The Influence of Pituitary Morphology on the Occurrence of Hormonal Disorders in Patients with Empty Sella or Partial Empty Sella
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lundholm, M.D.; Yogi-Morren, D. A comprehensive review of empty sella and empty sella syndrome. Endocr. Pract. 2024, 30, 497–502. [Google Scholar] [PubMed]
- Prabhat, N.; Kaur, K.; Takkar, A.; Ahuja, C.; Katoch, D.; Goyal, M.; Dutta, P.; Bhansali, A.; Lal, V. Pituitary Dysfunction in Idiopathic Intracranial Hypertension: An Analysis of 80 Patients. Can. J. Neurol. Sci. 2024, 51, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Rice-Canetto, T.E.; Carroll, P.; Reier, L.; Siddiqi, J. Asymptomatic Empty Sella: A Literature Review and Suggestions for Evaluation in Clinical Practice. Cureus 2024, 18, e75965. [Google Scholar]
- Saindane, A.M.; Lim, P.P.; Aiken, A.; Chen, Z.; Hudgins, P.A. Factors determining the clinical significance of an “empty” sella turcica. Am. J. Roentgenol. 2013, 200, 1125–1131. [Google Scholar]
- Sage, M.R.; Blumbergs, P.C. Primary empty sella turcica: A radiological-anatomical correlation. Australas. Radiol. 2000, 44, 341–348. [Google Scholar]
- Carosi, G.; Brunetti, A.; Mangone, A.; Baldelli, R.; Tresoldi, A.; Del Sindaco, G.; Lavezzi, E.; Sala, E.; Mungari, R.; Maria Fatti, L.M.; et al. A multicenter cohort study in patients with primary empty sella: Hormonal and neuroradiological features over a long follow-up. Front. Endocrinol. 2022, 13, 925378. [Google Scholar]
- Sorrentino, F.P.; Chiloiro, S.; Giampietro, A.; Bianchi, A.; Pontecorvi, A.; De Marinis, L. Empty sella syndrome: An update. Pituitary 2024, 30, 13. [Google Scholar]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Tartaglione, T.; Capobianco, A.; Anile, C.; De Marinis, L. Diagnosis of endocrine disease: Primary empty sella: A comprehensive review. Eur. J. Endocrinol. 2017, 177, 275–285. [Google Scholar]
