Protective Effects of Nerolidol on Thrombotic Events, Systemic Inflammation, Oxidative Stress, and DNA Damage Following Pulmonary Exposure to Diesel Exhaust Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Particles and Nerolidol
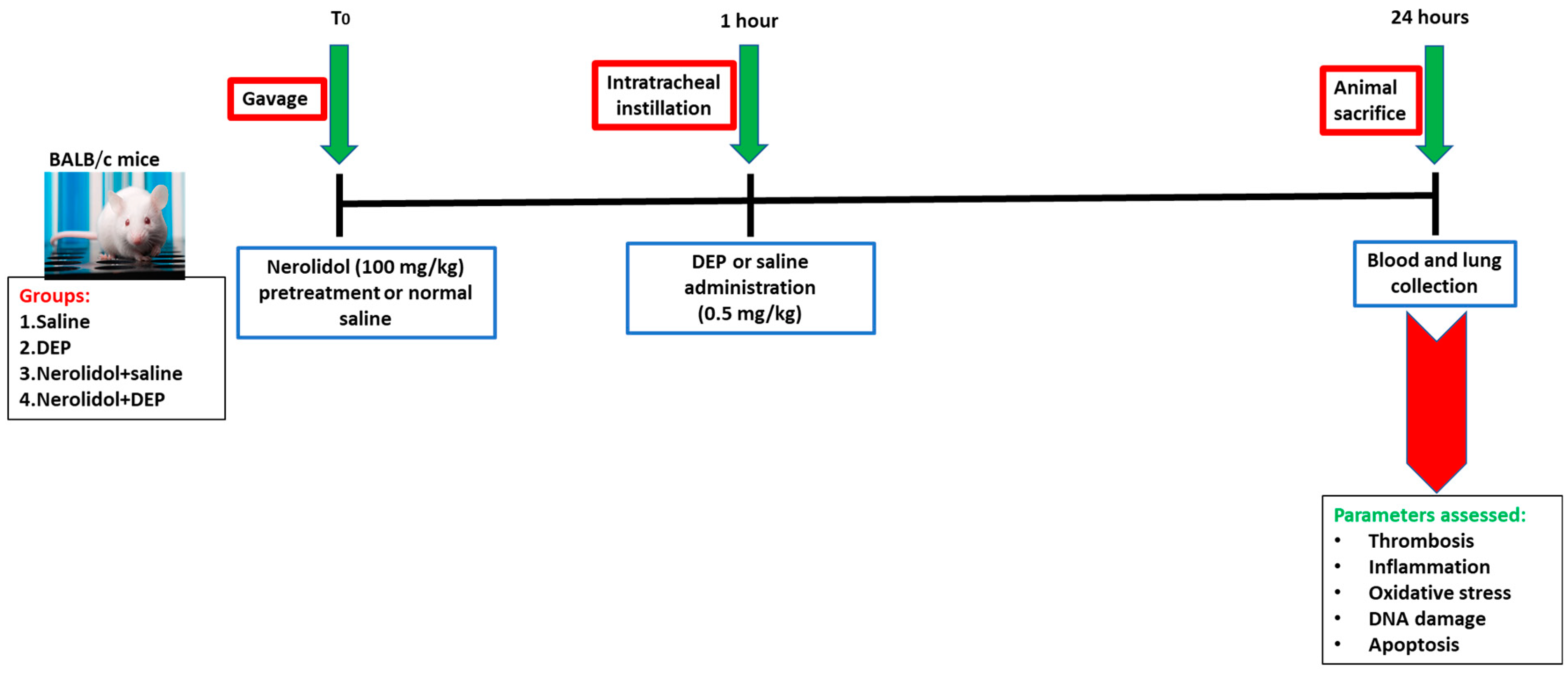
2.2. Animals and Treatments
- Group 1: Received normal saline given by oral gavage one hour before the i.t. instillation of saline.
- Group 2: Received normal saline given by oral gavage one hour before i.t. instillation of DEPs (0.5 mg/kg).
- Group 3: Received nerolidol given by oral gavage (100 mg/kg) one hour before the i.t. instillation of saline.
- Group 4: Received nerolidol given by gavage (100 mg/kg) one hour before i.t. instillation of DEPs (0.5 mg/kg).
