutations was performed; they were divided into three age groups: 3–10, 11–15, and 16–20 years. The chest leads of the ECG were defined as follows: V1-3, anterior leads; V4-6, lateral leads; V7-9, posterior leads; and V3R-V5R, right-sided chest leads. Cardiac dysfunction was defined as a left ventricular (LV) ejection fraction <53% on the same day, and echocardiography was performed. LV dilation was defined as dilation beyond the normal range, considering the body surface area. Results: In 167 of 184 patients (91%), fQRS was present in one or more chest leads. The number of fQRS leads in the anterior and lateral walls was significantly higher in 16–20-year-olds than in 3–10-year-olds. The total number of chest leads with fQRS was 4.9 ± 3.1 in the cardiac dysfunction group and 3.5 ± 2.5 in the preserved group. The cardiac dysfunction group had a significantly greater number of fQRS leads than did the preserved group (p = 0.003). The group with LV dilation had a significantly greater number of fQRS leads than did the non-dilation group (p = 0.009). Conclusions: The fQRS site is associated with age, cardiac dysfunction, and LV dilation. Multivariate regression analysis revealed that the number of anterior leads of the fQRS correlated with age and that of lateral leads of the fQRS with cardiac dysfunction and LV dilation. The number of fQRS leads on the lateral wall marks cardiac dysfunction and LV dilation.
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked inherited muscle disease that occurs in 1 in 5000 live birth boys [1]. DMD presents with a wide variety of complications, among which, respiratory and cardiac dysfunctions are the most common. Respiratory management with non-invasive positive pressure ventilation has improved the life expectancy of patients with DMD by an average of approximately 30 years [2,3,4]. Cardiac management of DMD is recommended using angiotensin-converting enzyme inhibitors and beta-blockers during cardiac dysfunction [5]. Consequently, the life expectancy of patients with DMD has improved. Nonetheless, cardiac dysfunction remains the leading cause of death in DMD [6].
Myocardial fibrosis precedes cardiac dysfunction in patients with DMD [7,8,9,10,11]. The frequency of fibrosis in the posterior and lateral walls is high [12], suggesting that LV fibrosis is the starting point for cardiac dysfunction. Myocardial fibrosis was assessed using cardiac magnetic resonance imaging (MRI). However, cardiac MRI presents many limitations, such as a long examination time and high invasiveness due to the use of contrast media, which limits the number of facilities that can perform the procedure. Therefore, a simple index for assessing myocardial fibrosis is necessary.
The fragmented QRS (fQRS) was first reported in 1969 as a notch waveform resulting from abnormal depolarization of the myocardium [13]. Subsequently, it was shown that the fQRS is associated with fibrosis and scarring of the myocardium [14,15,16,17,18,19,20,21]. In other words, fQRS may be a useful precursor of cardiac dysfunction in DMD. However, no reports have examined in detail the timing or site of appearance, such as the posterior leads or right ventricular side, in a large number of cases.
