Caveolin Scaffolding Domain (CSD) Peptide LTI-2355 Modulates the Phagocytic and Synthetic Activity of Lung-Derived Myeloid Cells in Idiopathic Pulmonary Fibrosis (IPF) and Post-Acute Sequelae of COVID Fibrosis (PASC-F)
Abstract
1. Introduction
2. Methods and Materials
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Alveolar macrophage |
| CAV | Caveolin |
| CD | Cluster of differentiation |
| CH3IL1 | Chitinase 3-like-1 |
| COPD | Chronic obstructive pulmonary disease |
| CSD | Caveolin scaffolding domain |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ECM | Extracellular matrix |
| FABP | Fatty acid-binding protein |
| IL | Interleukin |
| IPF | Idiopathic pulmonary fibrosis |
| MERTK | MER proto-oncogene tyrosine kinase |
| MMR/MRC | Mannose receptor |
| MMP | Matrix metalloprotease |
| Mo-MA | Monocyte-derived macrophage |
| PASC-F | Post-acute sequelae of COVID fibrosis |
| RCU | Red calibrated unit |
| SA | Staphylococcus aureus |
| sCD | Soluble CD |
| SPP/OPN | Osteopontin |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TR-AM | Tissue-resident alveolar macrophage |
References
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; Deiuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.L.; Bittar, H.T.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Ayaub, E.; Poli, S.; Ng, J.; Adams, T.; Schupp, J.; Quesada-Arias, L.; Poli, F.; Cosme, C.; Robertson, M.; Martinez-Manzano, J.; et al. Single Cell RNA-seq and Mass Cytometry Reveals a Novel and a Targetable Population of Macrophages in Idiopathic Pulmonary Fibrosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Schupp, J.C.; Adams, T.; Neumark, N.; Poli