- Auer, M.K.; Stieg, M.R.; Crispin, A.; Sievers, C.; Stalla, G.K.; Kopczak, A. Primary Empty Sella Syndrome and the Prevalence of Hormonal Dysregulation. Dtsch. Arztebl. Int. 2018, 115, 99–105. [Google Scholar]
- Guitelman, M.; Garcia Basavilbaso, N.; Vitale, M.; Chervin, A.; Katz, D.; Miragaya, K.; Herrera, J.; Cornalo, D.; Servidio, M.; Boero, L.; et al. Primary empty sella (PES): A review of 175 cases. Pituitary 2013, 16, 270–274. [Google Scholar]
- Zuhur, S.S.; Kuzu, I.; Ozturk, F.Y.; Uysal, E.; Altuntas, Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: Do all cases need endocrinological evaluation? Turk. Neurosurg. 2014, 24, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Lupi, I.; Manetti, L.; Raffaelli, V.; Grasso, L.; Sardella, C.; Cosottini, M.; Iannelli, A.; Gasperi, M.; Bogazzi, F.; Caturegli, P.; et al. Pituitary autoimmunity is associated with hypopituitarism in patients with primary empty sella. J. Endocrinol. Investig. 2011, 34, 240–244. [Google Scholar]
- Cannavò, S.; Curtò, L.; Venturino, M.; Squadrito, S.; Almoto, B.; Narbone, M.C.; Rao, R.; Trimarchi, F. Abnormalities of hypothalamic-pituitary-thyroid axis in patients with primary empty sella. J. Endocrinol. Investig. 2002, 25, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Cotta, O.R.; Ferone, D.; Torre, M.L.; Ferraù, F.; Di Somma, C.; Boschetti, M.; Teti, C.; Maria CSavanelli Alibrandi, A.; Trimarchi, F.; et al. Role of pituitary dysfunction on cardiovascular risk in primary empty sella patients. Clin. Endocrinol. 2013, 79, 211–216. [Google Scholar] [CrossRef]