A standard 12-lead electrocardiography (ECG) cannot record the posterior wall and right ventricular side; nonetheless, an 18-lead ECG can record the posterior wall and right ventricular side. In this study, DMD was recorded in multiple directions using the chest leads of a synthesized 18-ECG (syn18-ECG) that computationally synthesized waveforms from a standard 12-lead ECG [22], and the number and sites of fQRS leads and their changes with cardiac dysfunction and aging were also recorded.
2. Materials and Methods
2.1. Study Design and Patient Recruitment
Patients with DMD aged 20 years or younger who visited the Department of Pediatrics, Kobe University Hospital, between July 2007 and March 2021 were included in this study. Patient data were retrospectively reviewed at the final visit. The DMD mutation was determined by multiplex ligation-dependent probe amplification or sequencing using genomic DNA or cDNA extracted from peripheral blood samples, as described before [23]. Patients with DMD or chromosomal abnormalities were excluded. Furthermore, patients with DMD were divided into three age groups (3–10, 11–15, and 16–20 years) to analyze age-related changes. Serum creatine kinase (CK) was measured using an automatic analyzer, JCA-BM8040 (JEOL Ltd., Tokyo, Japan), with Cygnus Auto CK (Shino-Test Corporation, Tokyo, Japan). Plasma brain natriuretic peptide was measured using the fully automated chemiluminescent enzyme immunoassay AIA-CL2400 (Eiken chemical Co., Ltd. Tokyo, Japan).
2.2. Electrocardiography
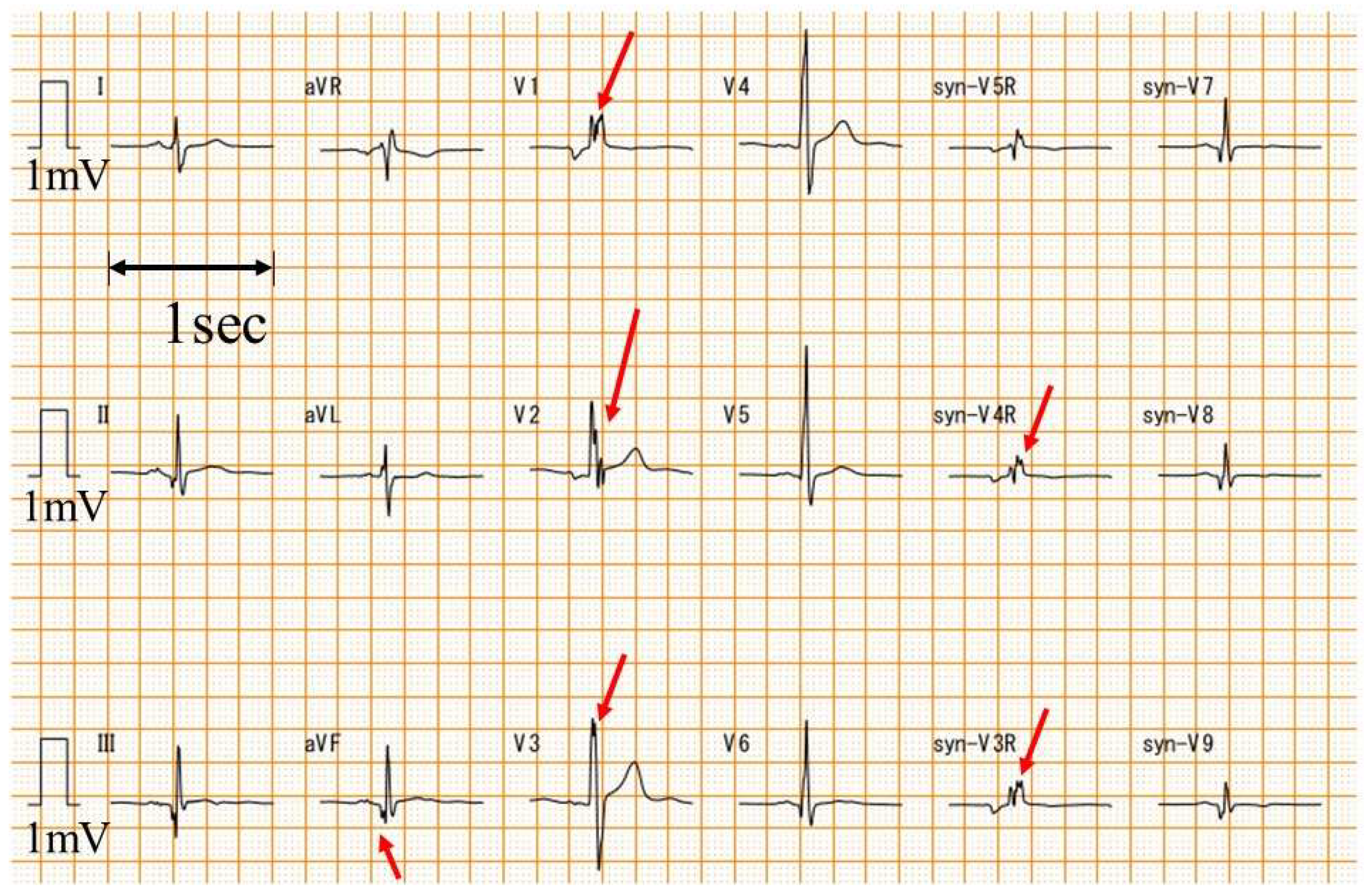
A standard 12-lead ECG was converted to syn18-ECG on an ECG-2550 (Nihon Kohden, Tokyo, Japan) using the logic of daming [22]. Heart rate, QRS axis, and QRS duration were automatically calculated from the ECG-2550 using ECAPS12C (Nihon Kohden, Tokyo, Japan). V1-V3, V4-V6, V7-V9, and V3R-V5R of the syn18-ECG were defined as the anterior, lateral, posterior, and right-sided chest leads, respectively. The fQRS was analyzed by one examiner (T.Y.) with extensive experience in ECG diagnosis in patients with DMD who did not know the value of LV ejection fraction (LVEF) before the fQRS analysis. The fQRS was defined as a notched QRS (Figure 1) [24].
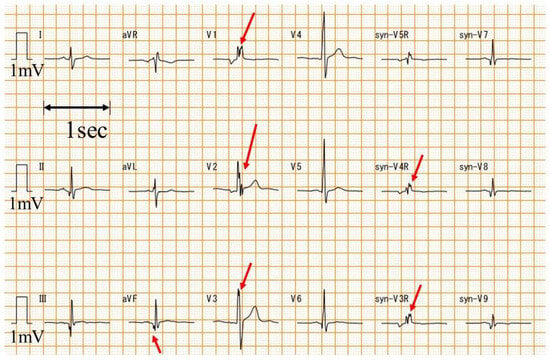
Figure 1.
Examples of fQRS. An example of fQRS is presented. The red arrow is the fQRS. syn-: synthesized ECG was recorded with a paper speed of 25 mm/s and an amplitude of 10 mm/mV.
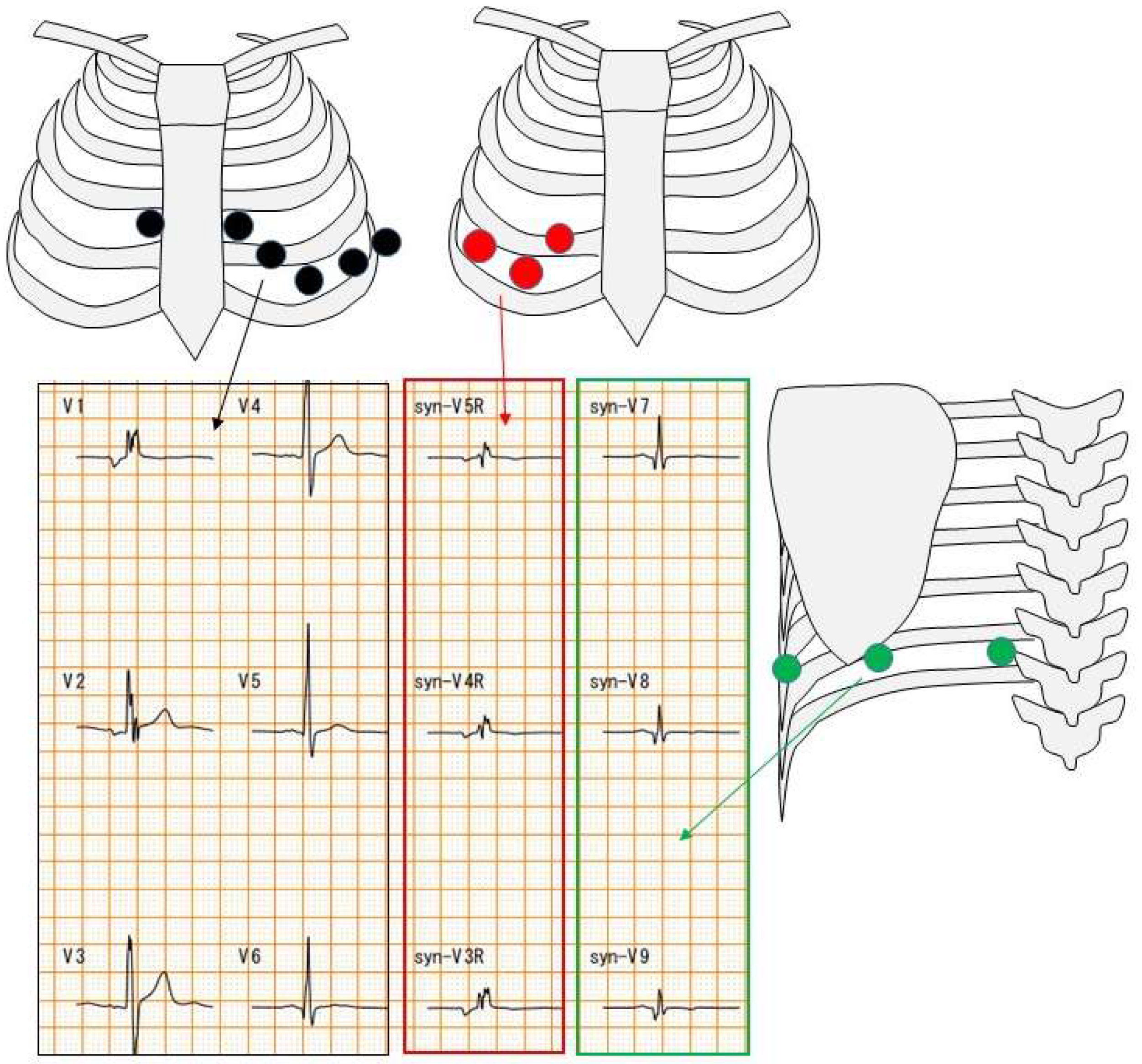
The difference between a 12-lead ECG and an 18-lead ECG is the number of chest leads. A 12-lead ECG has six chest leads. On the other hand, an 18-lead ECG has six 12-lead ECG electrodes as well as three electrodes on the right side and three electrodes on the back (Figure 2).