2.2.1. Pial Microvessels Thrombosis Model
2.2.2. Whole Blood Platelet Aggregation Ex Vivo
2.2.3. The In Vitro Measurement of Activated Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT)
2.2.4. Blood Collection for Biochemical Analysis
2.3. Statistical Analysis
3. Results
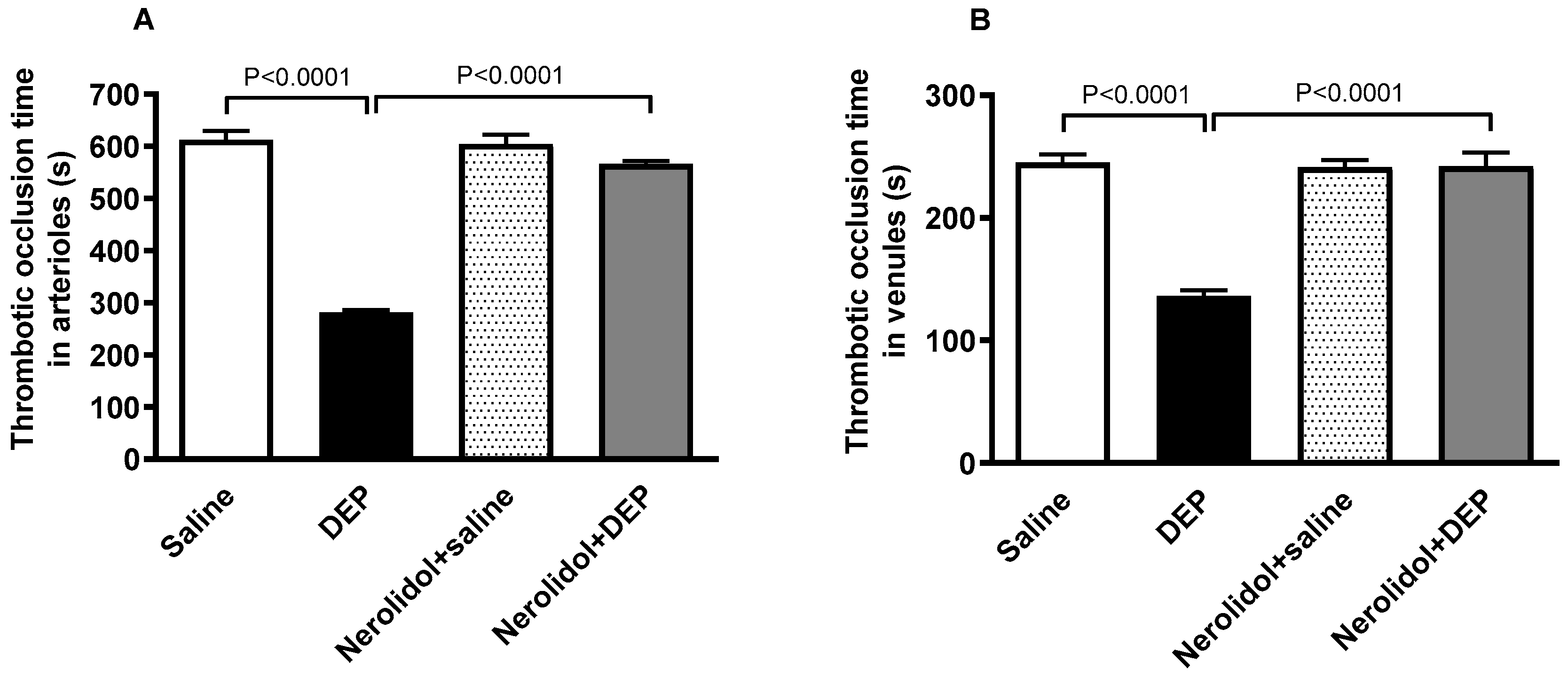
3.1. In Vivo Thrombosis in Pial Microvessels
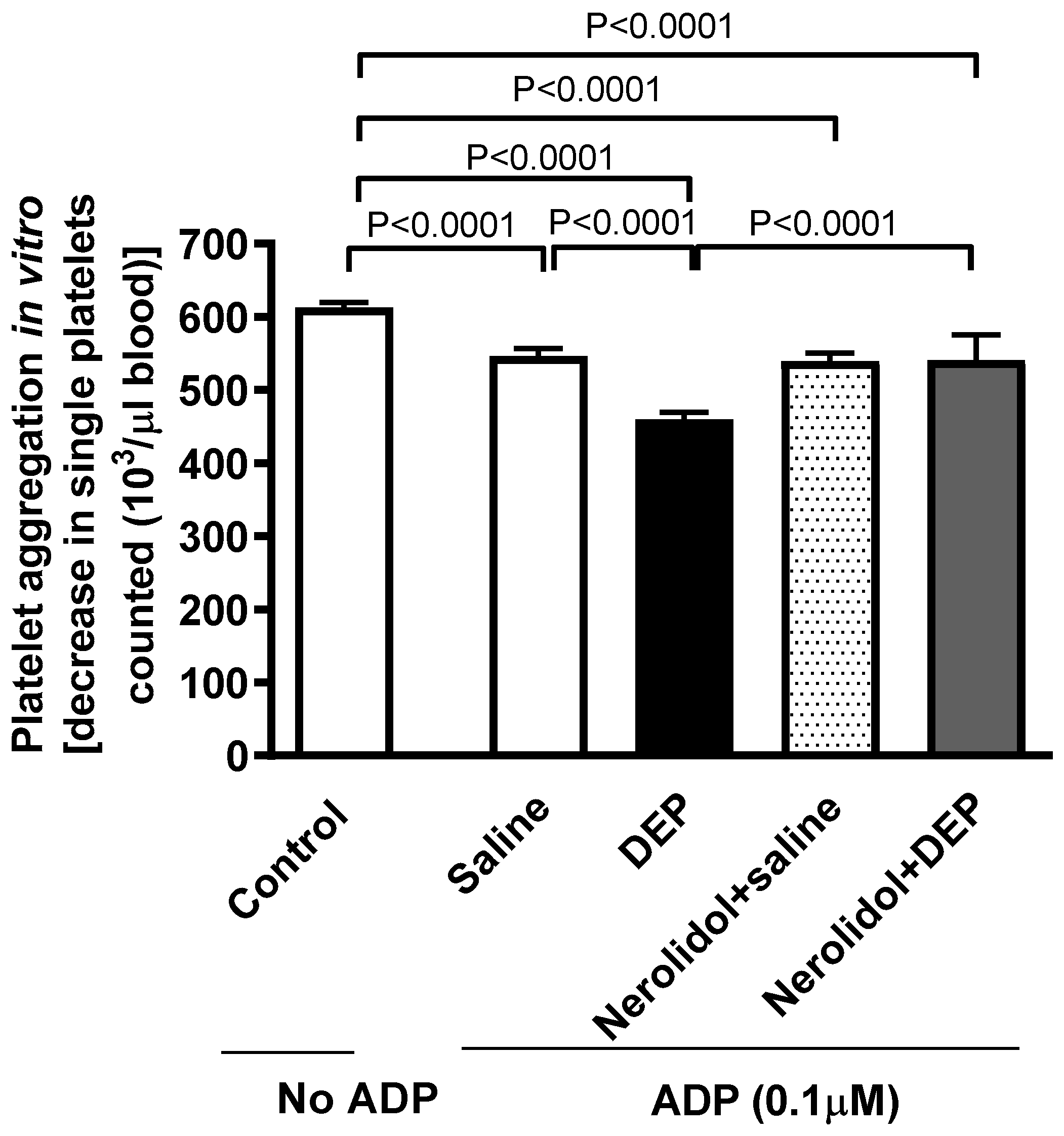
3.2. Ex Vivo Platelet Aggregation in Whole Blood
3.3. Activated Partial Thromboplastin Time and Prothrombin Time
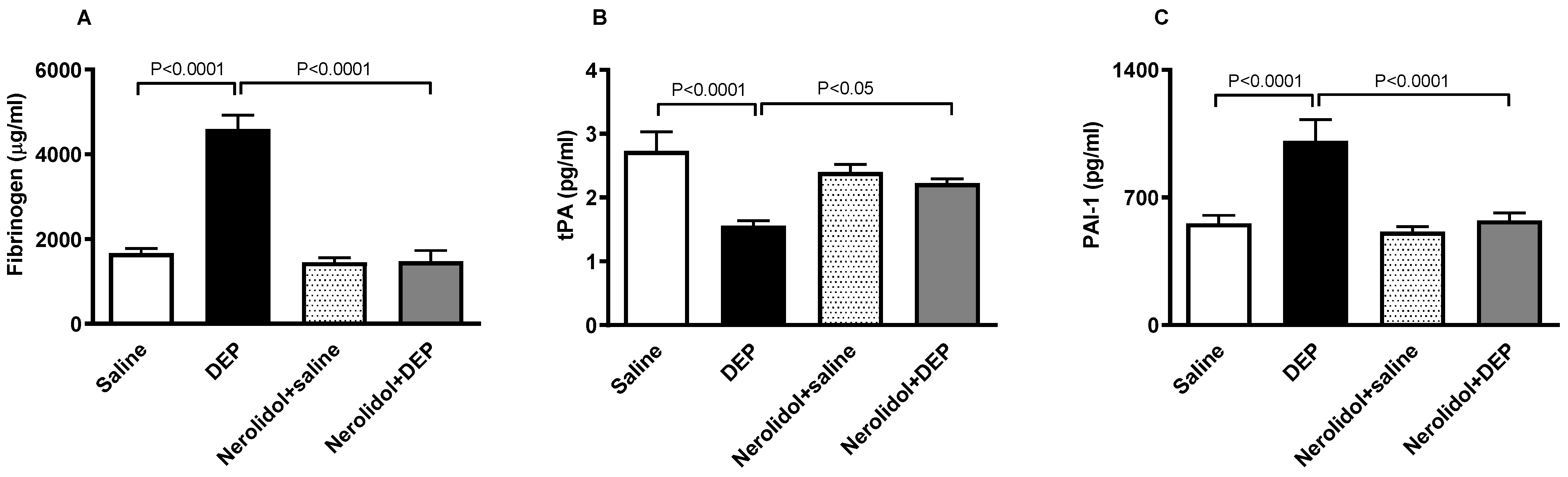
3.4. Markers of Coagulation and Fibrinolysis
3.5. Markers of Inflammation and Oxidative Stress
3.6. Markers of Oxidative Stress
3.7. Markers of Vascular Dysfunction
3.8. HIF-1α, Galectin-3 and NGAL Concentrations in the Plasma
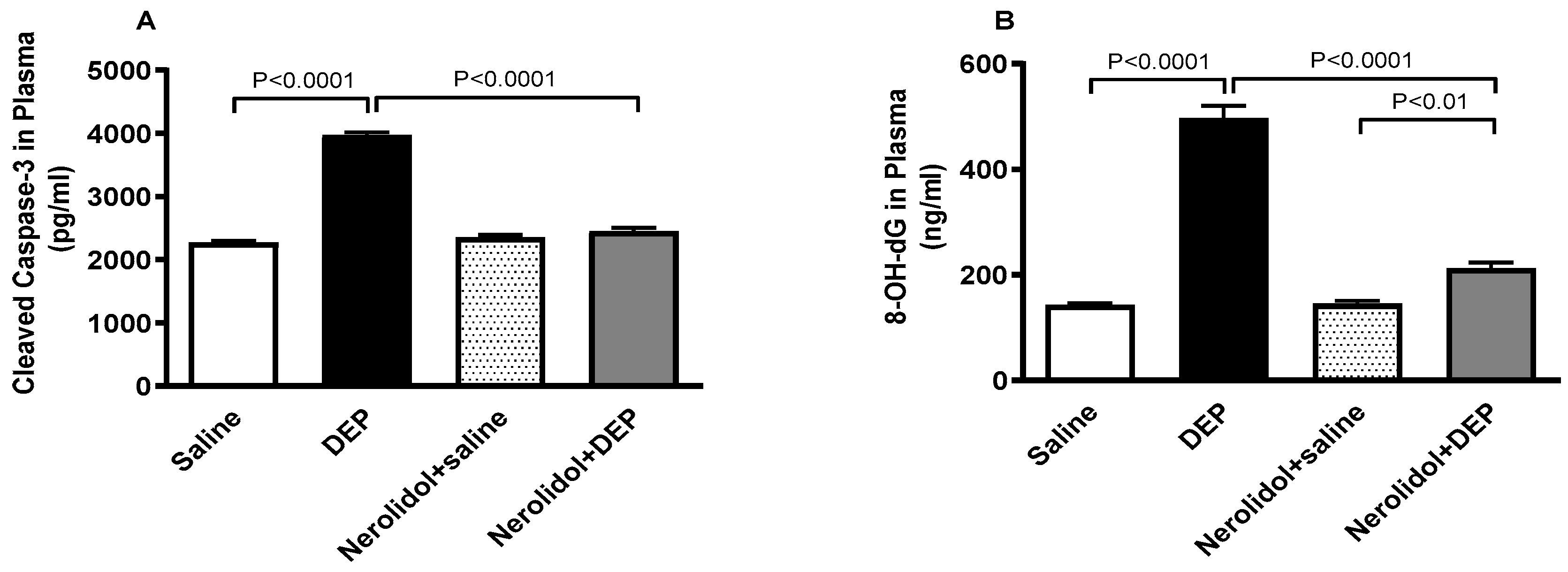
3.9. Markers of Apoptosis and Oxidative DNA Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, M.S.; Osman, W.J.; Garelnabi, E.A.; Osman, Z.; Osman, B.; Khalid, H.S.; Mohamed, M.A. Secondary metabolites as anti-inflammatory agents. J. Phytopharm. 2014, 3, 275–285. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: The Paul Wood Lecture, British Cardiovascular Society 2021. Heart 2022, 108, 1267–1273. [Google Scholar] [CrossRef]
- Fathieh, S.; Grieve, S.M.; Negishi, K.; Figtree, G.A. Potential Biological Mediators of Myocardial and Vascular Complications of Air Pollution—A State-of-the-Art Review. Heart Lung Circ. 2023, 32, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef]
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Cardiovasc. Res. 2020, 116, 279–294. [Google Scholar] [CrossRef]
- Xu, Y.; Barregard, L.; Nielsen, J.; Gudmundsson, A.; Wierzbicka, A.; Axmon, A.; Jönsson, B.A.; Kåredal, M.; Albin, M. Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part. Fibre Toxicol. 2013, 10, 60. [Google Scholar] [CrossRef]