- Akkus, G.; Sözütok, S.; Odabaş, F.; Onan, B.; Evran, M.; Karagun, B.; Sert, M.; Tetiker, T. Pituitary Volume in Patients with Primary Empty Sella and Clinical Relevance to Pituitary Hormone Secretion: A Retrospective Single Center Study. Curr. Med. Imaging 2021, 17, 1018–1024. [Google Scholar] [CrossRef]
- Fleckman, A.M.; Schubart, U.K.; Danziger, A.; Fleischer, N. Empty sella of normal size in Sheehan’s syndrome. Am. J. Med. 1983, 75, 585–591. [Google Scholar] [CrossRef]
- Terano, T.; Seya, A.; Tamura, Y.; Yoshida, S.; Hirayama, T. Characteristics of the pituitary gland in elderly subjects from magnetic resonance images: Relationship to pituitary hormone secretion. Clin. Endocrinol. 1996, 45, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Grams, A.E.; Gempt, J.; Stahl, A.; Förschler, A. Female pituitary size in relations to age and hormonal factors. Neuroendocrinology 2010, 92, 128–132. [Google Scholar] [CrossRef]
- Berntsen, E.M.; Haukedal, M.D.; Häberg, A.K. Normative data for pituitary size and volume in the general populatin between 50 and 66 years. Pituitary 2021, 24, 737–745. [Google Scholar] [CrossRef]
- Atmaca, M.; Baykara, S.; Mermi, O.; Yildirim, H.; Akaslan, U. Pituitary volumes are changed in patients with conversion disorder. Brain Imaging Behav. 2016, 10, 92–95. [Google Scholar] [CrossRef]
- Pepe, J.S.; Brouwer, R.M.; van Leeuwen, M. HPG—axis hormones during puberty: A study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology 2010, 35, 133–140. [Google Scholar]
- Simmons, J.G.; Whittle, S.L.; Patton, G.C. Study protocol: Imaging brain development in the Childhood to adolescence Transition study (iCATS). BMC Pediatr. 2014, 14, 115. [Google Scholar] [CrossRef]