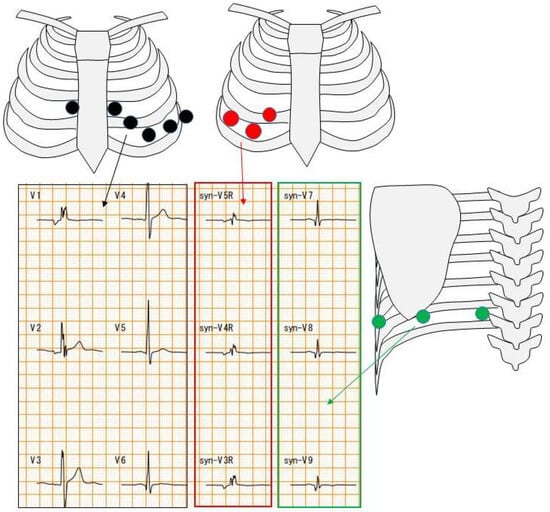
Figure 2.
Chest electrode attachment site. Black circles indicate chest leads for 12-lead ECG; red and green circles indicate chest leads for 18-lead ECG. Red square indicates right-sided chest waveforms and green square indicates posterior waveforms.
2.3. Echocardiography
Patients were recorded in the supine position for echocardiography using Aplio XG (Canon Medical, Otawara, Japan). Echocardiographic images were obtained by recording three consecutive beats using a left parasternal and apical approach according to the recommendations of the American Society of Echocardiography [25].
LVEF was measured using the apical approach with a modified Simpson method. In the case of LVEF < 53%, DMD was defined as cardiac dysfunction [25]. LV dilation was described as an LV end-diastolic dimension above the normal range, considering the body surface area [25,26].
2.4. Statistical Analysis
Values are expressed as mean ± SD. The t-test, U-test, and Fisher test were used for two-group comparisons and the Kruskal–Wallis and Friedman tests were used for three or more group comparisons, followed by a comparison between the two groups using the Bonferroni method. Multivariate logistic regression analysis was performed to analyze independent factors associated with cardiac dysfunction and age. p values less than 0.05 were defined as statistically significant differences. All analyses were performed using commercially available software R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
2.5. Ethics
This study was approved by the Ethics Committee of Kobe University (no. 1534) and Nagahama Institute of Bio-Science and Technology (no. 008).
3. Results
3.1. Patients
A total of 184 patients with DMD were included, with an age at entry of 14.0 ± 4.6 years (range, 3–20 years). Exon deletions in the DMD gene were the most common (107, 58%), followed by nonsense mutations in 38 patients (21%). Other exon duplications, small insertions/deletions, splice site mutations, and deep intron mutations were observed in 18 (10%), 16 (9%), 4 (2%), and 1 (1%) patients, respectively. LVEF at entry was 52.5 ± 12.3%.
3.2. Number of fQRSs
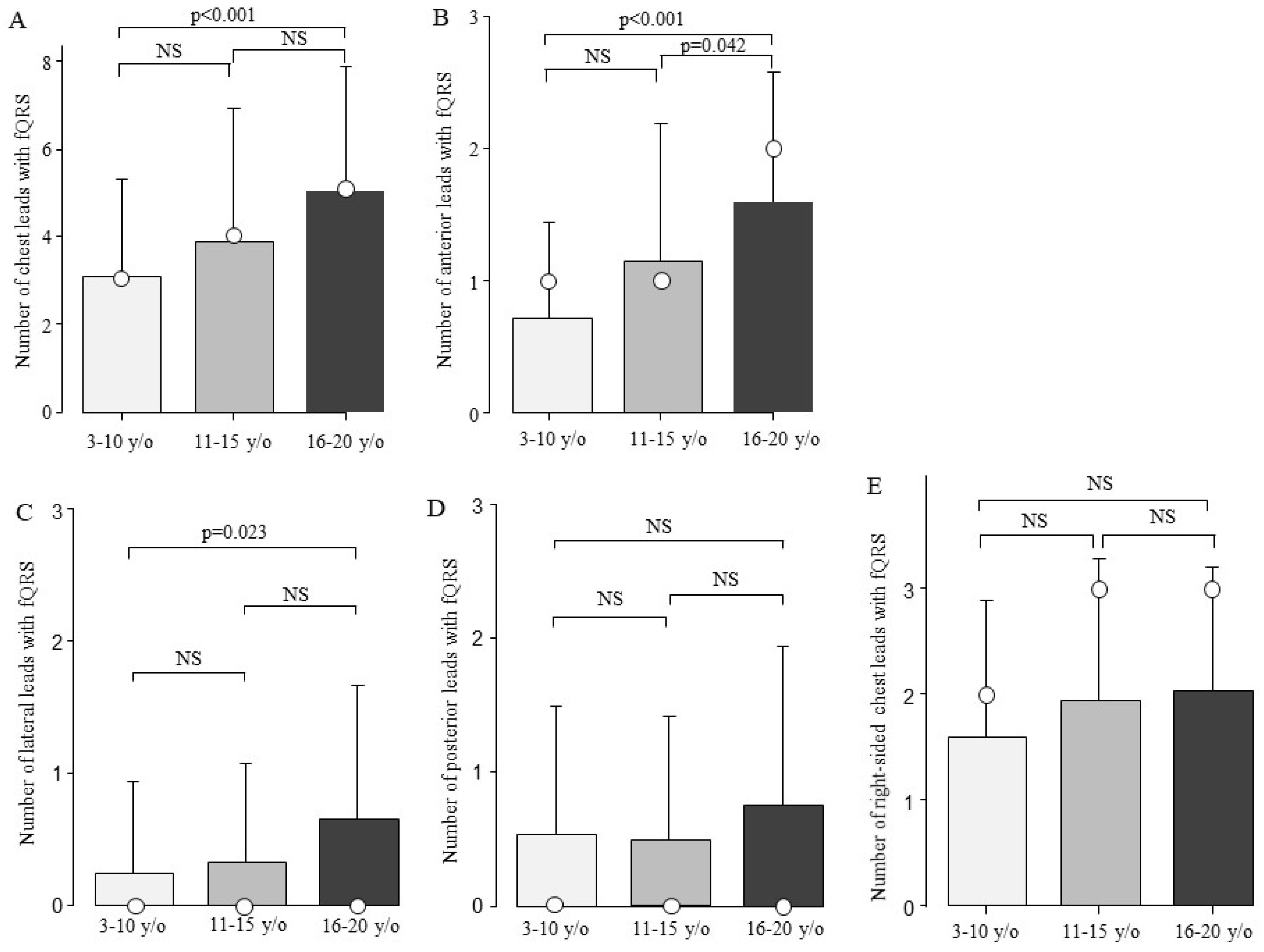
