Investigation into the Use of Surufatinib and Donafenib as Novel Multi-Kinase Inhibitors Therapeutic Agents in Managing Advanced Differentiated Thyroid Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
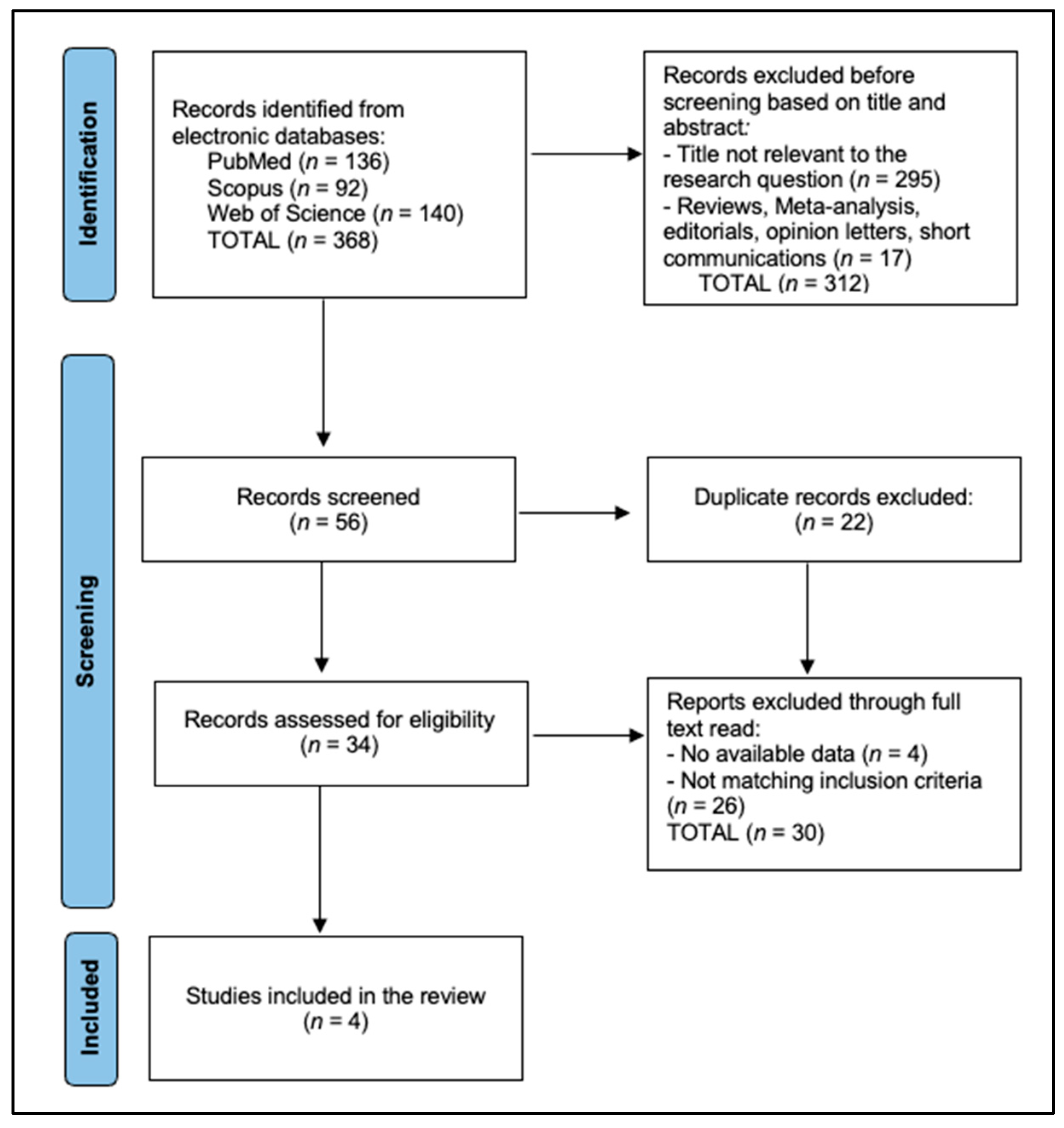
2.3. Selection Process
2.4. Data Collection and Quality Assessment
3. Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.M.; Schneider, D.F.; Leverson, G.; Chen, H.; Sippel, R.S. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: A population-based study of 10,740 cases. Thyroid 2013, 23, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, L.G.; Tuttle, R.M.; Davies, L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 709–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, C.J.; Lai, K.Y.; Lu, Y.L.; Wu, M.H.; Liu, F.H.; Lin, S.F. Outcomes of Patients With Metastatic Differentiated Thyroid Cancer After Excellent Response to Treatment. Front. Endocrinol. 2022, 13, 923182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-Refractory Thyroid Cancer: Molecular Basis of Redifferentiation Therapies, Management, and Novel Therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, J.M.; Ahn, B.C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251–6277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Satapathy, S.; Bal, C. Theranostic Options for Radioiodine-Refractory Differentiated Thyroid Carcinoma: Recent Advances, Challenges, and Road Ahead. Front. Endocrinol. 2022, 13, 924841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parvathareddy, S.K.; Siraj, A.K.; Siraj, N.; Ahmed, S.O.; Al-Rasheed, M.; Qadri, Z.; Siddiqui, K.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Kuraya, K.S. Radioactive iodine refractoriness in Middle Eastern differentiated thyroid cancer: Clinical outcome and risk factor analysis. Front. Endocrinol. 2024, 15, 1326976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Jundi, M.; Thakur, S.; Gubbi, S.; Klubo-Gwiezdzinska, J. Novel Targeted Therapies for Metastatic Thyroid Cancer-A Comprehensive Review. Cancers 2020, 12, 2104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laursen, R.; Wehland, M.; Kopp, S.; Pietsch, J.; Infanger, M.; Grosse, J.; Grimm, D. Effects and Role of Multikinase Inhibitors in Thyroid Cancer. Curr. Pharm. Des. 2016, 22, 5915–5926. [Google Scholar] [CrossRef] [PubMed]
- Shonka, D.C., Jr.; Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Abdelhamid Ahmed, A.H.; Bible, K.C.; Brose, M.S.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head. Neck 2022, 44, 1277–1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agosto Salgado, S.; Kaye, E.R.; Sargi, Z.; Chung, C.H.; Papaleontiou, M. Management of Advanced Thyroid Cancer: Overview, Advances, and Opportunities. Am. Soc. Clin. Oncol. Educ. Book. 2023, 43, e389708. [Google Scholar] [CrossRef] [PubMed]