De Frias, S.; Ahangari, F.; Deiuliis, G.; Chu, S.; Yan, X.; Kaminski, N.; Prasse, A.; et al. Macrophage Programs in BAL and Lung Parenchyma of the Healthy and in IPF Patients. In Proceedings of the American Thoracic Society 2019 International Conference, Dallas, TX, USA, 17–22 May 2019. [Google Scholar]
- Bingham, G.C.; Muehling, L.M.; Li, C.; Huang, Y.; Abebayehu, D.; Noth, I.; Sun, J.; Woodfolk, J.A.; Barker, T.H.; Bonham, C. Reduction in circulating monocytes correlates with persistent post-COVID pulmonary fibrosis in multi-omic comparison of long-haul COVID and IPF. medRxiv 2022. [Google Scholar] [CrossRef]
- Chen, S.T.; Park, M.D.; del Valle, D.M.; Buckup, M.; Tabachnikova, A.; Thompson, R.C.; Simons, N.W.; Mouskas, K.; Lee, B.; Geanon, D.; et al. A shift in lung macrophage composition is associated with COVID-19 severity and recovery. Sci. Transl. Med. 2022, 14, 5168. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef]
- Wendisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021, 184, 6243–6261.e27. [Google Scholar] [CrossRef]
- Bosteels, C.; Van Damme, K.F.A.; De Leeuw, E.; Declercq, J.; Maes, B.; Bosteels, V.; Hoste, L.; Naesens, L.; Debeuf, N.; Deckers, J.; et al. Loss of GM-CSF-dependent instruction of alveolar macrophages in COVID-19 provides a rationale for inhaled GM-CSF treatment. Cell Rep. Med. 2022, 3, 100833. [Google Scholar] [CrossRef]
- Wicher, S.A.; Prakash, Y.S.; Pabelick, C.M. Caveolae, caveolin-1 and lung diseases of aging. Expert Rev. Respir. Med. 2019, 13, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Volonte, D.; Galbiati, F. Caveolin-1, a master regulator of cellular senescence. Cancer Metastasis Rev. 2020, 39, 397–414. [Google Scholar] [CrossRef]