The fQRS was present in one of the chest leads in 167 of the 184 patients (91%). fQRS was most frequently observed in right-sided chest leads (n = 139, 76%). The second most common site was the anterior leads (n = 131, 71%), followed by the posterior leads (n = 55, 30%) and lateral leads (n = 47, 26%) (Supplementary Figure S1). There were no significant differences in the type of genetic mutation, CK value, HR, QRS duration, or QRS axis between patients with and without fQRS (Table 1). The patients with fQRS were significantly older than those without fQRS (p = 0.003). Next, the patients with DMD were compared in terms of the number of fQRS leads among the three age groups. The total number of fQRS on the chest leads increased with age: 3.1 ± 2.2 in the 3–10-year-old group, 3.9 ± 3.1 in the 11–15-year-old group, and 5.0 ± 2.9 in the 16–20-year-old group. The total number of fQRS on the chest leads showed a significant difference between ages 3–10 and 16–20 (Figure 3A, p < 0.001). The DMD was examined for age-related changes at the chest lead site (Figure 3B–E). Moreover, there was a significant increase in the number of fQRS leads in the anterior and lateral walls between the ages of 3–10 and 16–20 years (Figure 3B,C). In addition, the number of anterior fQRS leads also significantly increased between the ages of 11–15 and 16–20 (Figure 3B, p = 0.042).

Table 1.
Comparison of fQRS.
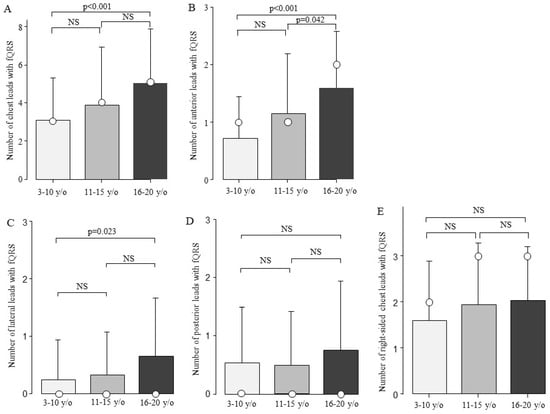
Figure 3.
Comparison of the number of fQRS leads by age. The number of fQRS leads according to age is shown. The number of fQRS increased with age at all sites. Bars are expressed as mean ± SD. The white circles indicate the medians. (A) All leads; (B) anterior leads; (C) lateral leads; (D) posterior leads; and (E) right-sided chest leads. NS: Not significant.
3.3. Relationship Between the Number of fQRS and Cardiac Function
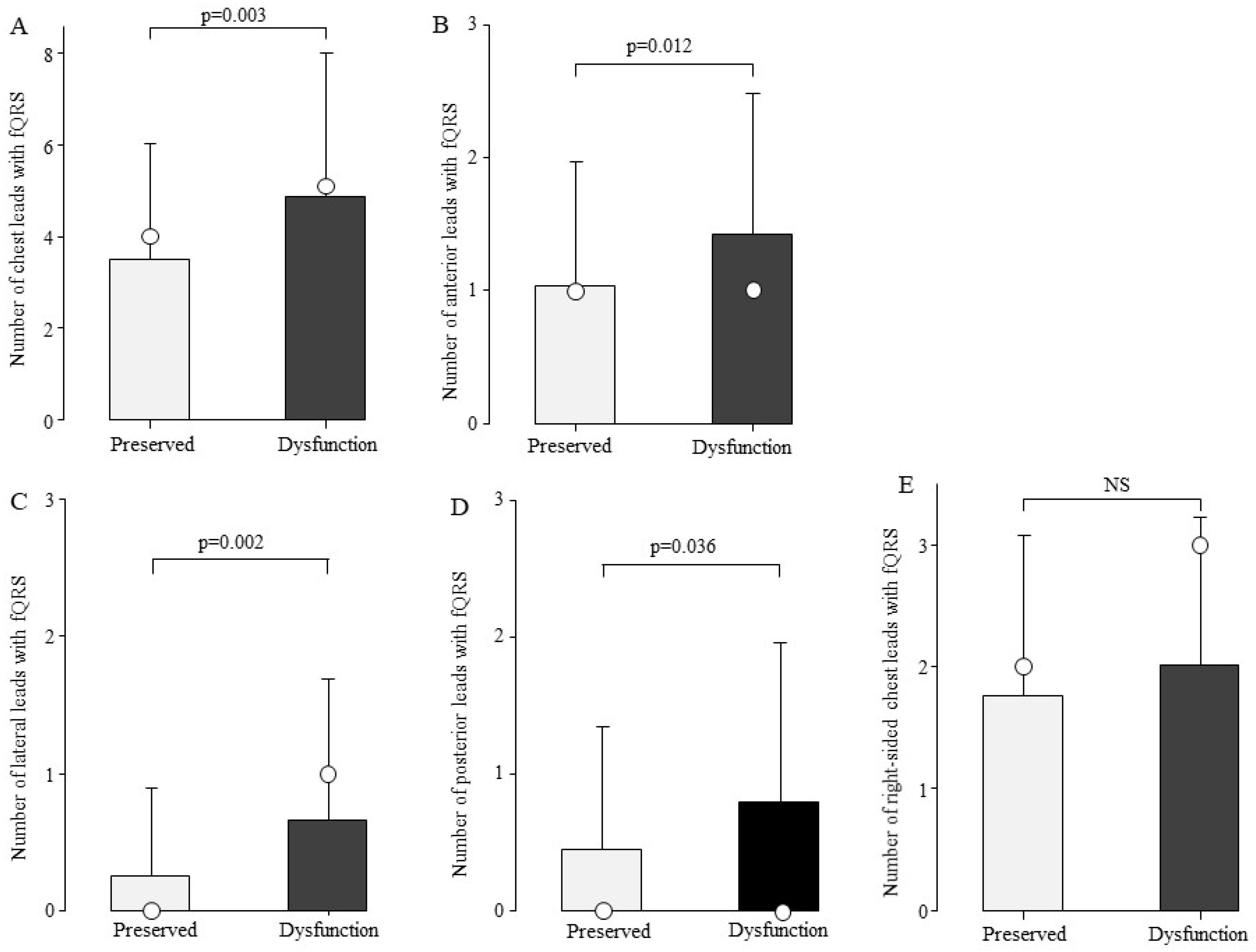
To examine the association between the number of fQRS and cardiac function, we compared the number of fQRS leads in the preserved cardiac function (n = 100) and cardiac dysfunction groups (n = 84). The total number of chest leads with fQRS was 4.9 ± 3.1 in the cardiac dysfunction group and 3.5 ± 2.5 in the preserved cardiac function group, with significantly more fQRS frequently in the cardiac dysfunction group (Figure 4A, p = 0.003). fQRS comparisons were then made by site (Figure 4B–E). The number of fQRS leads by site was significantly higher in the cardiac dysfunction group in the anterior, lateral, and posterior leads (Figure 4B–D).