- Goulart, B.H.L.; Unger, J.M.; Chennupati, S.; Fedorenko, C.R.; Ramsey, S.D. Out-of-Pocket Costs for Tyrosine Kinase Inhibitors and Patient Outcomes in EGFR- and ALK-Positive Advanced Non-Small-Cell Lung Cancer. JCO Oncol. Pract. 2021, 17, e130–e139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huot, J.R.; Essex, A.L.; Gutierrez, M.; Barreto, R.; Wang, M.; Waning, D.L.; Plotkin, L.I.; Bonetto, A. Chronic Treatment with Multi-Kinase Inhibitors Causes Differential Toxicities on Skeletal and Cardiac Muscles. Cancers 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talon, B.; Calip, G.S.; Lee, T.A.; Sharp, L.K.; Patel, P.; Touchette, D.R. Trend in Tyrosine Kinase Inhibitor Utilization, Price, and Out-of-Pocket Costs in Patients With Chronic Myelogenous Leukemia. JCO Oncol. Pract. 2021, 17, e1811–e1820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, S.; Li, J.; Gao, S.; Han, Y.; Han, X.; Wu, Y.; Bi, J.; Xu, M.; Bi, W. Sulfatinib, a novel multi-targeted tyrosine kinase inhibitor of FGFR1, CSF1R, and VEGFR1-3, suppresses osteosarcoma proliferation and invasion via dual role in tumor cells and tumor microenvironment. Front. Oncol. 2023, 13, 1158857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, X.; Liang, W.; Lv, G.; Ding, C.; Lai, H.; Li, L.; Zeng, Q.; Lv, B.; Sheng, L. Efficacy and safety of targeted therapeutics for patients with radioiodine-refractory differentiated thyroid cancer: Systematic review and network meta-analysis. Front. Pharmacol. 2022, 13, 933648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Ji, Q.; Bai, C.; Zheng, X.; Zhang, Y.; Shi, F.; Li, X.; Tang, P.; Xu, Z.; Huang, R.; et al. Surufatinib in Chinese Patients with Locally Advanced or Metastatic Differentiated Thyroid Cancer and Medullary Thyroid Cancer: A Multicenter, Open-Label, Phase II Trial. Thyroid. 2020, 30, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Huang, N.S.; Wei, W.J.; Hu, J.Q.; Cao, Y.M.; Shen, Q.; Lu, Z.W.; Wang, Y.L.; Wang, Y.; Ji, Q.H. The Efficacy and Safety of Surufatinib Combined with Anti PD-1 Antibody Toripalimab in Neoadjuvant Treatment of Locally Advanced Differentiated Thyroid Cancer: A Phase II Study. Ann. Surg. Oncol. 2023, 30, 7172–7180. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qin, S.; Yang, H.; Shi, F.; Yang, A.; Han, X.; Liu, B.; Li, Z.; Ji, Q.; Tang, L.; et al. Multicenter Randomized Double-Blind Phase III Trial of Donafenib in Progressive Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Clin. Cancer Res. 2023, 29, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Yang, H.; Ding, Y.; Cheng, Y.Z.; Shi, F.; Tan, J.; Deng, Z.Y.; Chen, Z.D.; Wang, R.F.; Ji, Q.H.; et al. Donafenib in Progressive Locally Advanced or Metastatic Radioactive Iodine-Refractory Differentiated Thyroid Cancer: Results of a Randomized, Multicenter Phase II Trial. Thyroid 2021, 31, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Surufatinib: First Approval. Drugs 2021, 81, 727–732. [Google Scholar] [CrossRef]
- Keam, S.J.; Duggan, S. Donafenib: First Approval. Drugs 2021, 81, 1915–1920. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Wu, Y. Donafenib as neoadjuvant therapy in locally advanced thyroid cancer: Protocol for the DONATHYCA phase II prospective single-arm trial in China. BMJ Open 2024, 14, e081090. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Smit, J.W.A.; Lin, C.C.; Tori, M.; Bowles, D.W.; Worden, F.; Shen, D.H.; Huang, S.M.; Tsai, H.J.; Alevizaki, M.; et al. Multikinase Inhibitors for the Treatment of Asymptomatic Radioactive Iodine-Refractory Differentiated Thyroid Cancer: Global Noninterventional Study (RIFTOS MKI). Thyroid 2022, 32, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Smit, J.; Lin, C.; Pitoia, F.; Fellous, M.; DeSanctis, Y.; Schlumberger, M.; Tori, M.; Sugitani, I. Timing of multikinase inhibitor initiation in differentiated thyroid cancer. Endocr.-Relat. Cancer 2017, 24, 237–242. [Google Scholar] [CrossRef]