- Volonte, D.; Zhang, K.; Lisanti, M.P.; Galbiati, F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol. Biol. Cell 2002, 13, 2502–2517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.W.; Zhang, Y.; Hong, P.K.; Zhou, Z.; Feghali-Bostwick, C.A.; Liu, F.; Ifedigbo, E.; Xu, X.; Oury, T.D.; Kaminski, N.; et al. Caveolin-1: A critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 2006, 203, 2895. [Google Scholar] [CrossRef]
- Lin, X.; Barravecchia, M.; Matthew Kottmann, R.; Sime, P.; Dean, D.A. Caveolin-1 gene therapy inhibits inflammasome activation to protect from bleomycin-induced pulmonary fibrosis. Sci. Rep. 2019, 9, 19643. [Google Scholar] [CrossRef] [PubMed]
- Melms, J.C.; Biermann, J.; Huang, H.; Wang, Y.; Nair, A.; Tagore, S.; Katsyv, I.; Rendeiro, A.F.; Amin, A.D.; Schapiro, D.; et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 2021, 595, 114–119. [Google Scholar] [CrossRef]
- Sikkema, L.; Ramírez-Suástegui, C.; Strobl, D.C.; Gillett, T.E.; Zappia, L.; Madissoon, E.; Markov, N.S.; Zaragosi, L.E.; Ji, Y.; Ansari, M.; et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 2023, 29, 1563–1577. [Google Scholar] [CrossRef]
- Chianese, M.; Screm, G.; Salton, F.; Confalonieri, P.; Trotta, L.; Barbieri, M.; Ruggero, L.; Mari, M.; Reccardini, N.; Geri, P.; et al. Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals 2024, 17, 709. [Google Scholar] [CrossRef]