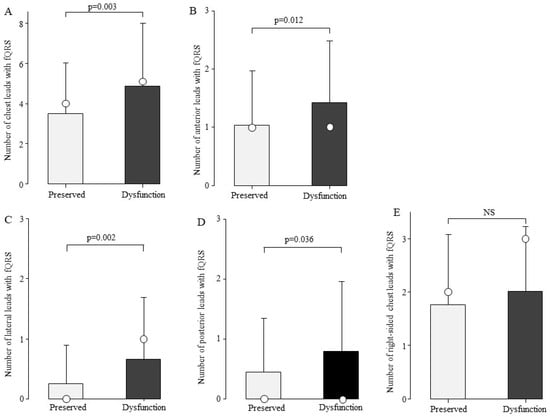
Figure 4.
Comparison of the number of fQRS leads by cardiac function. At all sites, the number of fQRS in patients with cardiac dysfunction was greater than that in the preserved group. Bars are expressed as mean ± SD. The white circles indicate the medians. (A) All leads; (B) anterior leads; (C) lateral leads; (D) posterior leads; and (E) right-sided chest leads. NS: Not significant.
3.4. Relationship Between the Number of fQRS and LV Dilation
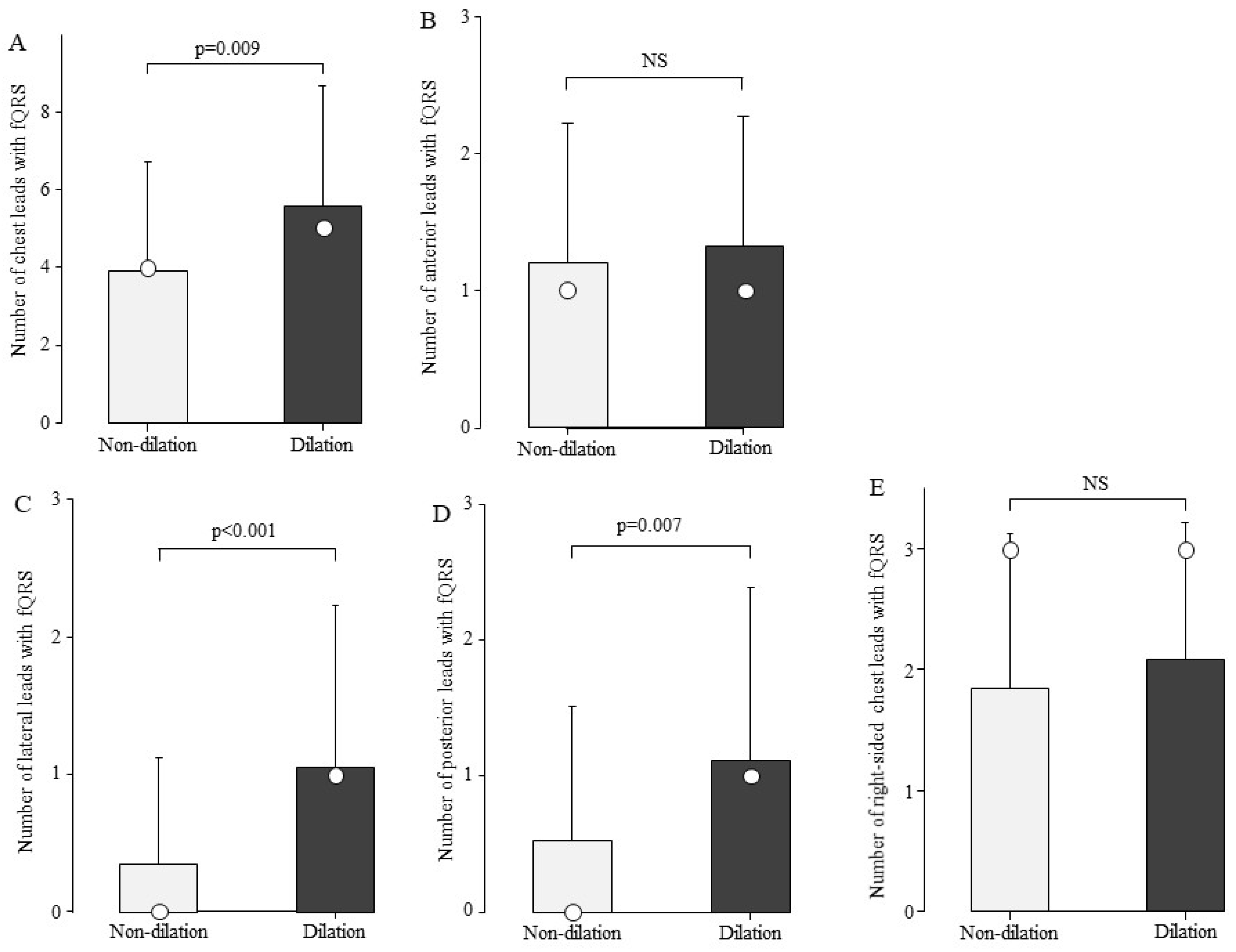
We examined the association between the number of fQRS and LV dilation. The total number of chest leads in the fQRS was 4.9 ± 3.1 in the group with LV dilation (n = 25) and 3.5 ± 2.5 in the group without LV dilation (n = 159). The group with LV dilation had a significantly higher number of fQRS leads than the group without fQRS (Figure 5A, p = 0.009). The fQRS was examined for its association with LV dilation by site (Figure 5B–E). In the group with LV dilatation, the number of leads with fQRS by site was significantly higher in the lateral and posterior leads (Figure 5C,D).