- Wilson, S.J.; Miller, M.R.; Newby, D.E. Effects of Diesel Exhaust on Cardiovascular Function and Oxidative Stress. Antioxid. Redox Signal 2018, 28, 819–836. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. Thrombosis and systemic and cardiac oxidative stress and DNA damage induced by pulmonary exposure to diesel exhaust particles and the effect of nootkatone thereon. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H917–H927. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; D’Abrosca, B.; Golino, A.; Mastellone, C.; Piccolella, S.; Fiorentino, A.; Monaco, P. Antioxidant evaluation of polyhydroxylated nerolidols from redroot pigweed (Amaranthus retroflexus) leaves. LWT Food Sci. Technol. 2008, 41, 1665–1671. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Gonçalves, M.S.S.; Silva, E.A.P.; Santos, D.M.; Santana, I.R.; Souza, D.S.; Araujo, A.M.; Heimfarth, L.; Vasconcelos, C.M.L.; Santos, V.C.O.; Santos, M.R.V.; et al. Nerolidol attenuates isoproterenol-induced acute myocardial infarction in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 353–363. [Google Scholar] [CrossRef]
- Lin, Y.M.; Badrealam, K.F.; Kuo, W.W.; Lai, P.F.; Shao-Tsu Chen, W.; Hsuan Day, C.; Ho, T.J.; Viswanadha, V.P.; Shibu, M.A.; Huang, C.Y. Nerolidol improves cardiac function in spontaneously hypertensive rats by inhibiting cardiac inflammation and remodelling associated TLR4/NF-κB signalling cascade. Food Chem. Toxicol. 2021, 147, 111837. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.L.; Shen, H.T.; Su, C.H.; Chen, W.Y.; Huang-Liu, R.; Chen, C.J.; Chen, S.P.; Kuan, Y.H. Nerolidol Suppresses the Inflammatory Response during Lipopolysaccharide-Induced Acute Lung Injury via the Modulation of Antioxidant Enzymes and the AMPK/Nrf-2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 9605980. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Maskari, S.; Ali, B.H.; Al-Amri, I.S. Cardiovascular and lung inflammatory effects induced by systemically administered diesel exhaust particles in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L664–L670. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdorster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Fujisawa, Y.; Yatera, K.; Fujita, K.; Maru, J.; Endoh, S.; Honda, K. Basic study of intratracheal instillation study of nanomaterials for the estimation of the hazards of nanomaterials. Ind. Health 2018, 56, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Beegam, S.; Zaaba, N.E.; Elzaki, O.; Nemmar, A. α-Bisabolol alleviates diesel exhaust particle-induced lung injury and mitochondrial dysfunction by regulating inflammatory, oxidative stress, and apoptotic biomarkers through the c-Jun N-terminal kinase signaling pathway. Front. Pharmacol. 2024, 15, 1485101. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, N.B.; Yüce, H.; Taşlıdere, A.; Şahin, Y.; Çiftçi, O. The Ameliorate Effects of Nerolidol on Thioacetamide-induced Oxidative Damage in Heart and Kidney Tissue. Turk. J. Pharm. Sci. 2022, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Jaiswal, G.; Brar, J.; Kumar, P. Neuroprotective effect of nerolidol in traumatic brain injury associated behavioural comorbidities in rats. Toxicol. Res. 2021, 10, 40–50. [Google Scholar] [CrossRef]
- Raj, V.; Venkataraman, B.; Ojha, S.K.; Almarzooqi, S.; Subramanian, V.S.; Al-Ramadi, B.K.; Adrian, T.E.; Subramanya, S.B. Cis-Nerolidol Inhibits MAP Kinase and NF-κB Signaling Pathways and Prevents Epithelial Tight Junction Dysfunction in Colon Inflammation: In Vivo and In Vitro Studies. Molecules 2023, 28, 2982. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Ali, B.H. Chronic exposure to water-pipe smoke induces cardiovascular dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H329–H339. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; John, A.; Raza, H.; Ali, B.H. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H740–H746. [Google Scholar] [CrossRef]