- Libra, A.; Sciacca, E.; Muscato, G.; Sambataro, G.; Spicuzza, L.; Vancheri, C. Highlights on Future Treatments of IPF: Clues and Pitfalls. Int. J. Mol. Sci. 2024, 25, 8392. [Google Scholar] [CrossRef]
- Bonilla, H.; Peluso, M.J.; Rodgers, K.; Aberg, J.A.; Patterson, T.F.; Tamburro, R.; Baizer, L.; Goldman, J.D.; Rouphael, N.; Deitchman, A.; et al. Therapeutic Trials for Long COVID-19: A Call to Action from the Interventions Taskforce of the RECOVER Initiative. Front. Immunol. 2023, 14, 1129459. [Google Scholar] [CrossRef]
- Isshiki, T.; Vierhout, M.; Naiel, S.; Ali, P.; Yazdanshenas, P.; Kumaran, V.; Yang, Z.; Dvorkin-Gheva, A.; Rullo, A.F.; Kolb, M.R.; et al. Therapeutic Strategies Targeting Pro-Fibrotic Macrophages in Interstitial Lung Disease. Biochem. Pharmacol. 2023, 211, 115501. [Google Scholar] [CrossRef]
- Monteleone, G.; Bergantini, L.; D’Alessandro, M.; Pianigiani, T.; Simonetti, J.; Iovene, B.; Varone, F.; Sgalla, G.; Richeldi, L.; Bargagli, E.; et al. The Management of Familial Pulmonary Fibrosis in Different Medical Settings: Where Does That Leave Us? An Italian Nationwide Survey. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2024, 41, e2024047. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Korfei, M.; Mackenzie, B.; Meiners, S. The Ageing Lung under Stress. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2020, 29, 200126. [Google Scholar] [CrossRef] [PubMed]
- Reese, C.F.; Chinnakkannu, P.; Tourkina, E.; Hoffman, S.; Kuppuswamy, D. Multiple Subregions within the Caveolin-1 Scaffolding Domain Inhibit Fibrosis, Microvascular Leakage, and Monocyte Migration. PLoS ONE 2022, 17, e0264413. [Google Scholar] [CrossRef]