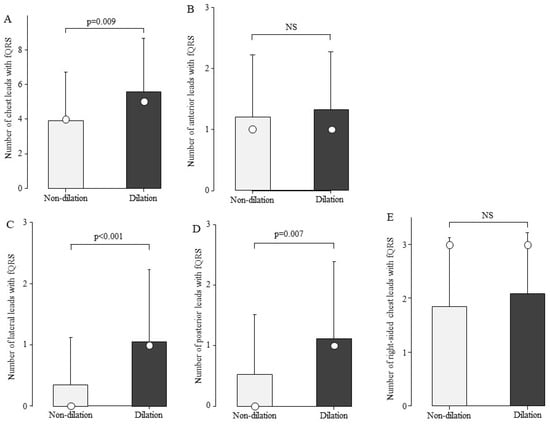
Figure 5.
Comparison of the number of fQRS leads by left ventricular dilation. The number of fQRS in the patients in the LV dilation group was greater than that in the non-dilation group at sites other than the anterior wall. Bars are expressed as mean ± SD. The white circles indicate the medians. (A) All leads; (B) anterior leads; (C) lateral leads; (D) posterior leads; and (E) right-sided chest leads. NS: Not significant.
3.5. Independent Determinants Related to Cardiac Dysfunction and LV Dilation
These results indicate that the fQRS site is associated with age, cardiac function, and LV dilation. As patients with DMD have age-related cardiac dysfunction and LV dilation, fQRS may be a confounding factor for age and cardiac function. Therefore, we performed a multivariate logistic regression analysis using age and cardiac dysfunction to examine the factors associated with the number of leads in the anterior, lateral, and posterior fQRS. The number of anterior leads of the fQRS was associated with age (Table 2, p < 0.001) and the number of lateral leads of the fQRS was associated with cardiac dysfunction (Table 2, p = 0.004). Next, multivariate logistic regression analysis was performed to examine the number of lateral and posterior fQRS leads and the factors associated with LV dilation. Only the number of lateral leads was associated with LV dilation (Table 3; p < 0.001).

Table 2.
Multivariate logistic analysis of fQRS with cardiac dysfunction and age.

Table 3.
Multivariate logistic analysis of fQRS with LV dilation.
4. Discussion
In this study, we used syn18-ECG to examine the number of fQRS leads in patients with DMD, the number of leads by site, age changes, and their association with cardiac dysfunction. The results revealed that (1) fQRS appearing in the anterior leads was associated with age and (2) fQRS appearing in the lateral leads was associated with cardiac dysfunction and LV dilation. To our knowledge, this is the first report on the clinical implications of the fQRS site in patients with DMD.
In this study, we showed for the first time that lateral-lead fQRS was associated with cardiac dysfunction in 184 patients with DMD. Myocardial fibrosis begins in the lateral walls of patients with DMD [7]. Because fQRS is associated with myocardial fibrosis [14,15,16,17,18,19,20,21], the lateral lead fQRS was considered to reflect myocardial fibrosis, leading to cardiac dysfunction.
There have been several reports on fQRS using standard 12-lead ECG in patients with DMD. A study comparing the clinical data of 37 patients with DMD divided into two groups according to the presence or absence of fQRS reported that patients with DMD with fQRS had a lower LVEF, higher left ventricular Tei index, and more frequent ventricular arrhythmias [27]. A study of 27 patients with DMD reported that the presence of fQRS was positively correlated with age and negatively correlated with LVEF [28]. In a study comparing fQRS and MRI in 195 patients with DMD, the number of fQRS leads was higher in the group with fibrosis than in the group without fQRS [29]. The cardiac dysfunction group also had a higher number of fQRS leads than the group with preserved cardiac function. These findings suggest that the appearance of fQRS is related to cardiac dysfunction. In patients with DMD, myocardial fibrosis begins at the lateral wall on cardiac MRI [12]. Because our number of patients was larger than that in previous studies, we believe that our results are consistent with cardiac MRI results examining myocardial fibrosis. By site, this is the first study to show that the lateral wall is associated with cardiac function and dilation. This finding is consistent with the fact that myocardial fibrosis begins in the lateral wall on MRI. Lateral-lead fQRS may reflect myocardial fibrosis.
In this study, syn18-lead was used. A standard 12-lead ECG makes it challenging to assess the posterior wall, which is the site of cardiac dysfunction in DMD. Therefore, we used syn18-lead to evaluate the posterior wall in patients with DMD. However, only the lateral leads showed a significant association between cardiac dysfunction and LV dilation. In contrast, the right-sided chest-lead and posterior-lead fQRS did not show a significant association between cardiac dysfunction and LV dilation. In other words, fQRS associated with cardiac dysfunction or LV dilation can be determined using a standard 12-lead ECG.
This study found that the number of anterior leads in fQRS was associated with age. No studies have been conducted on myocardial fibrosis of the right ventricle in patients with DMD. The right ventricle may be more prone to myocardial fibrosis because the right ventricular wall is thinner than the LV wall [30]. Therefore, fQRS is likely the most common condition in right-sided chest leads. There are only three right-sided chest leads, and the number of fQRS can only be in a narrow range of only four levels (0–3). In the present results, the 11–15 and 16–20 age groups showed a median of 3 (Figure 3E), with no measurable age change beyond 11 years. The reason why the association between age and number was not apparent may have been influenced by the narrow assessment range. In arrhythmogenic right ventricular cardiomyopathy, epsilon waves, which represent conduction disturbances due to fatty degeneration of the myocardium [31], also appear and are detected in the anterior leads [32,33]. Thus, the anterior leads may exhibit an ECG signal that reflects the right ventricle.
On echocardiography, we expected the posterior leads fQRS to be related to cardiac function because asynergy begins in the LV posterior wall [34,35,36,37]; however, no association was found. This may have been because the posterior leads were farther from the myocardium than the lateral leads and may not have adequately captured the changes. Multivariate regression analysis revealed that posterior leads tended to be associated with cardiac dysfunction (p = 0.058), which requires further investigation.
The fQRS of V4-6 was considered a surrogate indicator of myocardial fibrosis associated with cardiac dysfunction. The recording of V4-V6 is possible with a 12-lead ECG, which can be performed in many hospitals. Thus, fQRS can be used to assess myocardial fibrosis in many hospitals. Furthermore, the fQRS is determined by the shape of the QRS on electrocardiography; therefore, there is no need for measurement or other time-consuming procedures. Therefore, the fQRS is considered useful in the management of myocardial damage in DMD because it is an easy marker to record and evaluate.
