The Hospital Frailty Risk Score as a Predictor of Mortality, Complications, and Resource Utilization in Heart Failure: Implications for Managing Critically Ill Patients
Abstract
1. Introduction
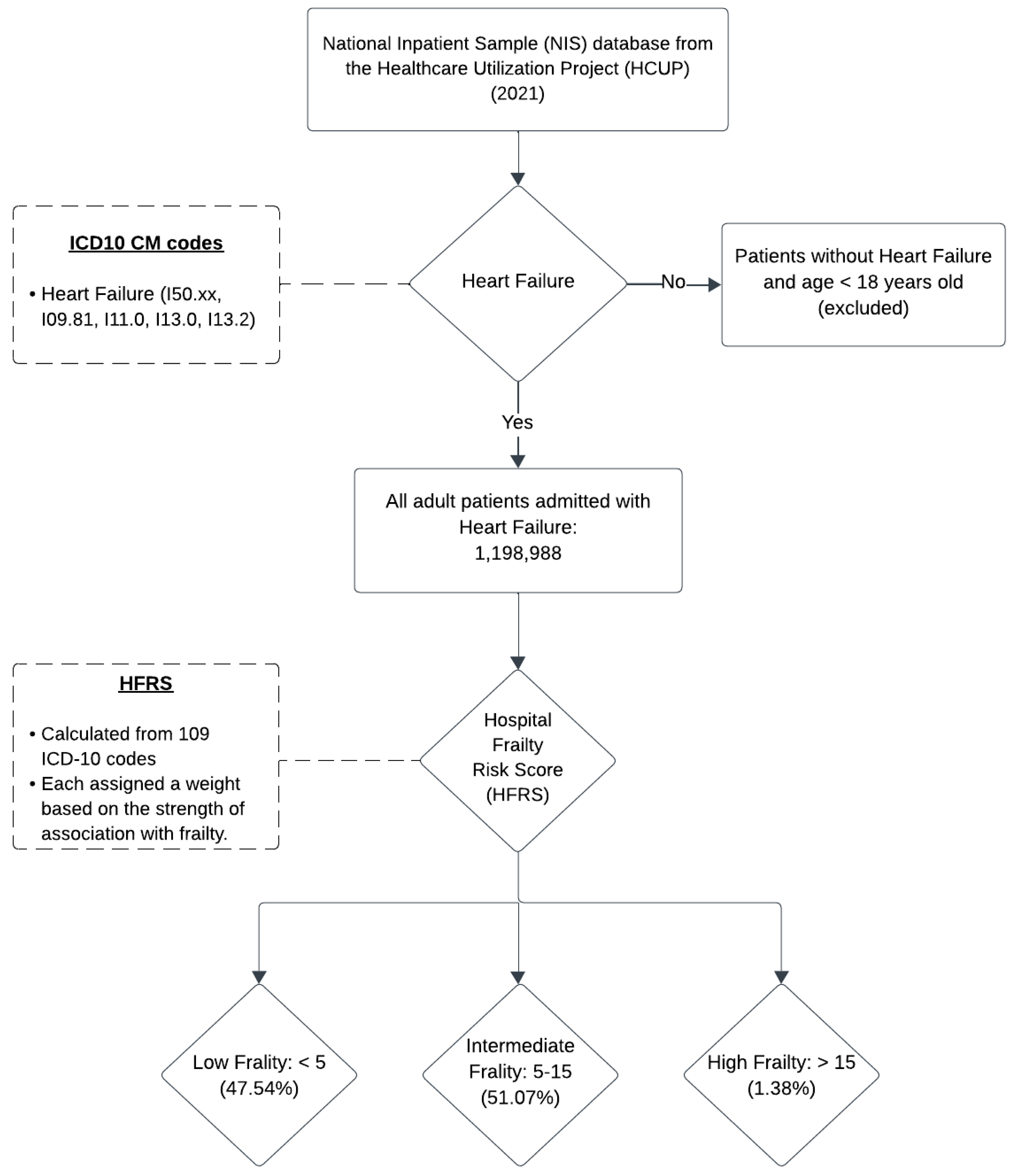
2. Methods
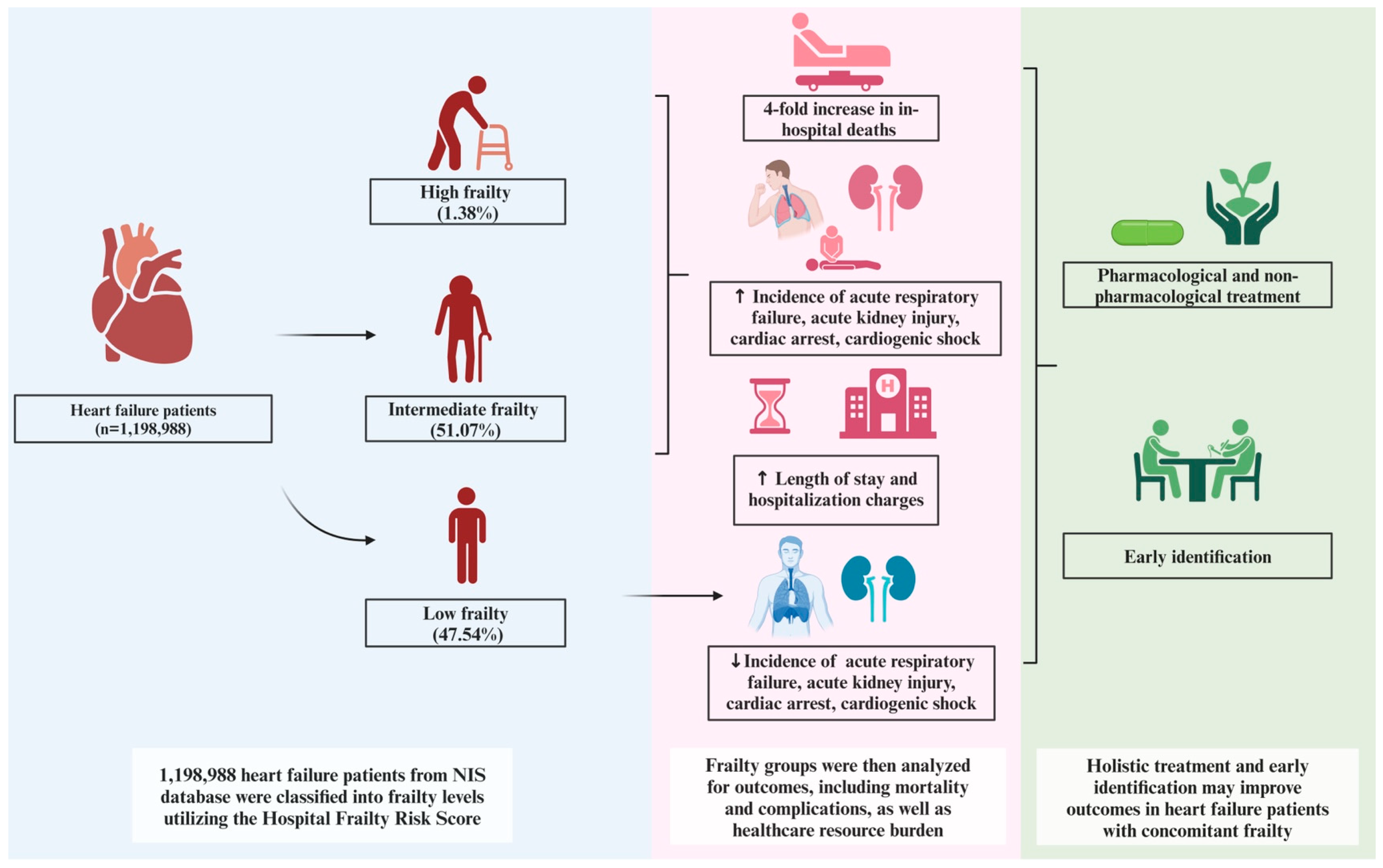
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome: Mortality
3.3. Secondary Outcomes
3.3.1. Resource Utilization: Length of Stay and Hospital Charges
3.3.2. Complications
3.3.3. Predictors of In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, Q.-L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salmon, T.; Essa, H.; Tajik, B.; Isanejad, M.; Akpan, A.; Sankaranarayanan, R. The Impact of Frailty and Comorbidities on Heart Failure Outcomes. Card. Fail. Rev. 2022, 8, e07. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kitzman, D.; Reeves, G. Frailty is Intertwined with Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019, 7, 1001–1011. [Google Scholar] [CrossRef]
- Dewan, P.; Jackson, A.; Jhund, P.S.; Shen, L.; Ferreira, J.P.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.; et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—An analysis of PARADIGM-HF and ATMOSPHERE. Eur. J. Heart Fail. 2020, 22, 2123–2133. [Google Scholar] [CrossRef]
- Pandey, A.; Segar, M.W.; Singh, S.; Reeves, G.R.; O’connor, C.; Piña, I.; Whellan, D.; Kraus, W.E.; Mentz, R.J.; Kitzman, D.W. Frailty Status Modifies the Efficacy of Exercise Training Among Patients with Chronic Heart Fail-ure and Reduced Ejection Fraction: An Analysis From the HF-ACTION Trial. Circulation 2022, 146, 80–90. [Google Scholar] [CrossRef]
- Yang, X.; Lupón, J.; Vidán, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayés-Genís, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef]
- Goldwater, D.S.; Pinney, S.P. Frailty in Advanced Heart Failure: A Consequence of Aging or a Separate Entity? Clin. Med. Insights Cardiol. 2015, 9 (Suppl. S2), 39–46. [Google Scholar] [CrossRef]