- Jin, J.; Quinton, T.M.; Zhang, J.; Rittenhouse, S.E.; Kunapuli, S.P. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood 2002, 99, 193–198. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Zaaba, N.E.; Alblooshi, S.; Alseiari, S.; Ali, B.H. The Salutary Effects of Catalpol on Diesel Exhaust Particles-Induced Thrombogenic Changes and Cardiac Oxidative Stress, Inflammation and Apoptosis. Biomedicines 2022, 10, 99. [Google Scholar] [CrossRef]
- Ferdous, Z.; Beegam, S.; Zaaba, N.E.; Elzaki, O.; Tariq, S.; Greish, Y.E.; Ali, B.H.; Nemmar, A. Exacerbation of Thrombotic Responses to Silver Nanoparticles in Hypertensive Mouse Model. Oxidative Med. Cell. Longev. 2022, 2022, 2079630. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Vijayasarathy, C.; Raza, H.; Mullick, J.; Avadhani, N.G. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem. Pharmacol. 1998, 56, 831–839. [Google Scholar] [CrossRef]
- Raza, H.; Prabu, S.K.; Robin, M.A.; Avadhani, N.G. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: Tissue-specific variations and roles in oxidative stress. Diabetes 2004, 53, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Wennmalm, A.; Benthin, G.; Edlund, A.; Jungersten, L.; Kieler-Jensen, N.; Lundin, S.; Westfelt, U.N.; Petersson, A.S.; Waagstein, F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ. Res. 1993, 73, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.Y.; Sussmann, R.A.; Kimura, E.A.; Cassera, M.B.; Katzin, A.M. Quantification of nerolidol in mouse plasma using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 100–103. [Google Scholar] [CrossRef]
- He, Y.S.; Sun, W.; Zhang, B.Y.; Xu, L.H.; Yang, J.; Gao, W.; Qi, L.W.; Li, P.; Wen, X.D. Application of a sensitive liquid chromatography-mass spectrometry method to a pharmacokinetic study of nerolidol in rat plasma. Anal. Methods 2016, 8, 785–789. [Google Scholar] [CrossRef]
- Beegam, S.; Zaaba, N.E.; Elzaki, O.; Alzaabi, A.; Alkaabi, A.; Alseiari, K.; Alshamsi, N.; Nemmar, A. Palliative effects of carnosol on lung-deposited pollutant particles-induced thrombogenicity and vascular injury in mice. Pharmacol. Res. Perspect. 2024, 12, e1201. [Google Scholar] [CrossRef]