This study has several limitations. First, because this was a single-center study, a patient selection bias may have existed. In the future, it will be necessary to verify these findings in many hospitals. Second, because this was a cross-sectional study, it was not possible to compare the timing of fQRS onset with that of cardiac dysfunction. Therefore, it remains unclear whether the appearance of fQRS precedes or results in cardiac dysfunction. Further studies with different research designs are required to clarify this issue. The syn18-lead ECG showed no chest electrodes on the inferior wall; therefore, the inferior wall was not evaluated in this study.
5. Conclusions
Lateral-lead fQRS was associated with cardiac dysfunction and LV dilation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13040804/s1, Figure S1: Comparison of fQRS leads by site. The number of fQRS was significantly greater in the right-sided chest leads than at other sites. Bars are expressed as mean ± SD. White open circle shows the median.
Author Contributions
Methodology, T.Y.; Validation, H.A.; Formal Analysis, Y.N. and R.B.; Data Curation, Y.I. and K.M.; Visualization, S.O.; Writing—Original Draft Preparation, T.Y.; Writing—Review and Editing, A.S., M.M. and H.A.; Supervision, M.M.; Project Administration, M.M. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (Kaken Pharmaceutical, #23K17203).
Institutional Review Board Statement
This study was approved by the Ethics Committees of Kobe University (no. 1534) and Nagahama Institute of Bio-Science and Technology (no. 008).
Informed Consent Statement
Written informed consent was obtained from the patient or parent of the patient for entry into this research.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We also thank Tamami Ichii, Masataka Inoue, Takumi Endo, Hikari Kitagawa, and Yuto Tajima for their assistance with the analyses.
Conflicts of Interest
M.M. is an advisor to JCR Pharmaceuticals Co., Ltd., Japan, and Daiichi Sankyo Co., Ltd. H.A. received consulting fees from Daiichi Sankyo Co., Ltd.; Nippon Shinya-ku Co., Ltd.; and Takeda Pharmaceutical Co., Ltd. and lecture fees from Nippon Shinyaku Co., Ltd.; Chugai Pharmaceutical Co. Ltd.; Biogen Japan Ltd.; Pfizer Japan Inc.; and JCR Pharmaceuticals Co., Ltd. The authors declare that this research was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
Abbreviations
| BPM | Beats Per Minute |
| CK | Creatine Kinase |
| DMD | Duchenne Muscular Dystrophy |
| ECG | Electrocardiography |
| fQRS | Fragmented QRS |
| HR | Heart Rate |
| IU/L | International Units per Liter |
| LV | Left Ventricle |
| LVEF | Left Ventricular Ejection Fraction |
| MRI | Magnetic Resonance Imaging |
| SD | Standard Deviation |
References
- Norwood, F.L.; Harling, C.; Chinnery, P.F.; Eagle, M.; Bushby, K.; Straub, V. Prevalence of genetic muscle disease in Northern England: In-depth analysis of a muscle clinic population. Brain 2009, 132, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Rosaria Cecio, M.; Torre, V.; Luca, F.D.E.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 121–125. [Google Scholar]
- Rall, S.; Grimm, T. Survival in Duchenne muscular dystrophy. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 117–120. [Google Scholar]
- Ishikawa, Y.; Miura, T.; Ishikawa, Y.; Aoyagi, T.; Ogata, H.; Hamada, S.; Minami, R. Duchenne muscular dystrophy: Survival by cardio-respiratory interventions. Neuromuscul. Disord. NMD 2011, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, L.; Kroksmark, A.K.; Tulinius, M.; Sofou, K. One in five patients with Duchenne muscular dystrophy dies from other causes than cardiac or respiratory failure. Eur. J. Epidemiol. 2022, 37, 147–156. [Google Scholar] [CrossRef]
- Silva, M.C.; Meira, Z.M.; Gurgel Giannetti, J.; da Silva, M.M.; Campos, A.F.; Barbosa Mde, M.; Starling Filho, G.M.; Ferreira Rde, A.; Zatz, M.; Rochitte, C.E. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J. Am. Coll. Cardiol. 2007, 49, 1874–1879. [Google Scholar] [CrossRef]
- Puchalski, M.D.; Williams, R.V.; Askovich, B.; Sower, C.T.; Hor, K.H.; Su, J.T.; Pack, N.; Dibella, E.; Gottliebson, W.M. Late gadolinium enhancement: Precursor to cardiomyopathy in Duchenne muscular dystrophy? Int. J. Cardiovasc. Imaging 2009, 25, 57–63. [Google Scholar] [CrossRef]
- Verhaert, D.; Richards, K.; Rafael-Fortney, J.A.; Raman, S.V. Cardiac involvement in patients with muscular dystrophies: Magnetic resonance imaging phenotype and genotypic considerations. Circ. Cardiovasc. Imaging 2011, 4, 67–76. [Google Scholar] [CrossRef]
- Hor, K.N.; Taylor, M.D.; Al-Khalidi, H.R.; Cripe, L.H.; Raman, S.V.; Jefferies, J.L.; O’Donnell, R.; Benson, D.W.; Mazur, W. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: Effect of age and left ventricular systolic function. J. Cardiovasc. Magn. Reson. 2013, 15, 107. [Google Scholar] [CrossRef]
- Tandon, A.; Villa, C.R.; Hor, K.N.; Jefferies, J.L.; Gao, Z.; Towbin, J.A.; Wong, B.L.; Mazur, W.; Fleck, R.J.; Sticka, J.J.; et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. J. Am. Heart Assoc. 2015, 4, e001338. [Google Scholar] [CrossRef]
- Bilchick, K.C.; Salerno, M.; Plitt, D.; Dori, Y.; Crawford, T.O.; Drachman, D.; Thompson, W.R. Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy. J. Cardiovasc. Magn. Reson. 2011, 13, 20. [Google Scholar] [CrossRef]
- Flowers, N.C.; Horan, L.G.; Thomas, J.R.; Tolleson, W.J. The anatomic basis for high-frequency components in the electrocardiogram. Circulation 1969, 39, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Buerkel, D.M.; Corbett, J.R.; Gurm, H.S. Fragmented QRS complex has poor sensitivity in detecting myocardial scar. Ann. Noninvasive Electrocardiol. Off. J. Int. Soc. Holter Noninvasive Electrocardiol. 2010, 15, 308–314. [Google Scholar] [CrossRef]