- Atci, I.B.; Yilmaz, H.; Karagoz, Y.; Kocak, A. Prognosis of Hormonal Deficits in Empty Sella Syndrome Using Neuroimaging. Asian J. Neurosurg. 2018, 13, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Ekhzaimy, A.A.; Mujammami, M.; Tharkar, S.; Alansary, M.A.; Al Otaibi, D. Clinical presentation, evaluation and case management of primary empty sella syndrome: A retrospective analysis of 10-year single-center patient data. BMC Endocr. Disord. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, L.; Bonadonna, S.; Bianchi, A.; Maira, G.; Giustina, A. Primary empty sella. J. Clin. Endocrinol. Metab. 2005, 90, 5471–5477. [Google Scholar] [CrossRef]
- Del Monte, P.; Foppiani, L.; Cafferata, C.; Marugo, A.; Bernasconi, D. Primary “empty sella” in adults: Endocrine findings. Endocr. J. 2006, 53, 803–809. [Google Scholar] [CrossRef]
- Leca, B.M.; Mytilinaiou, M.; Tsoli, M.; Epure, A.; Aylwin, S.J.B.; Kaltsas, G.; Randeva, H.S.; Dimitriadis, G.K. Identification of an optimal prolactin threshold to determine prolactinma size using receiver perating characteristic analysis. Sci. Rep. 2021, 11, 9801. [Google Scholar] [CrossRef]
- Alyami, N.; Alhenaki, G.; Al Atwah, S.; Alhenaki, N.; Smaisem, F.; Alotaibi, A.; Abu Risheh j Smaisem, M.; Alhenaki, A.; Alanazi, S.; Alshammeri, M.; et al. Correlation between MRI findings of pituitary gland and prolactin level among hyperprolactinemia adult female Saudi patients in rural areas: A retrospective multicentric study. Medicine 2025, 104, e40686. [Google Scholar] [CrossRef]
- Puliani, G.; Sbardella, E.; Cozzolino, A.; Sada, V.; Tozzi, R.; Andreoli Ch Fiorelli, M.; Di Basi, C.; Corallino, D.; Bala, A.; Paganini, A.M.; et al. Pituitary T1 signal intensity at magnetic resonance imaging is reduced in patients with obesity: Results from the CHIASM study. Int. J. Obes. 2023, 47, 948–955. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; De Marinis, L. Empty sella syndrome: Multiple endocrine disorders. Handb. Clin. Neurol. 2021, 181, 29–40. [Google Scholar]