- Miller, M.R. The cardiovascular effects of air pollution: Prevention and reversal by pharmacological agents. Pharmacol. Ther. 2022, 232, 107996. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Fan, C.; Xu, R.; Wang, Y.; Xu, C.; Xie, S.; Zhang, H.; Cui, X.; Peng, Z.; et al. Short-Term Exposure to Ambient Air Pollution and Mortality From Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 271–281. [Google Scholar] [CrossRef]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef]
- Kido, T.; Tamagawa, E.; Bai, N.; Suda, K.; Yang, H.H.; Li, Y.; Chiang, G.; Yatera, K.; Mukae, H.; Sin, D.D.; et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am. J. Respir. Cell Mol. Biol. 2011, 44, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Green, D.; Bellmeyer, A.; Baker, C.M.; Burgess, Z.; Rajamannan, N.; Christman, J.W.; Foiles, N.; Kamp, D.W.; Ghio, A.J.; et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Investig. 2007, 117, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Hamadi, N.K.; Ali, B.H. Prolonged Pulmonary Exposure to Diesel Exhaust Particles Exacerbates Renal Oxidative Stress, Inflammation and DNA Damage in Mice with Adenine-Induced Chronic Renal Failure. Cell Physiol. Biochem. 2016, 38, 1703–1713. [Google Scholar] [CrossRef]
- Nemmar, A.; Subramaniyan, D.; Ali, B.H. Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice. PLoS ONE 2012, 7, e39554. [Google Scholar] [CrossRef]
- Shaddick, G.; Thomas, M.L.; Amini, H.; Broday, D.; Cohen, A.; Frostad, J.; Green, A.; Gumy, S.; Liu, Y.; Martin, R.V.; et al. Data Integration for the Assessment of Population Exposure to Ambient Air Pollution for Global Burden of Disease Assessment. Environ. Sci. Technol. 2018, 52, 9069–9078. [Google Scholar] [CrossRef]
- WHO. WHO Report on Ambient (Outdoor) Air Pollution; WHO: Geneva, Switzerland, 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 9 March 2025).