- Warheit-Niemi, H.I.; Edwards, S.J.; SenGupta, S.; Parent, C.A.; Zhou, X.; O’Dwyer, D.N.; Moore, B.B. Fibrotic lung disease inhibits immune responses to staphylococcal pneumonia via impaired neutrophil and macrophage function. JCI Insight 2022, 7, e152690. [Google Scholar] [CrossRef]
- Ai, F.; Zhao, G.; Lv, W.; Liu, B.; Lin, J. Dexamethasone induces aberrant macrophage immune function and apoptosis. Oncol. Rep. 2020, 43, 427. [Google Scholar] [CrossRef]
- Niemann, S.; Lucarini, L.; Mae Gowdy, K.; Yang, J.; Sang, X.; Wang, Y.; Xue, Z.; Qi, D.; Fan, G.; Tian, F.; et al. Macrophage-Targeted Lung Delivery of Dexamethasone Improves Pulmonary Fibrosis Therapy via Regulating the Immune Microenvironment. Front. Immunol. 2021, 12, 613907. [Google Scholar] [CrossRef]
- Kumaran Satyanarayanan, S.; el Kebir, D.; Soboh, S.; Butenko, S.; Sekheri, M.; Saadi, J.; Peled, N.; Assi, S.; Othman, A.; Schif-Zuck, S.; et al. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat. Commun. 2019, 10, 3471. [Google Scholar] [CrossRef]
- Schif-Zuck, S.; Gross, N.; Assi, S.; Rostoker, R.; Serhan, C.N.; Ariel, A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: Modulation by resolvins and glucocorticoids. Eur. J. Immunol. 2011, 41, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, D.; Lindenthal, L.; Sauer, J.; Chung, K.J.; Chavakis, T.; Griesser, E.; Baskaran, P.; Maier-Habelsberger, U.; Fundel-Clemens, K.; Schlotthauer, I.; et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis. J. Cell Biol. 2023, 222, e202207097. [Google Scholar] [CrossRef]