- Gasperi, M.; Aimaretti, G.; Cecconi, E.; Colao, A.; Di Somma, C.; Cannavò, S.; Baffoni, C.; Cosottini, M.; Curtò, L.; Trimarchi, F.; et al. Impairment of GH secretion in adults with primary empty sella. J. Endocrinol. Investig. 2002, 25, 329–333. [Google Scholar]
- Foresti, M.; Guidali, A.; Susanna, P. Primary empty sella. Incidence in 500 asymptomatic subjects examined with magnetic resonance. Radiol. Med. 1991, 81, 803–807. [Google Scholar] [PubMed]
- Ran Ch Peng, G.; Shen, R.; Liao, Q.; Wang, Q.; Zhou, L.; Zheng, H.; Long, M. Efficacy of GnRH Pulses in Hypogonadism secondary to Primary Empty Sella: Case report. Reprod. Sci. 2024, 31, 3892–3898. [Google Scholar]
- Belay, K.E.; Jemal, R.H.; Kebede, A.H.; Tulu, M.G.; Belay, A.E.; Haile, A.M.; Demisse, S.A. Arginine vasopressin deficiency (central diabetes insipidus) with partial empty sella: A case report. BMC Endocr. Disord. 2024, 24, 211. [Google Scholar]
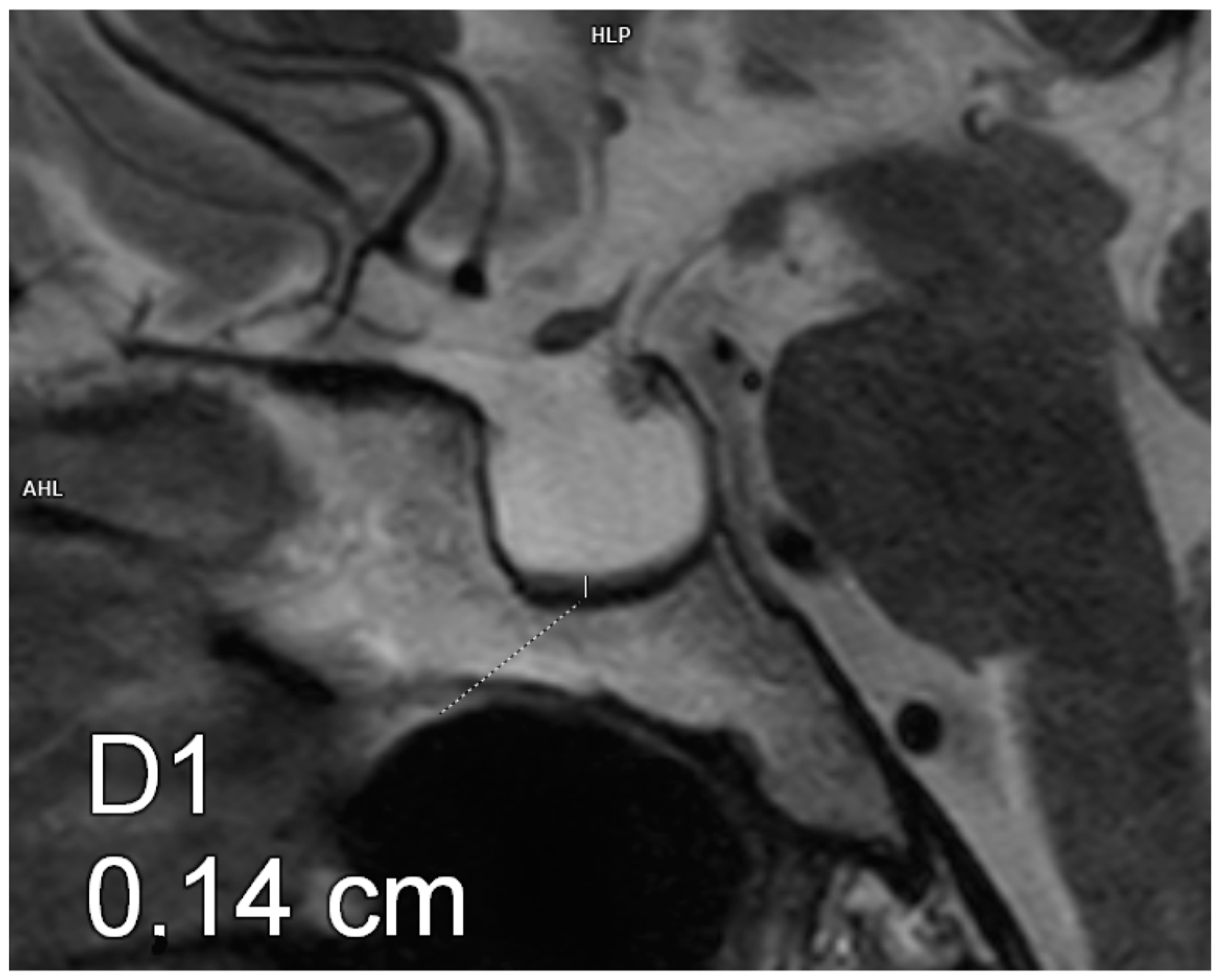
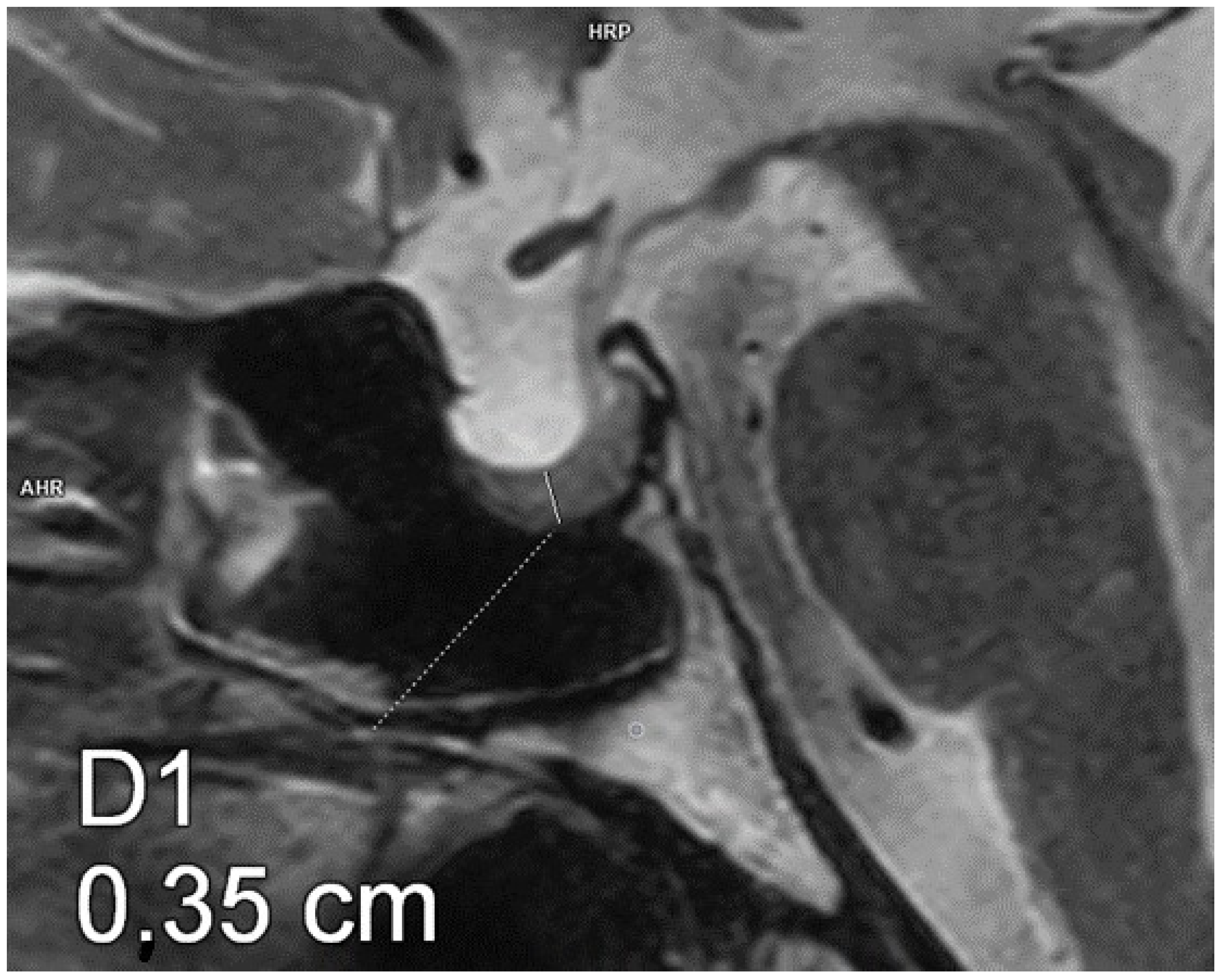
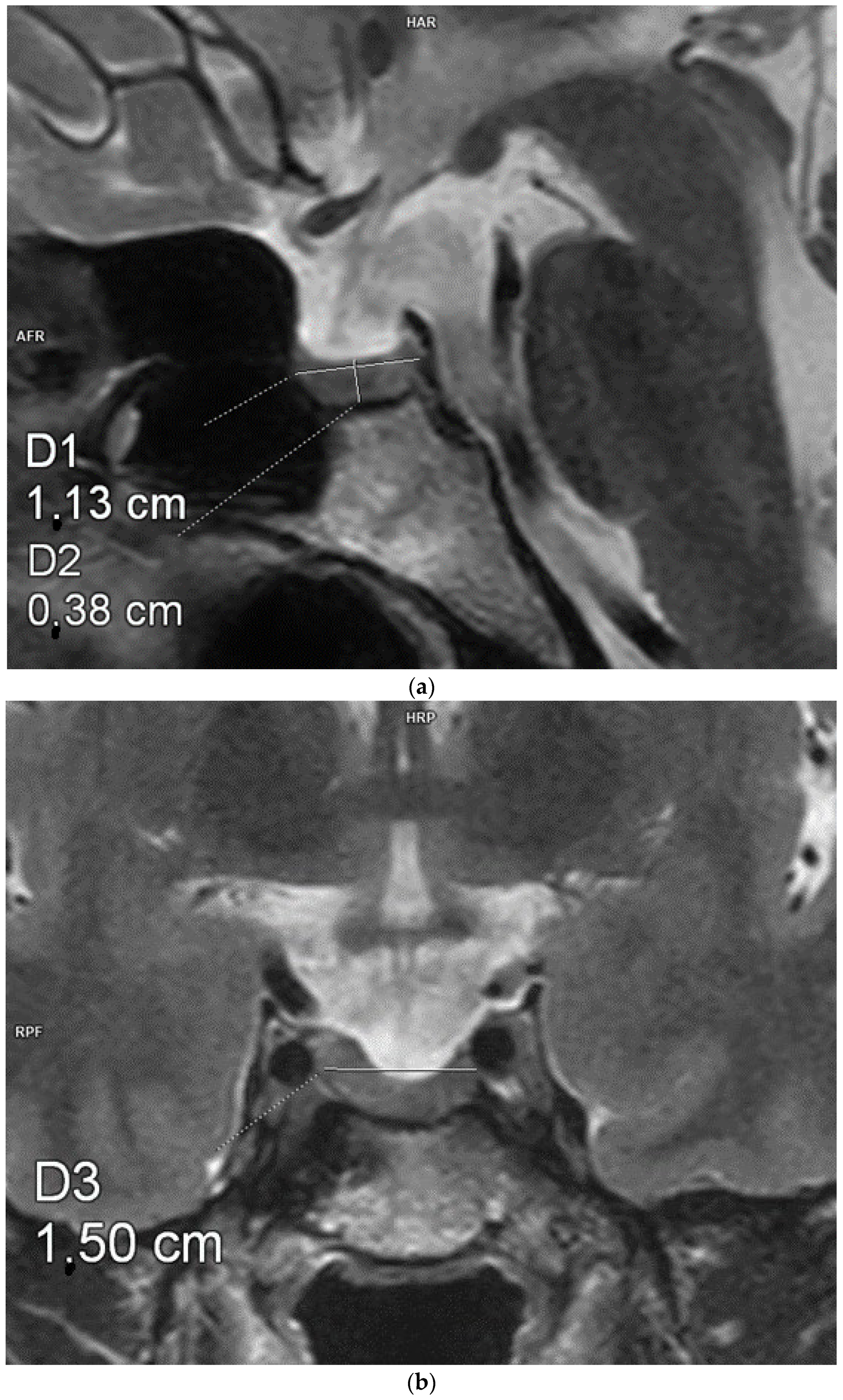

| VARIABLE | GROUP 1 (n = 20) | GROUP 2 (n = 23) | p-VALUE |
|---|---|---|---|
| Age [years] | 54.8 ± 12.4 | 59.5 ± 16.4 | 0.271 |
| Sex [M] | 6 (30%) | 3 (13%) | 0.173 |
| Body weight [kg] | 76.6 ± 14.55 | 78.9 ± 13.01 | 0.588 |
| BMI [kg/m2] | 25.8 ± 6.5 | 29.7 ± 4.8 | 0.039 |
| Waist circumference [cm] | 90.7 ± 11.7 | 94.7 ± 14.1 | 0.208 |
| Hypertension [n, %] | 6 (31.6%) | 11 (55%) | 0.141 |
| Normal thyroid function [n, %] | 19 (95%) | 22 (95.7%) | 0.919 |
| Primary hypothyroidism [n, %] | 4 (20%) | 6 (26.1%) | 0.522 |
| Hyperprolactinemia [n, %] | 2 (10%) | 2 (8.7%) | 0.883 |
| Secondary adrenocortical insufficiency [n, %] | 0 (0%) | 3 (13.04%) | 0.046 |
