Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro
Abstract
1. Introduction
2. Methods
2.1. Experiment Material
2.2. Grouping and Processing
2.3. CCK-8 Assay to Measure the Appropriate Concentration of LPS
2.4. Histopathology of the Jejunum
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blot
2.7. Molecular Docking
2.8. Statistical Analyses
3. Results
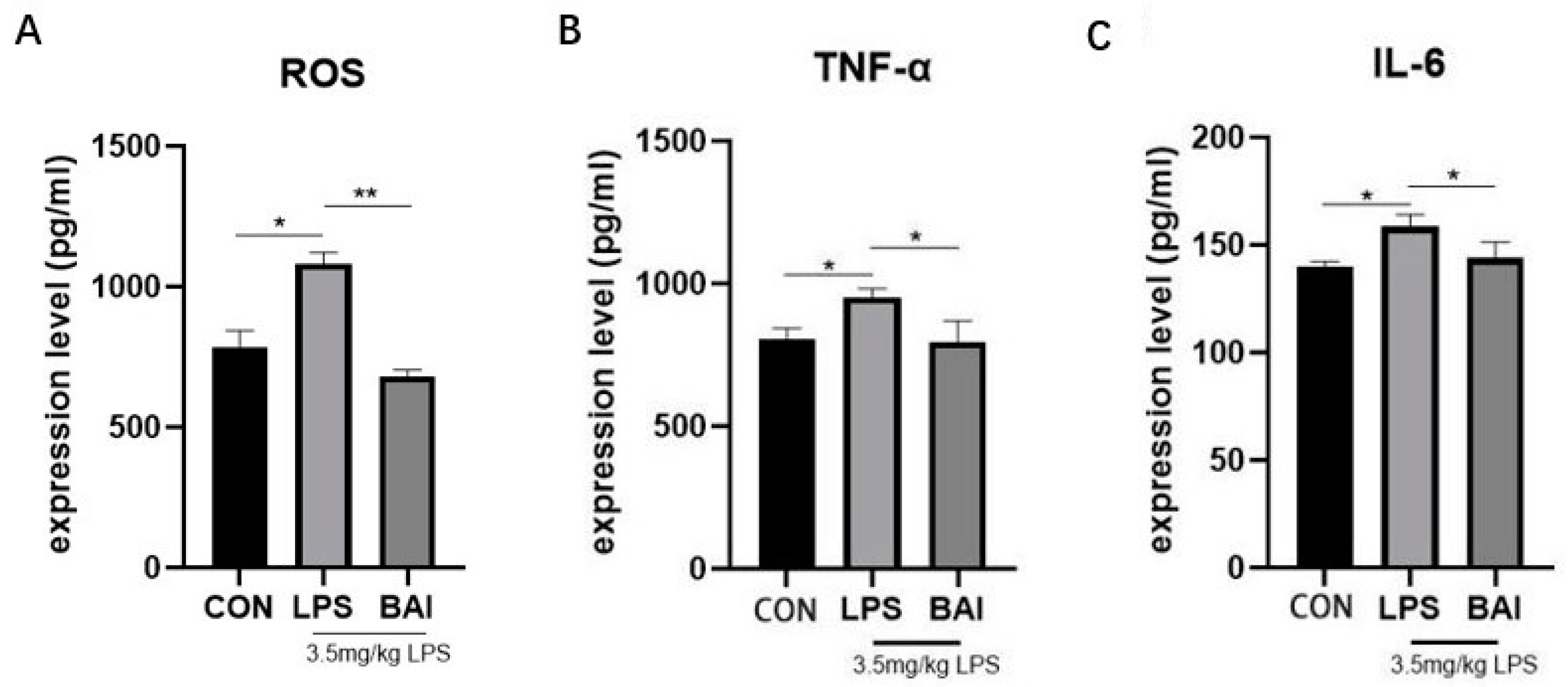