- Zhang, F.; Ayaub, E.A.; Wang, B.; Puchulu-Campanella, E.; Li, Y.-H.; Hettiarachchi, S.U.; Lindeman, S.D.; Luo, Q.; Rout, S.; Srinivasarao, M.; et al. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol. Med. 2020, 12, e12034. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Ogger, P.P.; Byrne, A.J. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol. 2020, 14, 282–295. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 6, eaax6337. [Google Scholar] [CrossRef]
- Scodeller, P.; Simón-Gracia, L.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A.; et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 14655. [Google Scholar] [CrossRef]
- Takamura, N.; Yamaguchi, Y.; Watanabe, Y.; Asami, M.; Komitsu, N.; Aihara, M. Downregulated Caveolin-1 expression in circulating monocytes may contribute to the pathogenesis of psoriasis. Sci. Rep. 2019, 9, 125. [Google Scholar] [CrossRef]
- Haczku, A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J. Allergy Clin. Immunol. 2008, 122, 861–879. [Google Scholar] [CrossRef]
- Lambrecht, B.N. Alveolar macrophage in the driver’s seat. Immunity 2006, 24, 366–368. [Google Scholar] [CrossRef]
- Bellamri, N.; Morzadec, C.; Joannes, A.; Lecureur, V.; Wollin, L.; Jouneau, S.; Vernhet, L. Alteration of human macrophage phenotypes by the anti-fibrotic drug nintedanib. Int. Immunopharmacol. 2019, 72, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Maier, C.; Zhang, Y.; Soare, A.; Dees, C.; Beyer, C.; Harre, U.; Chen, C.W.; Distler, O.; Schett, G.; et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Mizuguchi, S.; Minamiyama, Y.; Yamamoto-Oka, H.; Aota, T.; Kubo, S.; Nishiyama, N.; Shibata, T.; Takemura, S. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J. Clin. Biochem. Nutr. 2018, 63, 58. [Google Scholar] [CrossRef]