- Vitale, C.; Spoletini, I.; Rosano, G.M. The Dual Burden of Frailty and Heart Failure. Int. J. Heart Fail. 2024, 6, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Sharma, Y.; Horwood, C.; Hakendorf, P.; Shahi, R.; Thompson, C. External Validation of the Hospital Frailty-Risk Score in Predicting Clinical Outcomes in Older Heart-Failure Patients in Australia. J. Clin. Med. 2022, 11, 2193. [Google Scholar] [CrossRef]
- Chau, C.S.M.; Ee, S.C.E.; Huang, X.; Siow, W.S.; Tan, M.B.H.; Sim, S.K.R.; Chang, T.Y.; Kwok, K.M.; Ng, K.; Yeo, L.F.; et al. Frailty-aware surgical care: Validation of Hospital Frailty Risk Score (HFRS) in older surgi-cal patients. Ann. Acad. Med. Singap. 2024, 53, 90–100. [Google Scholar] [CrossRef]
- Dodson, J.A.; Chaudhry, S.I. Geriatric conditions in heart failure. Curr. Cardiovasc. Risk Rep. 2012, 6, 404–410. [Google Scholar] [CrossRef]
- Gastelurrutia, P.; Lupón, J.; Altimir, S.; de Antonio, M.; González, B.; Cabanes, R.; Rodríguez, M.; Urrutia, A.; Domingo, M.; Zamora, E.; et al. Fragility is a key determinant of survival in heart failure patients. Int. J. Cardiol. 2014, 175, 62–66. [Google Scholar] [CrossRef]
- Chiarantini, D.; Volpato, S.; Sioulis, F.; Bartalucci, F.; Del Bianco, L.; Mangani, I.; Pepe, G.; Tarantini, F.; Berni, A.; Marchionni, N.; et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J. Card. Fail. 2010, 16, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Segar, M.W.; Usman, M.S.; Singh, S.; Greene, S.J.; Fonarow, G.C.; Anker, S.D.; Felker, G.M.; Januzzi, J.L., Jr.; Butler, J.; et al. Frailty, guideline-directed medical therapy, and outcomes in HFrEF: From the GUIDE-IT trial. JACC Heart Fail. 2022, 10, 266–275. [Google Scholar] [PubMed]
- Vitale, C.; Spoletini, I.; Rosano, G.M.C. Frailty in Heart Failure: Implications for Management. Card. Fail. Rev. 2018, 4, 104–106. [Google Scholar] [CrossRef]