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Dinsdale, D.; Vermylen, J.; Hoylaerts, M.F.; Nemery, B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 2003, 107, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011, 118, 2405–2412. [Google Scholar] [CrossRef]
- Tabor, C.M.; Shaw, C.A.; Robertson, S.; Miller, M.R.; Duffin, R.; Donaldson, K.; Newby, D.E.; Hadoke, P.W. Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis. Part. Fibre Toxicol. 2016, 13, 6. [Google Scholar] [CrossRef]
- Hantrakool, S.; Sriwichai, M.; Shaengkhamnang, B.; Leetrakool, N.; Niprapan, P.; Kawichai, S.; Wannakul, S.; Panyasit, N.; Tuntivate, P.; Wongtagan, O.; et al. The effects of ambient particulate matter air pollution on platelets and hemostasis. Front. Public. Health 2024, 12, 1410406. [Google Scholar] [CrossRef]
- Franchini, M.; Mengoli, C.; Cruciani, M.; Bonfanti, C.; Mannucci, P.M. Association between particulate air pollution and venous thromboembolism: A systematic literature review. Eur. J. Intern. Med. 2016, 27, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hantrakool, S.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Particulate Matter on Inflammation and Thrombosis: Past Evidence for Future Prevention. Int. J. Environ. Res. Public. Health 2022, 19, 8771. [Google Scholar] [CrossRef] [PubMed]
- Budinger, G.R.; McKell, J.L.; Urich, D.; Foiles, N.; Weiss, I.; Chiarella, S.E.; Gonzalez, A.; Soberanes, S.; Ghio, A.J.; Nigdelioglu, R.; et al. Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PLoS ONE 2011, 6, e18525. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Y. Antidiabetic effects of nerolidol through promoting insulin receptor signaling in high-fat diet and low dose streptozotocin-induced type 2 diabetic rats. Hum. Exp. Toxicol. 2022, 41, 9603271221126487. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Sharma, C.; Goyal, S.N.; Ojha, S. Nerolidol Attenuates Oxidative Stress, Inflammation, and Apoptosis by Modulating Nrf2/MAPK Signaling Pathways in Doxorubicin-Induced Acute Cardiotoxicity in Rats. Antioxidants 2021, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.M.A.; Amir, N.; Adeghate, E.; Ojha, S. Nerolidol, a sesquiterpene, attenuates oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol. Cell Biochem. 2021, 476, 3497–3512. [Google Scholar] [CrossRef]
- Wang, K.; Lei, L.; Li, G.; Lan, Y.; Wang, W.; Zhu, J.; Liu, Q.; Ren, L.; Wu, S. Association between Ambient Particulate Air Pollution and Soluble Biomarkers of Endothelial Function: A Meta-Analysis. Toxics 2024, 12, 76. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Wang, T.; Leng, Y.F.; Zhang, Y.; Xue, X.; Kang, Y.Q.; Zhang, Y. Oxidative stress and hypoxia-induced factor 1α expression in gastric ischemia. World J. Gastroenterol. 2011, 17, 1915–1922. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, L.; Cui, T.; Ahmed, R.Z.; Yu, H.; Zhang, R.; Wei, Y.; Li, D.; Zheng, Y.; Chen, W.; et al. Oxygen sensors mediated HIF-1α accumulation and translocation: A pivotal mechanism of fine particles-exacerbated myocardial hypoxia injury. Environ. Pollut. 2022, 300, 118937. [Google Scholar] [CrossRef]
- Lin, H.; Chen, M.; Gao, Y.; Wang, Z.; Jin, F. Tussilagone protects acute lung injury from PM2.5 via alleviating Hif-1α/NF-κB-mediated inflammatory response. Environ. Toxicol. 2022, 37, 1198–1210. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef] [PubMed]
- de Couto, G.; Ouzounian, M.; Liu, P.P. Early detection of myocardial dysfunction and heart failure. Nat. Rev. Cardiol. 2010, 7, 334–344. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Zheng, Y.; Yang, J.; Liu, Y.; Qi, Z.; Wu, M.; Fan, Z.; Yin, K.; Chen, Y.; et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: A translational study. Eur. Heart J. 2022, 43, 3556–3574. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D., Jr.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3-binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Zaaba, N.E.; Elzaki, O.; Pathan, A.; Ali, B.H. Waterpipe smoke inhalation induces lung injury and aortic endothelial dysfunction in mice. Physiol. Res. 2023, 72, 337–347. [Google Scholar] [CrossRef]