| VARIABLE | GROUP 1 (n = 20) | GROUP 2 (n = 23) | p-VALUE |
|---|---|---|---|
| Pituitary AP diameter [mm] | 11.4 ± 1.4 | 13.2 ± 1.9 | 0.003 |
| Pituitary transverse diameter [mm] | 14.8 ± 2.9 | 17.2 ± 2.9 | 0.016 |
| Pituitary CC diameter [mm] | 3.9 ± 0.62 | 2.2 ± 0.6 | 0.001 |
| Pituitary volume [mm3] | 332.8 ± 107.6 | 243.5 ± 70.9 | 0.001 |
| Pituitary concavity | 12 (60%) | 17 (73.9%) | 0.331 |
| Pituitary concavity in the coronal plane | 12 (60%) | 17 (73.9%) | 0.331 |
| Pituitary concavity in the sagittal plane | 11 (55%) | 17 (73.9%) | 0.194 |
| Pituitary stalk displacement | 7 (35%) | 17 (73.9%) | 0.010 |
| Anteroposterior pituitary stalk displacement | 7 (35%) | 16 (69.6%) | 0.023 |
| Lateral pituitary stalk displacement | 0 (0%) | 3 (13%) | 0.094 |
| Upper and middle sella turcica filled with CSF | 15 (75%) | 21 (91.3%) | 0.149 |
| VARIABLE | GROUP 1 (n = 20) | GROUP 2 (n = 23) | p-VALUE |
|---|---|---|---|
| Specific gravity of urine [g/mL] | 1.01 ± 0.001 | 1.01 ± 0.01 | 0.809 |
| Serum sodium [mmol/L] | 139.8 ± 3.65 | 141.3 ± 2.32 | 0.195 |
| Serum potassium [mmol/L] | 4.44 ± 0.38 | 4.51 ± 0.44 | 0.538 |
| Serum osmolality [mOsm/kg] | 291.3 ± 8.1 | 289.6 ± 4.9 | 0.621 |
| Urine osmolality [mOsm/kg] | 569.3 ± 215.7 | 570.4 ± 221.1 | 0.971 |
| TSH [µIU/mL] | 1.83 ± 0.92 | 2.06 ± 0.96 | 0.446 |
| fT3 [pg/mL] | 3.1 ± 0.4 | 3.1 ± 0.4 | 0.609 |
| fT4 [ng/dL] | 1.27 ± 0.2 | 1.23 ± 0.16 | 0.681 |
| Prolactin [ng/mL] | 12.6 ± 10.6 | 13.9 ± 7.1 | 0.171 |
| Cortisol at 8 a.m. [µg/dL] | 12.9 ± 3.5 | 14.7 ± 4.5 | 0.362 |
| ACTH at 8 a.m. [pg/mL] | 27.5 ± 13.2 | 21.8 ± 17.6 | 0.039 |
| Growth hormone [ng/mL] | 2.13 ± 4.1 | 0.83 ± 1.3 | 0.461 |
| IGF-1 [ng/mL] | 143.6 ± 47.1 | 152.6 ± 65.5 | 0.991 |
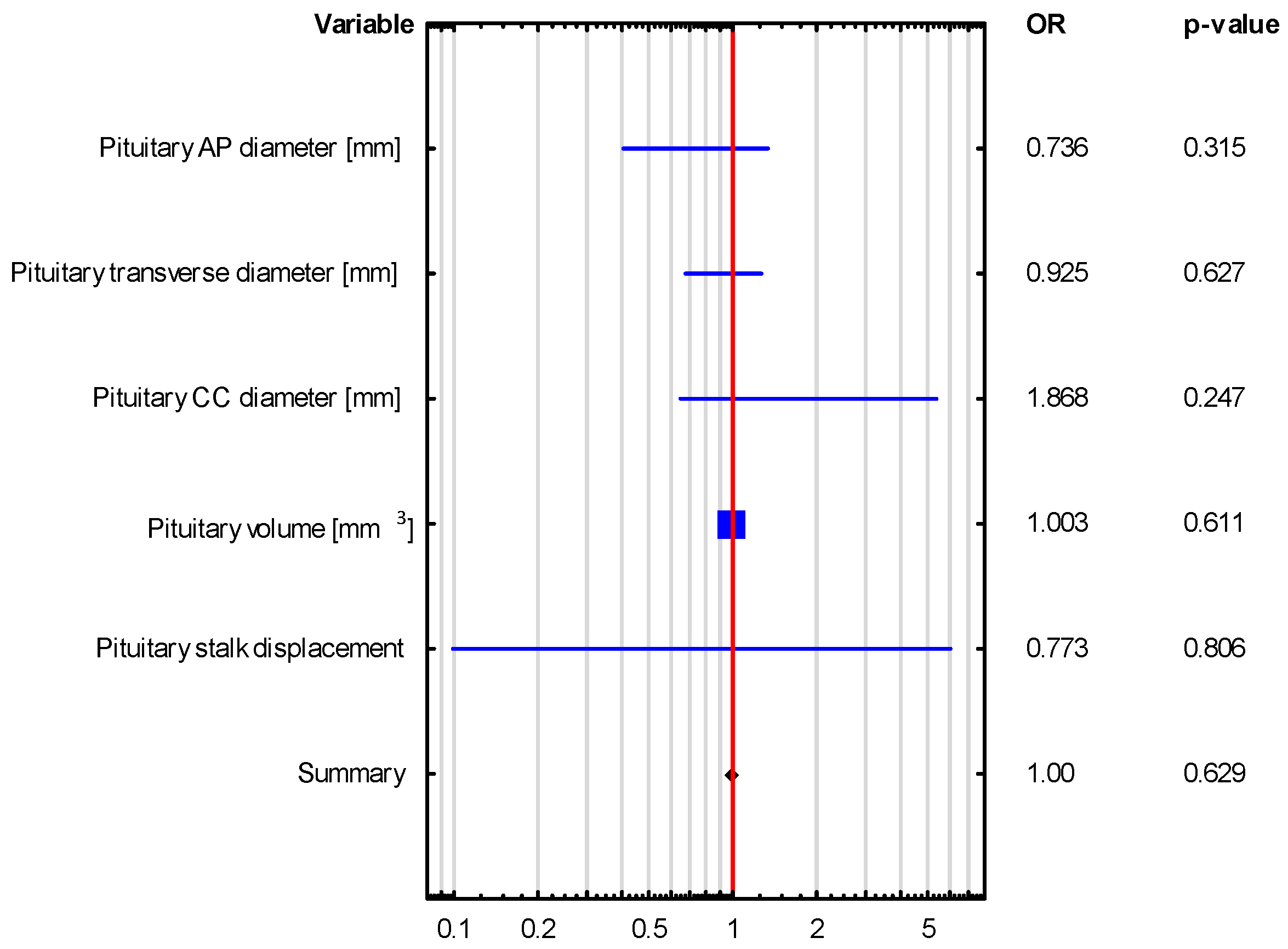