- Carey, M.G.; Luisi, A.J., Jr.; Baldwa, S.; Al-Zaiti, S.; Veneziano, M.J.; deKemp, R.A.; Canty, J.M., Jr.; Fallavollita, J.A. The Selvester QRS Score is more accurate than Q waves and fragmented QRS complexes using the Mason-Likar configuration in estimating infarct volume in patients with ischemic cardiomyopathy. J. Electrocardiol. 2010, 43, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, U.; Kabakçi, G.; Aytemir, K.; Dural, M.; Sahiner, L.; Yorgun, H.; Sunman, H.; Bariş Kaya, E.; Tokgözoğlu, L.; Oto, A. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J. Cardiovasc. Electrophysiol. 2013, 24, 1260–1266. [Google Scholar] [CrossRef]
- Bae, M.H.; Jang, S.Y.; Choi, W.S.; Kim, K.H.; Park, S.H.; Lee, J.H.; Kim, H.K.; Yang, D.H.; Huh, S.; Park, H.S.; et al. A new revised cardiac risk index incorporating fragmented QRS complex as a prognostic marker in patients undergoing noncardiac vascular surgery. Am. J. Cardiol. 2013, 112, 122–127. [Google Scholar] [CrossRef]
- Timonen, P.; Magga, J.; Risteli, J.; Punnonen, K.; Vanninen, E.; Turpeinen, A.; Tuomainen, P.; Kuusisto, J.; Vuolteenaho, O.; Peuhkurinen, K. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int. J. Cardiol. 2008, 124, 293–300. [Google Scholar] [CrossRef]
- Kawano, H.; Tsuneto, A.; Koide, Y.; Tasaki, H.; Sueyoshi, E.; Sakamoto, I.; Hayashi, T. Magnetic resonance imaging in a patient with peripartum cardiomyopathy. Intern. Med. 2008, 47, 97–102. [Google Scholar] [CrossRef]
- Marijianowski, M.M.; Teeling, P.; Mann, J.; Becker, A.E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment. J. Am. Coll. Cardiol. 1995, 25, 1263–1272. [Google Scholar] [CrossRef]
- Gunja-Smith, Z.; Morales, A.R.; Romanelli, R.; Woessner, J.F., Jr. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am. J. Pathol. 1996, 148, 1639–1648. [Google Scholar] [PubMed]
- Daming, W. Derived electrocardiograms on the posterior leads from 12-lead system: Method and evaluation. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; Volume 71, pp. 74–77. [Google Scholar]
- Takeshima, Y.; Yagi, M.; Okizuka, Y.; Awano, H.; Zhang, Z.; Yamauchi, Y.; Nishio, H.; Matsuo, M. Mutation spectrum of the dystrophin gene in 442 Duchenne/Becker muscular dystrophy cases from one Japanese referral center. J. Hum. Genet. 2010, 55, 379–388. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, N.; Kim, H.J.; Park, B.E.; Kim, H.N.; Jang, S.Y.; Bae, M.H.; Lee, J.H.; Yang, D.H.; Park, H.S.; et al. Effectiveness of a new cardiac risk scoring model reclassified by QRS fragmentation as a predictor of postoperative cardiac event in patients with severe renal dysfunction. BMC Cardiovasc. Disord. 2021, 21, 359. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Kampmann, C.; Wiethoff, C.M.; Wenzel, A.; Stolz, G.; Betancor, M.; Wippermann, C.-F.; Huth, R.-G.; Habermehl, P.; Knuf, M.; Emschermann, T.; et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 2000, 83, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Lee, J.W.; Lee, J.; Shin, Y.B.; Lee, H.D. Relationship Between Fragmented QRS Complexes and Cardiac Status in Duchenne Muscular Dystrophy: Multimodal Validation Using Echocardiography, Magnetic Resonance Imaging, and Holter Monitoring. Pediatr. Cardiol. 2017, 38, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Monteze, N.M.; Giannetti, J.G.; Meira, Z.M.A. Electrocardiographic and Autonomic Nervous System Changes after Changes in the Posture of Children and Adolescents with Duchenne Muscular Dystrophy. Arq. Bras. Cardiol. 2024, 121, e20230483. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Pieters, Z.H.; Lao, K.; Tonnis, R.; Jacobs, H.; Gunsaulus, M.; Buddhavarapu, A.; Hayes, E.; Wood, P.; Lee, M.; et al. QRS Fragmentation in duchenne muscular dystrophy correlates with delayed enhancement and cardiac dysfunction on CMR. J. Cardiovasc. Magn. Reson. 2025, 27, 101370. [Google Scholar] [CrossRef]
- Ho, S.Y. Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. Eur. J. Echocardiogr. J. Work. Group Echocardiogr. Eur. Soc. Cardiol. 2009, 10, iii3–iii7. [Google Scholar] [CrossRef]
- Marcus, F.I.; Fontaine, G.H.; Guiraudon, G.; Frank, R.; Laurenceau, J.L.; Malergue, C.; Grosgogeat, Y. Right ventricular dysplasia: A report of 24 adult cases. Circulation 1982, 65, 384–398. [Google Scholar] [CrossRef]
- Gemayel, C.; Pelliccia, A.; Thompson, P.D. Arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 2001, 38, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Bomma, C.; Tandri, H.; Roguin, A.; Dalal, D.; Prakasa, K.; Tichnell, C.; James, C.; Spevak, P.J.; Marcus, F.; et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: A need to broaden diagnostic criteria. Circulation 2004, 110, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tanaka, H.; Matsumoto, K.; Lee, T.; Awano, H.; Yagi, M.; Imanishi, T.; Hayashi, N.; Takeshima, Y.; Kawai, H.; et al. Utility of transmural myocardial strain profile for prediction of early left ventricular dysfunction in patients with Duchenne muscular dystrophy. Am. J. Cardiol. 2013, 111, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tanaka, H.; Takeshima, Y.; Hayashi, N.; Hirata, K.; Kawano, S. Combining passive leg-lifting with transmural myocardial strain profile for enhanced predictive capability for subclinical left ventricular dysfunction in Duchenne muscular dystrophy. J. Cardiol. 2015, 66, 212–217. [Google Scholar] [CrossRef]
- Ogata, H.; Nakatani, S.; Ishikawa, Y.; Negishi, A.; Kobayashi, M.; Ishikawa, Y.; Minami, R. Myocardial strain changes in Duchenne muscular dystrophy without overt cardiomyopathy. Int. J. Cardiol. 2007, 115, 190–195. [Google Scholar] [CrossRef]
- Mori, K.; Hayabuchi, Y.; Inoue, M.; Suzuki, M.; Sakata, M.; Nakagawa, R.; Kagami, S.; Tatara, K.; Hirayama, Y.; Abe, Y. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne’s progressive muscular dystrophy. Echocardiography 2007, 24, 598–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).