- Pei, C.; Wang, X.; Lin, Y.; Fang, L.; Meng, S. Inhibition of Galectin-3 Alleviates Cigarette Smoke Extract-Induced Autophagy and Dysfunction in Endothelial Progenitor Cells. Oxidative Med. Cell. Longev. 2019, 2019, 7252943. [Google Scholar] [CrossRef]
- André, E.; Stoeger, T.; Takenaka, S.; Bahnweg, M.; Ritter, B.; Karg, E.; Lentner, B.; Reinhard, C.; Schulz, H.; Wjst, M. Inhalation of ultrafine carbon particles triggers biphasic pro-inflammatory response in the mouse lung. Eur. Respir. J. 2006, 28, 275–285. [Google Scholar] [CrossRef]
- Sivalingam, Z.; Larsen, S.B.; Grove, E.L.; Hvas, A.M.; Kristensen, S.D.; Magnusson, N.E. Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease. Clin. Chem. Lab. Med. 2017, 56, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Schreinlechner, M.; Noflatscher, M.; Lener, D.; Bauer, A.; Kirchmair, R.; Marschang, P.; Theurl, M. NGAL Correlates with Femoral and Carotid Plaque Volume Assessed by Sonographic 3D Plaque Volumetry. J. Clin. Med. 2020, 9, 2811. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, W.; Xu, Z.H.; Wang, J.; Hu, X.; Lu, Z.Z.; Zhang, X.W. Neutrophil Gelatinase-Associated Lipocalin 2 Accelerates Hypoxia-Induced Endothelial Cell Injury via eNOS/NRF2 Signalling. Cell J. 2021, 23, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Gröger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State In Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Aguilar, O.G.; Uribe-Ramírez, M.; Narváez-Morales, J.; De Vizcaya-Ruiz, A.; Barbier, O. Early kidney damage induced by subchronic exposure to PM(2.5) in rats. Part. Fibre Toxicol. 2016, 13, 68. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of Apoptosis. Biochemistry 2015, 80, 1393–1405. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Al Salam, S.; Al Suleimani, Y.; Manoj, P.; Nemmar, A.; Ali, B.H. Gum Acacia Improves Renal Function and Ameliorates Systemic Inflammation, Oxidative and Nitrosative Stress in Streptozotocin-Induced Diabetes in Rats with Adenine-Induced Chronic Kidney Disease. Cell Physiol. Biochem. 2018, 45, 2293–2304. [Google Scholar] [CrossRef]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Abdelrahman, A.M.; Ashique, M.; Manoj, P.; Adham, S.A.; Hartmann, C.; Schupp, N.; et al. Effects of the SGLT-2 Inhibitor Canagliflozin on Adenine-Induced Chronic Kidney Disease in Rats. Cell Physiol. Biochem. 2019, 52, 27–39. [Google Scholar] [CrossRef]
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 19 (Suppl. S1), 241–244. [Google Scholar] [CrossRef] [PubMed]
- Marano, F.; Boland, S.; Bonvallot, V.; Baulig, A.; Baeza-Squiban, A. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol. Toxicol. 2002, 18, 315–320. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamadi, N.; Beegam, S.; Zaaba, N.E.; Elzaki, O.; Alderei, A.; Alfalahi, M.; Alhefeiti, S.; Alnaqbi, D.; Alshamsi, S.; Nemmar, A. Protective Effects of Nerolidol on Thrombotic Events, Systemic Inflammation, Oxidative Stress, and DNA Damage Following Pulmonary Exposure to Diesel Exhaust Particles. Biomedicines 2025, 13, 729. https://doi.org/10.3390/biomedicines13030729
Hamadi N, Beegam S, Zaaba NE, Elzaki O, Alderei A, Alfalahi M, Alhefeiti S, Alnaqbi D, Alshamsi S, Nemmar A. Protective Effects of Nerolidol on Thrombotic Events, Systemic Inflammation, Oxidative Stress, and DNA Damage Following Pulmonary Exposure to Diesel Exhaust Particles. Biomedicines. 2025; 13(3):729. https://doi.org/10.3390/biomedicines13030729
Chicago/Turabian StyleHamadi, Naserddine, Sumaya Beegam, Nur Elena Zaaba, Ozaz Elzaki, Alreem Alderei, Maha Alfalahi, Shamma Alhefeiti, Dana Alnaqbi, Salama Alshamsi, and Abderrahim Nemmar. 2025. "Protective Effects of Nerolidol on Thrombotic Events, Systemic Inflammation, Oxidative Stress, and DNA Damage Following Pulmonary Exposure to Diesel Exhaust Particles" Biomedicines 13, no. 3: 729. https://doi.org/10.3390/biomedicines13030729
APA StyleHamadi, N., Beegam, S., Zaaba, N. E., Elzaki, O., Alderei, A., Alfalahi, M., Alhefeiti, S., Alnaqbi, D., Alshamsi, S., & Nemmar, A. (2025). Protective Effects of Nerolidol on Thrombotic Events, Systemic Inflammation, Oxidative Stress, and DNA Damage Following Pulmonary Exposure to Diesel Exhaust Particles. Biomedicines, 13(3), 729. https://doi.org/10.3390/biomedicines13030729