- McDonagh, J.; Martin, L.; Ferguson, C.; Jha, S.R.; Macdonald, P.S.; Davidson, P.M.; Newton, P.J. Frailty assessment instruments in heart failure: A systematic review. Eur. J. Cardiovasc. Nurs. 2017, 17, 23–35. [Google Scholar] [CrossRef]
- Sciatti, E.; Lombardi, C.; Ravera, A.; Vizzardi, E.; Bonadei, I.; Carubelli, V.; Gorga, E.; Metra, M. Nutritional Deficiency in Patients with Heart Failure. Nutrients 2016, 8, 442. [Google Scholar] [CrossRef]
- Hamada, T.; Kubo, T.; Kawai, K.; Nakaoka, Y.; Yabe, T.; Furuno, T.; Yamada, E.; Kitaoka, H.; Kochi YOSACOI Study. Frailty interferes with the guideline-directed medical therapy in heart failure patients with reduced ejection fraction. ESC Heart Fail. 2022, 10, 223–233. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Squire, I.B.; Clark, A.L. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1249–1258. [Google Scholar] [CrossRef]
- Reeves, G.R.; Whellan, D.J.; O’Connor, C.M.; Duncan, P.; Eggebeen, J.D.; Morgan, T.M.; Hewston, L.A.; Pastva, A.; Patel, M.J.; Kitzman, D.W. A Novel Rehabilitation Intervention for Older Patients with Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail. 2017, 5, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
| Frail | Non-Frail | p Value | |
|---|---|---|---|
| Total (%) of heart failure admissions | 52.45 | 47.55 | |
| Age (Median) | 72.49 | 68.78 | <0.01 |
| Female gender (%) | 49.72 | 43.75 | <0.01 |
| Race | <0.01 | ||
| Caucasian | 65.92 | 63.03 | |
| African American | 20.23 | 23.12 | |
| Hispanic | 8.76 | 8.9 | |
| Asian or Pacific Islander | 2.38 | 2.17 | |
| Native American | 0.53 | 0.62 | |
| Others | 2.17 | 2.17 | |
| Median income in patient’s zip code (%) | <0.01 | ||
| USD 1–47,999 | 76.72 | 66.22 | |
| USD 48,000–60,999 | 10.9 | 15.29 | |
| USD 61,000–81,999 | 10.24 | 14.18 | |
| ≥USD 82,000 | 2.14 | 4.31 | |
| Charlson Comorbidity Index (%) | 0.12 | ||
| 1 | 4.43 | 13.85 | |
| 2 | 9.18 | 20.92 | |
| 3 or more | 86.39 | 65.23 | |
| Hospital region | <0.01 | ||
| Northeast | 18.21 | 18.58 | |