- Feola, T.; Cozzolino, A.; Centello, R.; Pandozzi, C.; Tarsitano, M.G.; Giannetta, E. Predictors of Response and Survival to Multikinase Inhibitors in Radioiodine Resistant Differentiated Thyroid Cancer. J. Pers. Med. 2021, 11, 674. [Google Scholar] [CrossRef]
- Treistman, N.; Nobre, G.M.; Tramontin, M.Y.; Silva, G.M.; Herchenhorn, D.; Araujo, L.H.; Andrade, F.A.; Corbo, R.; Bulzico, D.; Vaisman, F. Prognostic factors in patients with advanced differentiated thyroid cancer treated with multikinase inhibitors—A single Brazilian center experience. Arch. Endocrinol. Metab. 2021, 65, 411–420. [Google Scholar] [CrossRef]
- Su, J.; Wang, M.; Fu, Y.; Yan, J.; Shen, Y.; Jiang, J.; Wang, J.; Lu, J.; Zhong, Y.; Lin, X.; et al. Efficacy and safety of multi-kinase inhibitors in patients with radioiodine-refractory differentiated thyroid cancer: A systematic review and meta-analysis of clinical trials. Expert. Rev. Anticancer Ther. 2022, 22, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Hofmann, M.C.; Iyer, P.C.; Cabanillas, M.E.; Hu, M.I.; Busaidy, N.L.; Dadu, R. Review article: New treatments for advanced differentiated thyroid cancers and potential mechanisms of drug resistance. Front. Endocrinol. 2023, 14, 1176731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, L.; Lai, S.Y.; Dong, W.; Feng, L.; Dadu, R.; Regone, R.M.; Cabanillas, M.E. Sorafenib in metastatic thyroid cancer: A systematic review. Oncologist 2014, 19, 251–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wirth, L.J.; Durante, C.; Topliss, D.J.; Winquist, E.; Robenshtok, E.; Iwasaki, H.; Luster, M.; Elisei, R.; Leboulleux, S.; Tahara, M. Lenvatinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer: Treatment Optimization for Maximum Clinical Benefit. Oncologist 2022, 27, 565–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brose, M.S.; Panaseykin, Y.; Konda, B.; de la Fouchardiere, C.; Hughes, B.G.M.; Gianoukakis, A.G.; Joo Park, Y.; Romanov, I.; Krzyzanowska, M.K.; Leboulleux, S.; et al. A Randomized Study of Lenvatinib 18 mg vs 24 mg in Patients With Radioiodine-Refractory Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2022, 107, 776–787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Josephs, D.H.; Fisher, D.S.; Spicer, J.; Flanagan, R.J. Clinical pharmacokinetics of tyrosine kinase inhibitors: Implications for therapeutic drug monitoring. Ther. Drug Monit. 2013, 35, 562–587. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cao, K.; Ren, G.; Qin, Z.; Zhao, D.; Li, N.; Chen, X.; Xia, Y.; Lu, Y. Effects of the ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of afatinib in healthy Chinese volunteers. Xenobiotica 2020, 50, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Y.; Shi, Y.; Xing, P.; Wang, L.; Feng, Y.; Han, X.; He, X. Sorafenib in metastatic radioactive iodine-refractory differentiated thyroid cancer: A pilot study. Mol. Clin. Oncol. 2014, 2, 87–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study and Author | Year | Country | Study Design | Sample Size | Study Population | Study Quality |