- Ying, H.; Fang, M.; Hang, Q.Q.; Chen, Y.; Qian, X.; Chen, M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway. J. Cell. Mol. Med. 2021, 25, 8662–8675. [Google Scholar] [CrossRef]
- Ghebremedhin, A.; Bin Salam, A.; Adu-Addai, B.; Noonan, S.; Stratton, R.; Ahmed, M.S.U.; Khantwal, C.; Martin, G.R.; Lin, H.; Andrews, C.; et al. A Novel CD206 Targeting Peptide Inhibits Bleomycin-Induced Pulmonary Fibrosis in Mice. Cells 2023, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Kasam, R.K.; Reddy, G.B.; Jegga, A.G.; Madala, S.K. Dysregulation of mesenchymal cell survival pathways in severe fibrotic lung disease: The effect of nintedanib therapy. Front. Pharmacol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef]
- Lerbs, T.; Cui, L.; King, M.E.; Chai, T.; Muscat, C.; Chung, L.; Brown, R.; Rieger, K.; Shibata, T.; Wernig, G. CD47 prevents the elimination of diseased fibroblasts in scleroderma. JCI Insight 2020, 5, e140458. [Google Scholar] [CrossRef]
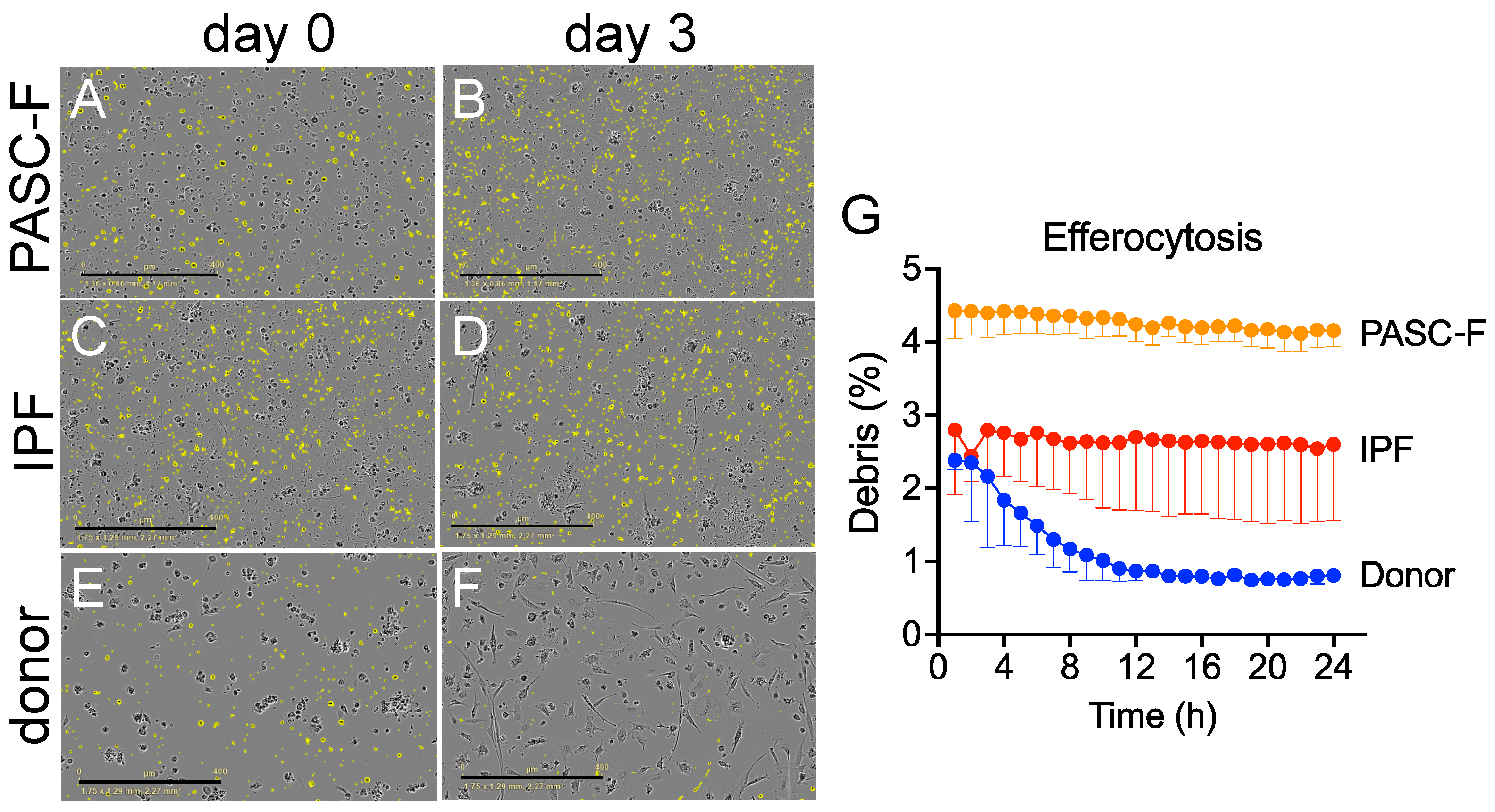
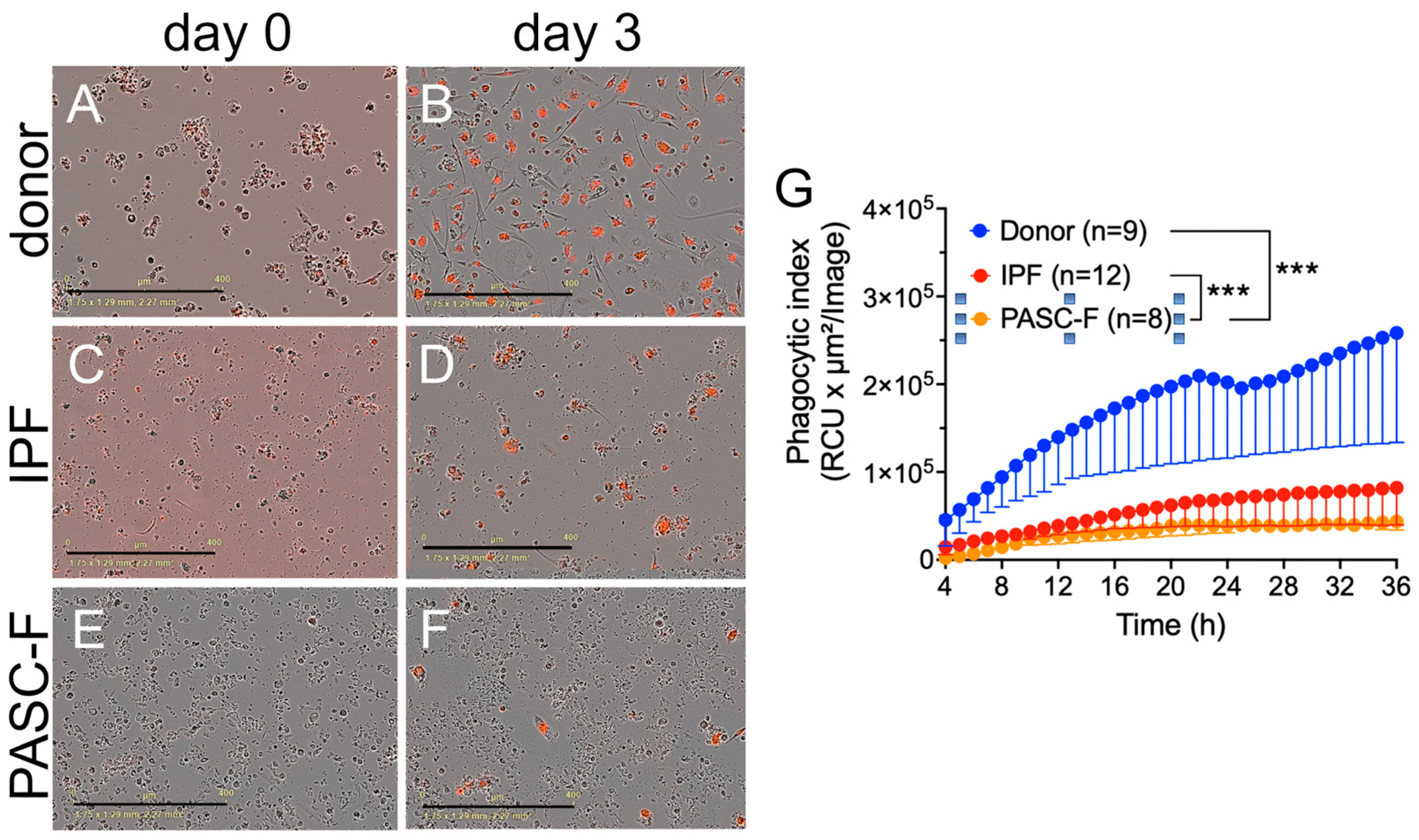


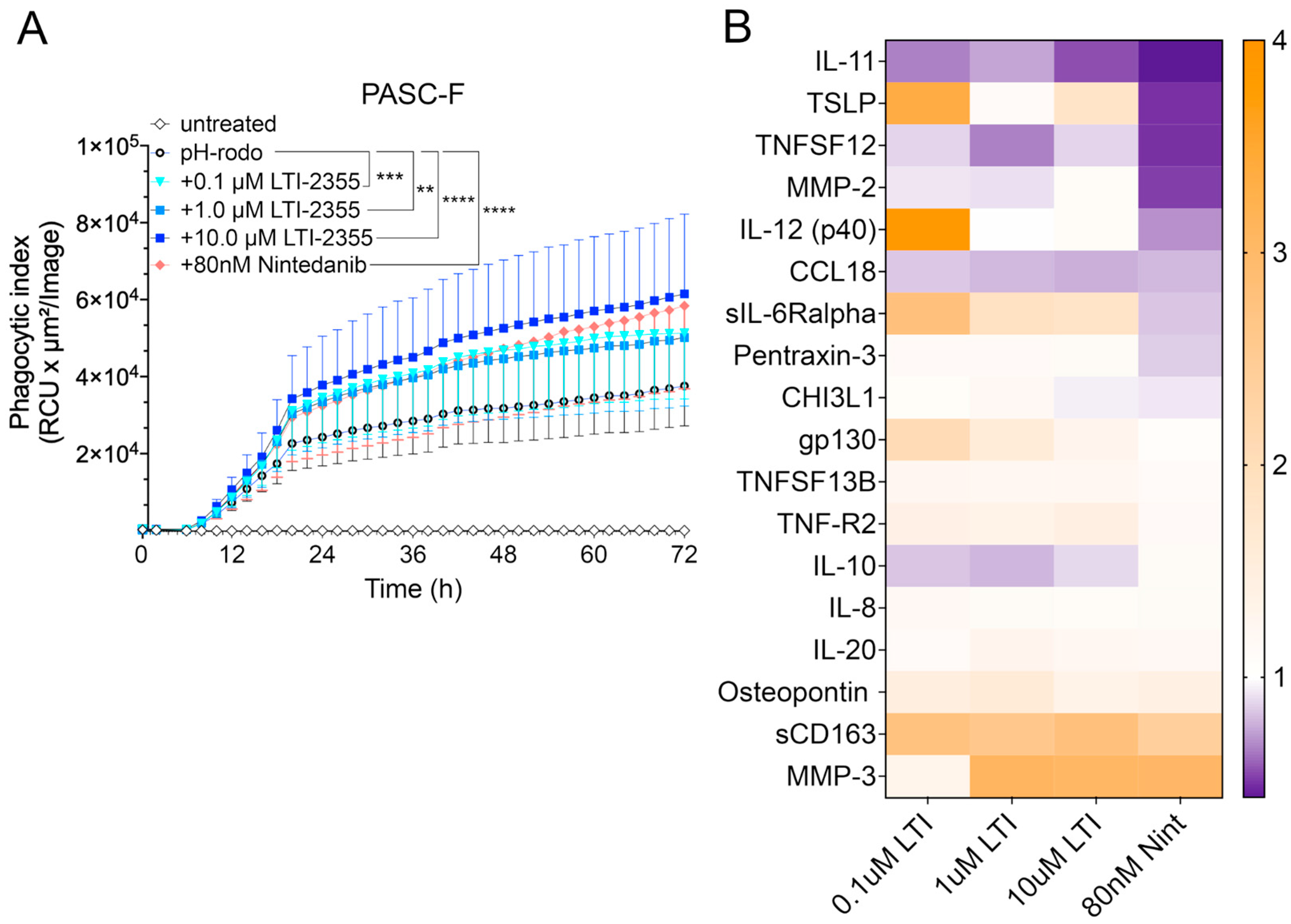
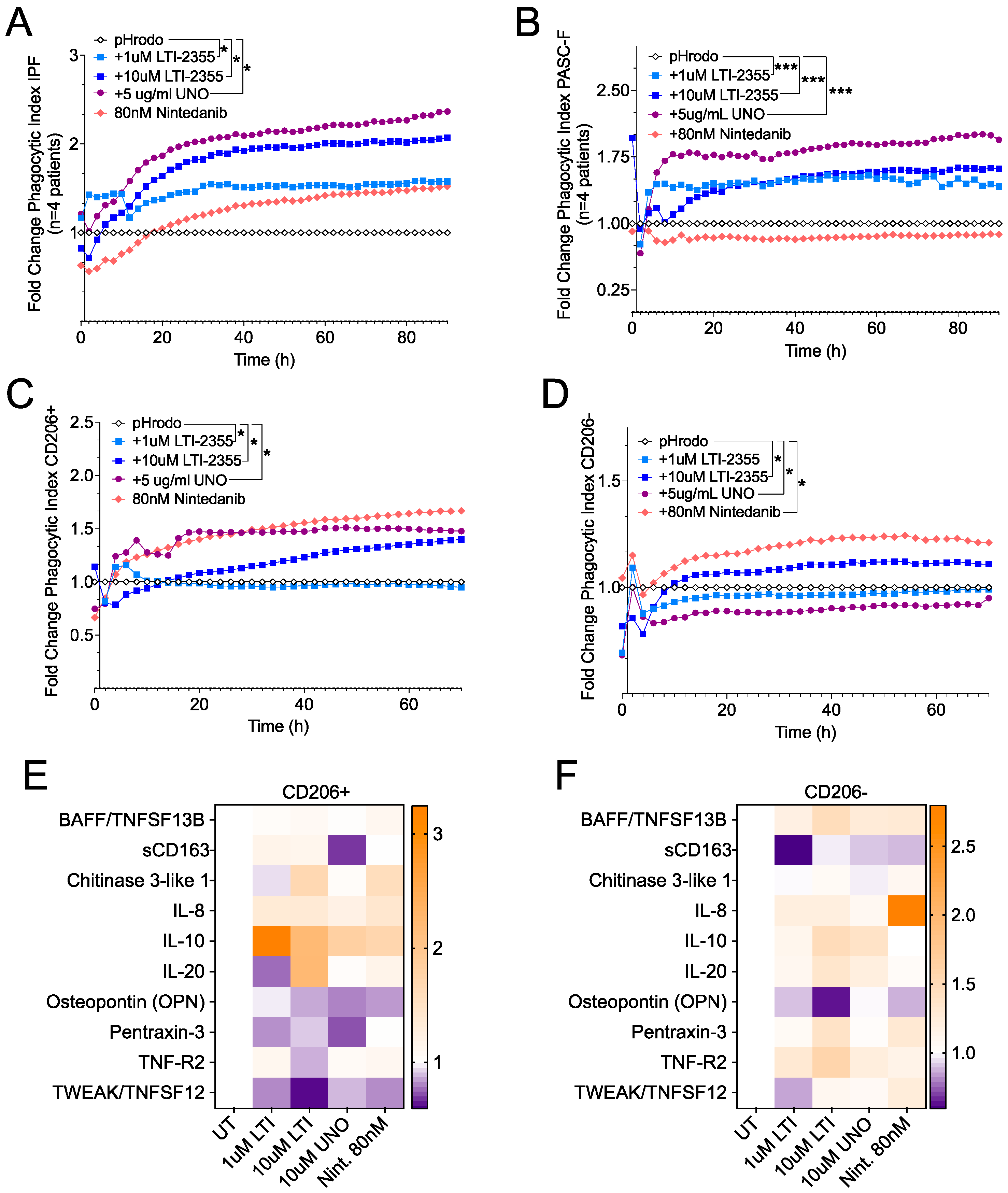
| Controls | IPF | PASC-F | |
|---|---|---|---|
| Age (mean, (SD)) | 51.6 (14.5) | 66.0 (15.8) | 56.5 (16.0) |
| Gender (F/M (% male)) | 0/7 (100%) | 2/7 (77.8%) | 1/7 (85.7%) |