| Midwest | 24.39 | 21.33 | |
| South | 40.18 | 42.54 | |
| West | 17.22 | 17.56 | |
| Hospital bed size (%) | <0.01 | ||
| Small | 23.81 | 25.62 | |
| Medium | 28.48 | 28.84 | |
| Large | 47.71 | 45.54 | |
| Hospital location (%) | <0.01 | ||
| Rural | 9.07 | 10.53 | |
| Urban | 90.93 | 89.47 | |
| Hospital teaching (%) | <0.01 | ||
| Non-teaching (%) | 27.33 | 29.64 | |
| Teaching (%) | 72.67 | 70.36 | |
| Insurance type (%) | <0.01 | ||
| Medicaid | 76.72 | 66.22 | |
| Medicare | 10.9 | 15.29 | |
| Private | 10.24 | 14.18 | |
| Uninsured | 2.14 | 4.31 |
| Variable | Low Frailty | Intermediate Frailty | High Frailty | p Value |
|---|---|---|---|---|
| Deaths (%) | 1.06 | 4.68 | 10.42 | <0.01 |
| Complications (%) | ||||
| Cardiogenic shock | 2.91 | 3.55 | 2.82 | <0.01 |
| Cardiac arrest | 0.73 | 0.89 | 1 | <0.01 |
| Acute kidney injury | 35.31 | 38.65 | 37.75 | <0.01 |
| Acute respiratory failure | 39.06 | 30.5 | 33.9 | <0.01 |
| Resource Use | ||||
| LOS, d | 4.24 (4.19–4.29) | 7.19 (7.09–7.28) | 12.1 (11.55–12.65) | <0.01 |
| Hospital cost, USD | 49,081 (47,667–50,495) | 84,472 (81,105–87,839) | 129,516 (116,874–142,158) | <0.01 |
| Outcomes (Intermediate vs. Low Frailty) | aOR; 95% CI | p Value |
|---|---|---|
| Mortality | 4.26 (3.98–4.56) | <0.01 |
| Cardiogenic Shock | 3.59 (3.33–3.86) | <0.01 |
| Cardiac Arrest | 4.48 (3.98–5.03) | <0.01 |
| Acute Kidney Injury | 4.86 (4.75–4.97) | <0.01 |
| Acute Respiratory Failure | 2.58 (2.53–2.64) | <0.01 |
| Outcomes (High vs. Low Frailty) | ||
| Mortality | 9.32 (8.14–10.67) | <0.01 |
| Cardiogenic Shock | 6.43 (5.43–7.61) | <0.01 |
| Cardiac Arrest | 9.54 (7.51–12.11) | <0.01 |
| Acute Kidney Injury | 12.02 (11.03–13.09) | <0.01 |
| Acute Respiratory Failure | 3.67 (3.39–3.97) | <0.01 |
| Variable | Adjusted Odds Ratio (aOR) | p Value |
|---|---|---|
| Age | 1.02 (1.01–1.03) | <0.01 |
| Female sex | 0.88 (0.83–0.93) | <0.01 |
| Charlson Comorbidity Index | 1.02 (1.01–1.04) | <0.01 |