|---|---|---|---|---|---|---|
| Chen et al. [21] | 2021 | China | Phase II, Open-Label Trial | 59 | Locally Advanced/ Metastatic DTC | High |
| Chen et al. [22] | 2023 | China | Phase II, Single-Arm Study | 10 | Locally Advanced DTC | High |
| Lin et al. [23] | 2023 | China | Phase III, Randomized Controlled | 191 | Progressive RAIR-DTC | High |
| Lin et al. [24] | 2020 | China | Phase II, Randomized Trial | 35 | Locally Advanced/Metastatic RAIR-DTC | High |
| Study and Author | Mean Age (Years) | Sex (Male/Female) | Histological Subtype | Prior Treatments |
|---|---|---|---|---|
| Chen et al. [21] | 19–78 (Median 59) | 28/31 | DTC and MTC | Surgery, RAI, Chemotherapy |
| Chen et al. [22] | 28–76 (Median 59) | 2/8 | Locally Advanced DTC | Surgery, RAI |
| Lin et al. [23] | 27–76 (Median 59) | 84/107 | RAIR-DTC | Surgery, RAI, TKI Therapy |
| Lin et al. [24] | 21–79 (Median 55) | 13/22 | RAIR-DTC | Surgery, RAI |
| Study and Author | Treatment Regimen | ORR (%) | Median PFS (Months) | DCR (%) |
|---|---|---|---|---|
| Chen et al. [21] | Surufatinib 300 mg daily | 23.2 | 11.1 | 87.5 |
| Chen et al. [22] | Surufatinib + Toripalimab | 60 | Not Reported | 100 |
| Lin et al. [23] | Donafenib 300 mg twice daily | 23.3 (vs. 1.7 placebo) | 12.9 (vs. 6.4 placebo) | 93.3 (vs. 79.3 placebo) |
| Lin et al. [24] | Donafenib 200 mg vs. 300 mg twice daily | 12.5 vs. 13.3 | 9.4 vs. 15.0 | 100 in both arms |
| Study and Author | Common Adverse Events | Grade ≥ 3 AEs (%) | Treatment Discontinuation Due to AEs (%) |
|---|---|---|---|
| Chen et al. [21] | Hypertension, Proteinuria, PPE | 43.8 | 13.6 |
| Chen et al. [22] | Hypertension, Bilirubin Increase | 20 | 0 |
| Lin et al. [23] | HFS, Hypertension, Diarrhea | 43.8 | 6.3 |
| Lin et al. [24] | PPE, Hypertension, Alopecia | 51.4 | Not Reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejeu, D.; Dejeu, P.; Muresan, A.; Bradea, P.; Dejeu, V. Investigation into the Use of Surufatinib and Donafenib as Novel Multi-Kinase Inhibitors Therapeutic Agents in Managing Advanced Differentiated Thyroid Cancer: A Systematic Review. Biomedicines 2025, 13, 752. https://doi.org/10.3390/biomedicines13030752
Dejeu D, Dejeu P, Muresan A, Bradea P, Dejeu V. Investigation into the Use of Surufatinib and Donafenib as Novel Multi-Kinase Inhibitors Therapeutic Agents in Managing Advanced Differentiated Thyroid Cancer: A Systematic Review. Biomedicines. 2025; 13(3):752. https://doi.org/10.3390/biomedicines13030752
Chicago/Turabian StyleDejeu, Danut, Paula Dejeu, Anita Muresan, Paula Bradea, and Viorel Dejeu. 2025. "Investigation into the Use of Surufatinib and Donafenib as Novel Multi-Kinase Inhibitors Therapeutic Agents in Managing Advanced Differentiated Thyroid Cancer: A Systematic Review" Biomedicines 13, no. 3: 752. https://doi.org/10.3390/biomedicines13030752
APA StyleDejeu, D., Dejeu, P., Muresan, A., Bradea, P., & Dejeu, V. (2025). Investigation into the Use of Surufatinib and Donafenib as Novel Multi-Kinase Inhibitors Therapeutic Agents in Managing Advanced Differentiated Thyroid Cancer: A Systematic Review. Biomedicines, 13(3), 752. https://doi.org/10.3390/biomedicines13030752