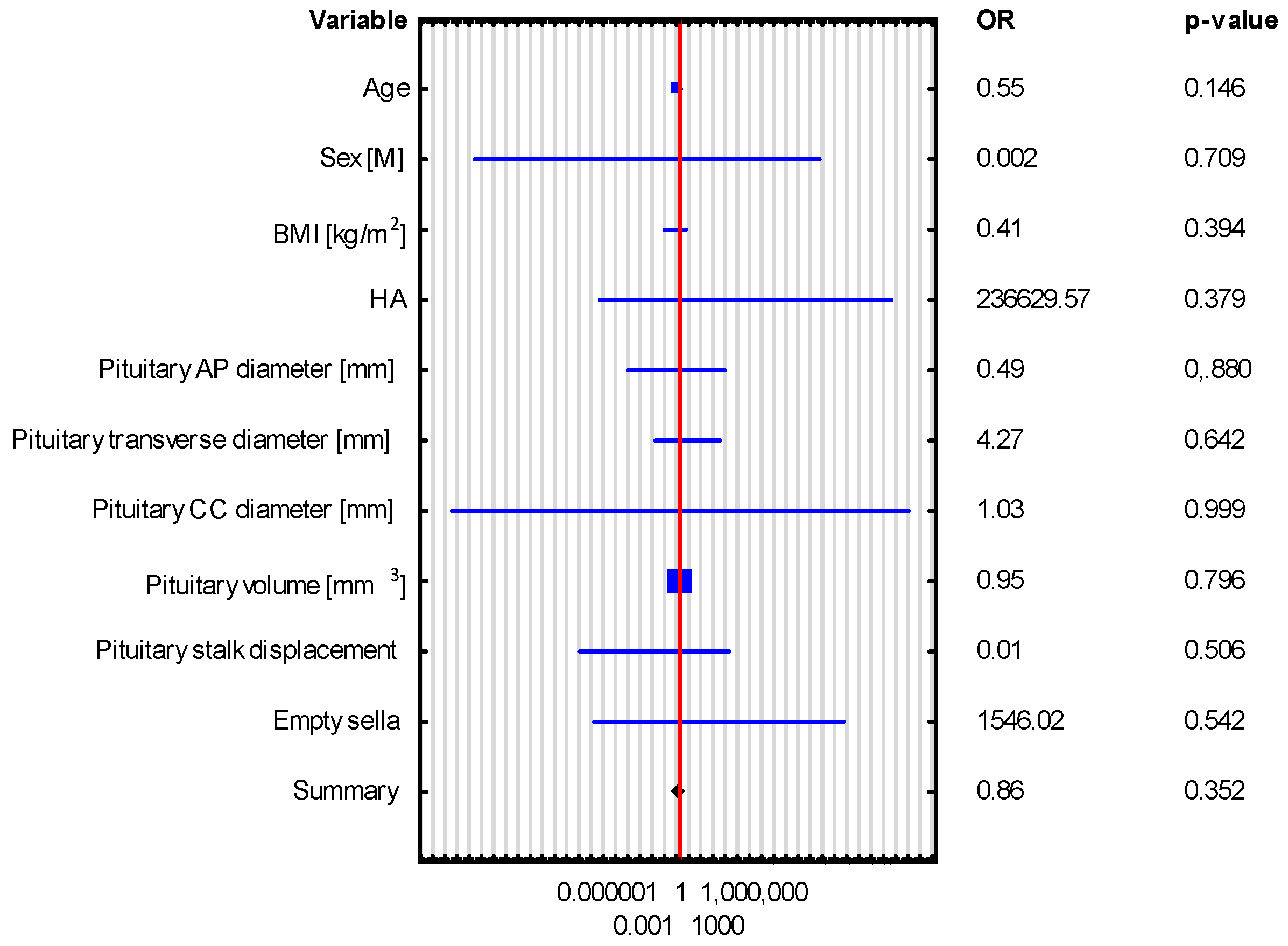
| VARIABLE | Adrenocortical Insufficiency OR (95% CI), p-Value | Hyperprolactinemia OR (95% CI), p-Value |
|---|---|---|
| The Whole Group n = 43 | The Whole Group n = 43 | |
| Pituitary AP diameter [mm] | 1.146 (0.639–2.053), p = 0.648 | 0.736 (0.405–1.338), p = 0.315 |
| Pituitary transverse diameter [mm] | 1.244 (0.846–1.830), p = 0.267 | 0.925 (0.674–1.268), p = 0.627 |
| Pituitary CC diameter [mm] | 0.48 (0.136–1.694), p = 0.254 | 1.868 (0.649–5.38), p = 0.247 |
| Pituitary volume [mm3] | 0.996 (0.984–1.009), p = 0.576 | 1.003 (0.992–1.013), p = 0.611 |
| Pituitary stalk displacement | 0.37 (0.031–4.418), p = 0.432 | 0.773 (0.099–6.061), p = 0.806 |
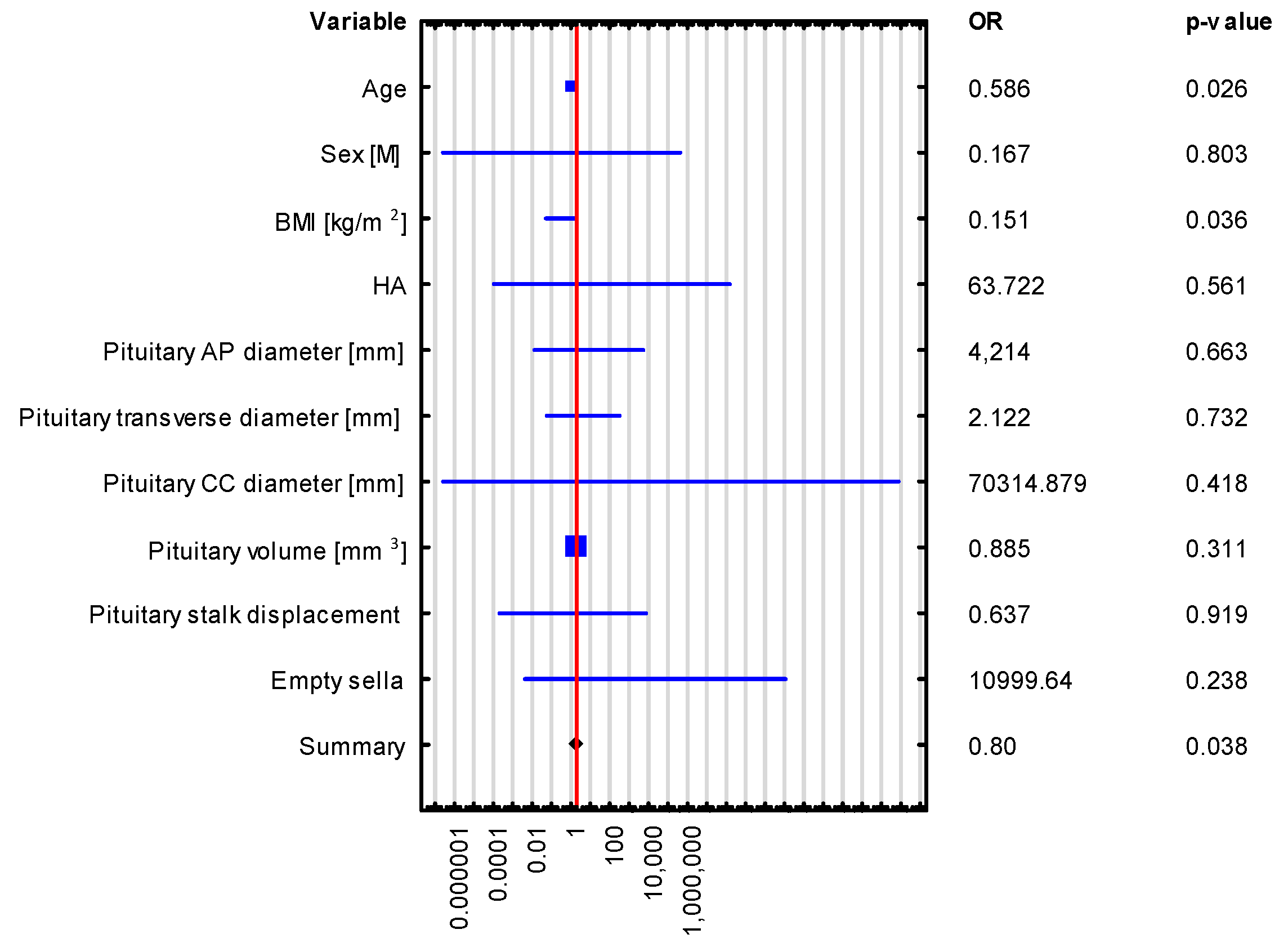
| VARIABLE | Adrenocortical Insufficiency OR (95% CI), p-Value | Hyperprolactinemia OR (95% CI), p-Value |
|---|---|---|
| The Whole Group n = 43 | The Whole Group n = 43 | |
| Age | 0.552 (0.247–1.231), p = 0.146 | 0.586 (0.365–0.939), p = 0.026 |
| Sex [M] | 0.002 (4.03245 × 10−17–1.39996 × 1011), p = 0.709 | 0.167 (1.34 × 10−7–208,177.659), p = 0.803 |
| BMI [kg/m2] | 0.411 (0.053–3.179), p = 0.394 | 0.151 (0.025–0.889), p = 0.036 |
| HTN | 236,629.574 (2.47841 × 10−7–2.25925 × 1017), p = 0.379 | 63.722 (5.13 × 10−5–79,090,557.431), p = 0.561 |
| Pituitary volume [mm3] | 0.954 (0.669–1.360), p = 0.796 | 0.885 (0.699–1.121), p = 0.311 |
| Pituitary AP diameter [mm] | 0.493 (3.75585 × 10−5–6481.229), p = 0.884 | 4.214 (0.006–2734.803), p = 0.663 |
| Pituitary transverse diameter [mm] | 4.273 (0.009–1957.818), p = 0.642 | 2.122 (0.028–158.944), p = 0.732 |
| Pituitary CC diameter [mm] | 1.028 (4.06766 × 10−18–2.59745 × 1017), p = 0.999 | 70,314.879 (1.33 × 10−7–3.70876 × 1016), p = 0.418 |