| Income Quartile | ||
| USD 1–47,999 | Reference | |
| USD 48,000–60,999 | 1.11 (1.04–1.18) | <0.01 |
| USD 61,000–81,999 | 1.02 (0.95–1.09) | 0.65 |
| ≥USD 82,000 | 0.98 (0.91–1.07) | 0.70 |
| Hospital Teaching Status | ||
| Non-Teaching | Reference | |
| Teaching | 1.12 (1.04–1.22) | <0.01 |
| Hospital Bed Size | ||
| Small | Reference | |
| Medium | 1.02 (0.94–1.11) | 0.64 |
| Large | 1.18 (1.09–1.28) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, N.; Kwak, E.S.; Mohamed, A.-R.; Aradhyula, V.; Qwaider, M.; Sherafati, A.; Assaly, R.; Eltahawy, E. The Hospital Frailty Risk Score as a Predictor of Mortality, Complications, and Resource Utilization in Heart Failure: Implications for Managing Critically Ill Patients. Biomedicines 2025, 13, 760. https://doi.org/10.3390/biomedicines13030760
Bansal N, Kwak ES, Mohamed A-R, Aradhyula V, Qwaider M, Sherafati A, Assaly R, Eltahawy E. The Hospital Frailty Risk Score as a Predictor of Mortality, Complications, and Resource Utilization in Heart Failure: Implications for Managing Critically Ill Patients. Biomedicines. 2025; 13(3):760. https://doi.org/10.3390/biomedicines13030760
Chicago/Turabian StyleBansal, Nahush, Eun Seo Kwak, Abdel-Rhman Mohamed, Vaishnavi Aradhyula, Mohanad Qwaider, Alborz Sherafati, Ragheb Assaly, and Ehab Eltahawy. 2025. "The Hospital Frailty Risk Score as a Predictor of Mortality, Complications, and Resource Utilization in Heart Failure: Implications for Managing Critically Ill Patients" Biomedicines 13, no. 3: 760. https://doi.org/10.3390/biomedicines13030760
APA StyleBansal, N., Kwak, E. S., Mohamed, A.-R., Aradhyula, V., Qwaider, M., Sherafati, A., Assaly, R., & Eltahawy, E. (2025). The Hospital Frailty Risk Score as a Predictor of Mortality, Complications, and Resource Utilization in Heart Failure: Implications for Managing Critically Ill Patients. Biomedicines, 13(3), 760. https://doi.org/10.3390/biomedicines13030760