| Smoking history (S/F/NS) | 1/1/5 | 0/2/7 | 0/1/7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creyns, B.; MacKenzie, B.; Jannini Sa, Y.A.P.; Coelho, A.L.; Christensen, D.; Parimon, T.; Windsor, B.; Hogaboam, C.M. Caveolin Scaffolding Domain (CSD) Peptide LTI-2355 Modulates the Phagocytic and Synthetic Activity of Lung-Derived Myeloid Cells in Idiopathic Pulmonary Fibrosis (IPF) and Post-Acute Sequelae of COVID Fibrosis (PASC-F). Biomedicines 2025, 13, 796. https://doi.org/10.3390/biomedicines13040796
Creyns B, MacKenzie B, Jannini Sa YAP, Coelho AL, Christensen D, Parimon T, Windsor B, Hogaboam CM. Caveolin Scaffolding Domain (CSD) Peptide LTI-2355 Modulates the Phagocytic and Synthetic Activity of Lung-Derived Myeloid Cells in Idiopathic Pulmonary Fibrosis (IPF) and Post-Acute Sequelae of COVID Fibrosis (PASC-F). Biomedicines. 2025; 13(4):796. https://doi.org/10.3390/biomedicines13040796
Chicago/Turabian StyleCreyns, Brecht, BreAnne MacKenzie, Yago Amigo Pinho Jannini Sa, Ana Lucia Coelho, Dale Christensen, Tanyalak Parimon, Brian Windsor, and Cory M. Hogaboam. 2025. "Caveolin Scaffolding Domain (CSD) Peptide LTI-2355 Modulates the Phagocytic and Synthetic Activity of Lung-Derived Myeloid Cells in Idiopathic Pulmonary Fibrosis (IPF) and Post-Acute Sequelae of COVID Fibrosis (PASC-F)" Biomedicines 13, no. 4: 796. https://doi.org/10.3390/biomedicines13040796
APA StyleCreyns, B., MacKenzie, B., Jannini Sa, Y. A. P., Coelho, A. L., Christensen, D., Parimon, T., Windsor, B., & Hogaboam, C. M. (2025). Caveolin Scaffolding Domain (CSD) Peptide LTI-2355 Modulates the Phagocytic and Synthetic Activity of Lung-Derived Myeloid Cells in Idiopathic Pulmonary Fibrosis (IPF) and Post-Acute Sequelae of COVID Fibrosis (PASC-F). Biomedicines, 13(4), 796. https://doi.org/10.3390/biomedicines13040796