| Pituitary stalk displacement | 0.008 (4.92937 × 10−9–12,431.538), p = 0.506 | 0.637 (0.0001–3722.328), p = 0.919 |
| Empty sella | 1546.021 (8.7857 × 10−8–2.72054 × 1013), p = 0.542 | 10,999.64 (0.002–56,464,947,364.589), p = 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałuża, B.; Furmanek, M.; Domański, J.; Żuk-Łapan, A.; Babula, E.; Poprawa, I.; Landowska, M.; Jarząbek, K.; Popczyńska, J.; Filipowicz, P.; et al. The Influence of Pituitary Morphology on the Occurrence of Hormonal Disorders in Patients with Empty Sella or Partial Empty Sella. Biomedicines 2025, 13, 762. https://doi.org/10.3390/biomedicines13040762
Kałuża B, Furmanek M, Domański J, Żuk-Łapan A, Babula E, Poprawa I, Landowska M, Jarząbek K, Popczyńska J, Filipowicz P, et al. The Influence of Pituitary Morphology on the Occurrence of Hormonal Disorders in Patients with Empty Sella or Partial Empty Sella. Biomedicines. 2025; 13(4):762. https://doi.org/10.3390/biomedicines13040762
Chicago/Turabian StyleKałuża, Bernadetta, Mariusz Furmanek, Jan Domański, Aleksandra Żuk-Łapan, Emilia Babula, Iga Poprawa, Małgorzata Landowska, Karolina Jarząbek, Justyna Popczyńska, Paulina Filipowicz, and et al. 2025. "The Influence of Pituitary Morphology on the Occurrence of Hormonal Disorders in Patients with Empty Sella or Partial Empty Sella" Biomedicines 13, no. 4: 762. https://doi.org/10.3390/biomedicines13040762
APA StyleKałuża, B., Furmanek, M., Domański, J., Żuk-Łapan, A., Babula, E., Poprawa, I., Landowska, M., Jarząbek, K., Popczyńska, J., Filipowicz, P., Wielgolewska, M., Walecki, J., & Franek, E. (2025). The Influence of Pituitary Morphology on the Occurrence of Hormonal Disorders in Patients with Empty Sella or Partial Empty Sella. Biomedicines, 13(4), 762. https://doi.org/10.3390/biomedicines13040762