3.1. Baicalin Decreased the Expressions of ROS in Serum and Inflammatory Factors in Jejunum Stimulated by LPS
3.2. Effect of Baicalin on the Tissue Morphology of the Jejunum in Mice Induced by LPS
3.3. Effects of Baicalin on Key Proteins in the MAPK Signaling Pathway in the Mice Jejunum Induced by LPS
3.4. ROS Scavenger (NAC) Reduces the Expression of Inflammatory Cytokines and ROS in LPS-Stimulated IPEC-J2 Cells
3.5. ROS Scavenger (NAC) Reduces the Expression of Key Proteins of the MAPK Signaling Pathway in LPS-Stimulated IPEC-J2 Cells
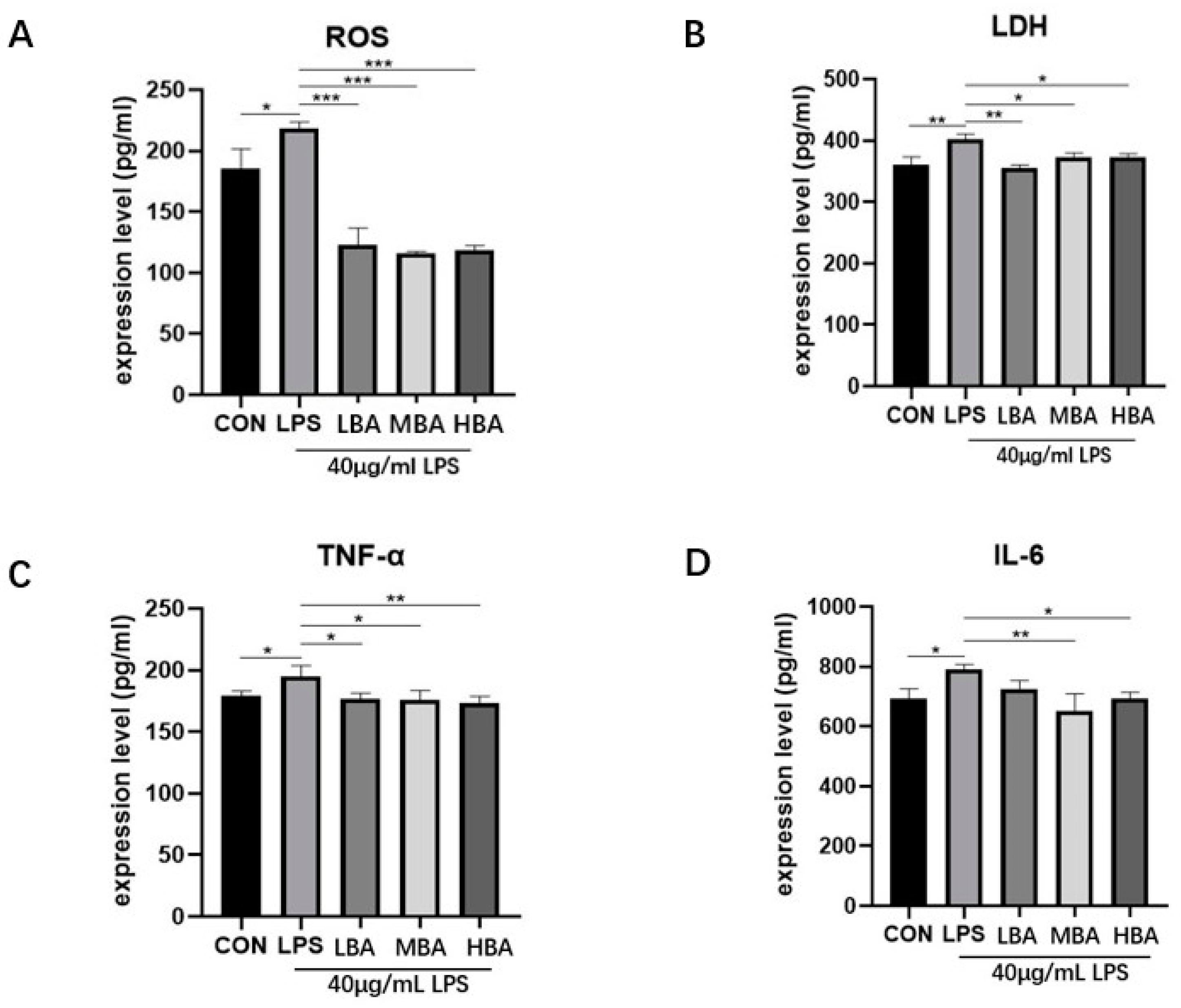
3.6. Baicalin Reduces the Expression of Inflammatory Factors, ROS and Lactate Dehydrogenase (LDH) in LPS-Stimulated IPEC-J2 Cells
3.7. Baicalin Reduces the Expression of Key Proteins of the MAPK Signaling Pathway in LPS-Stimulated IPEC Cells
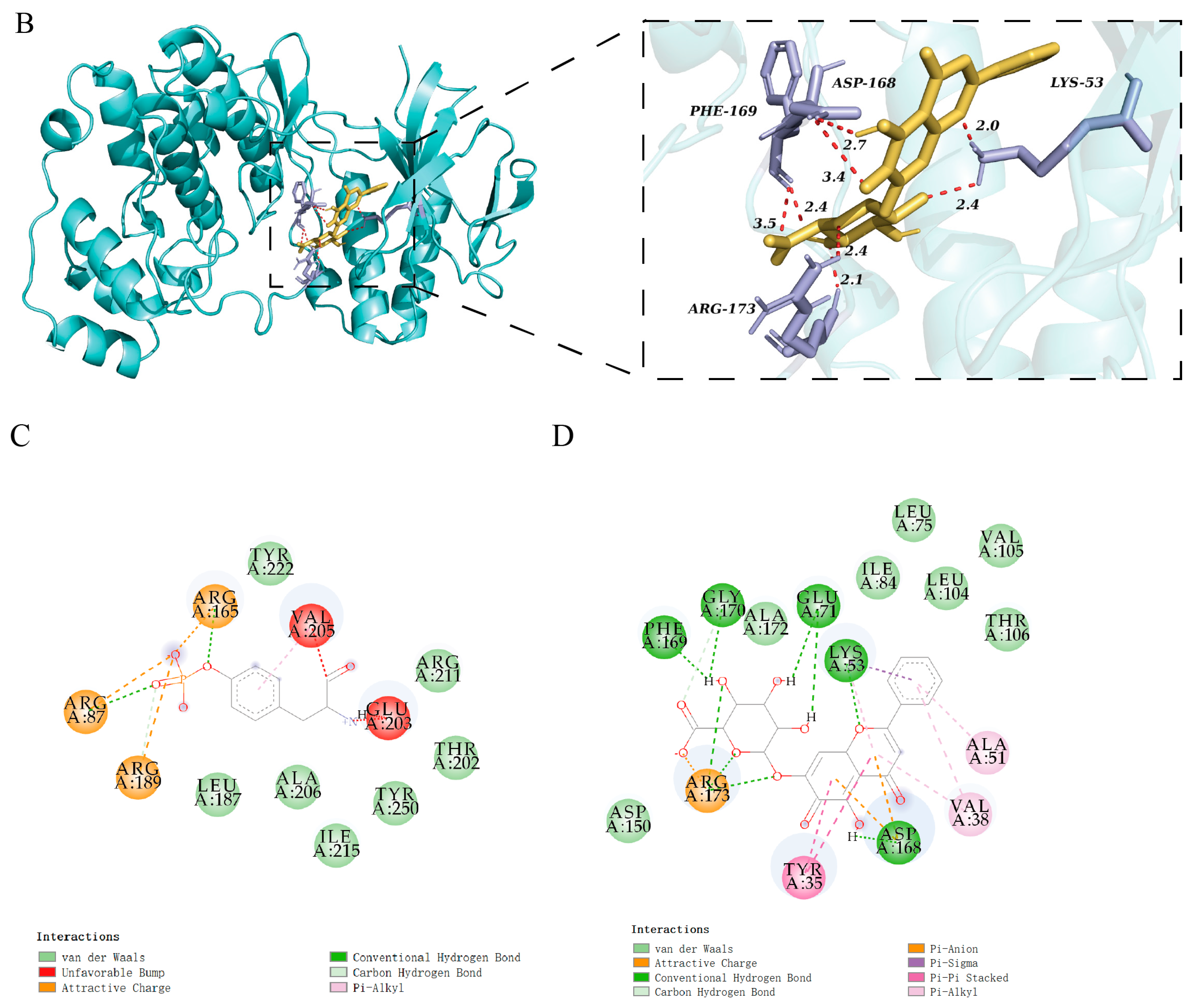
3.8. Molecular Docking of Baicalin Binding to Target Proteins
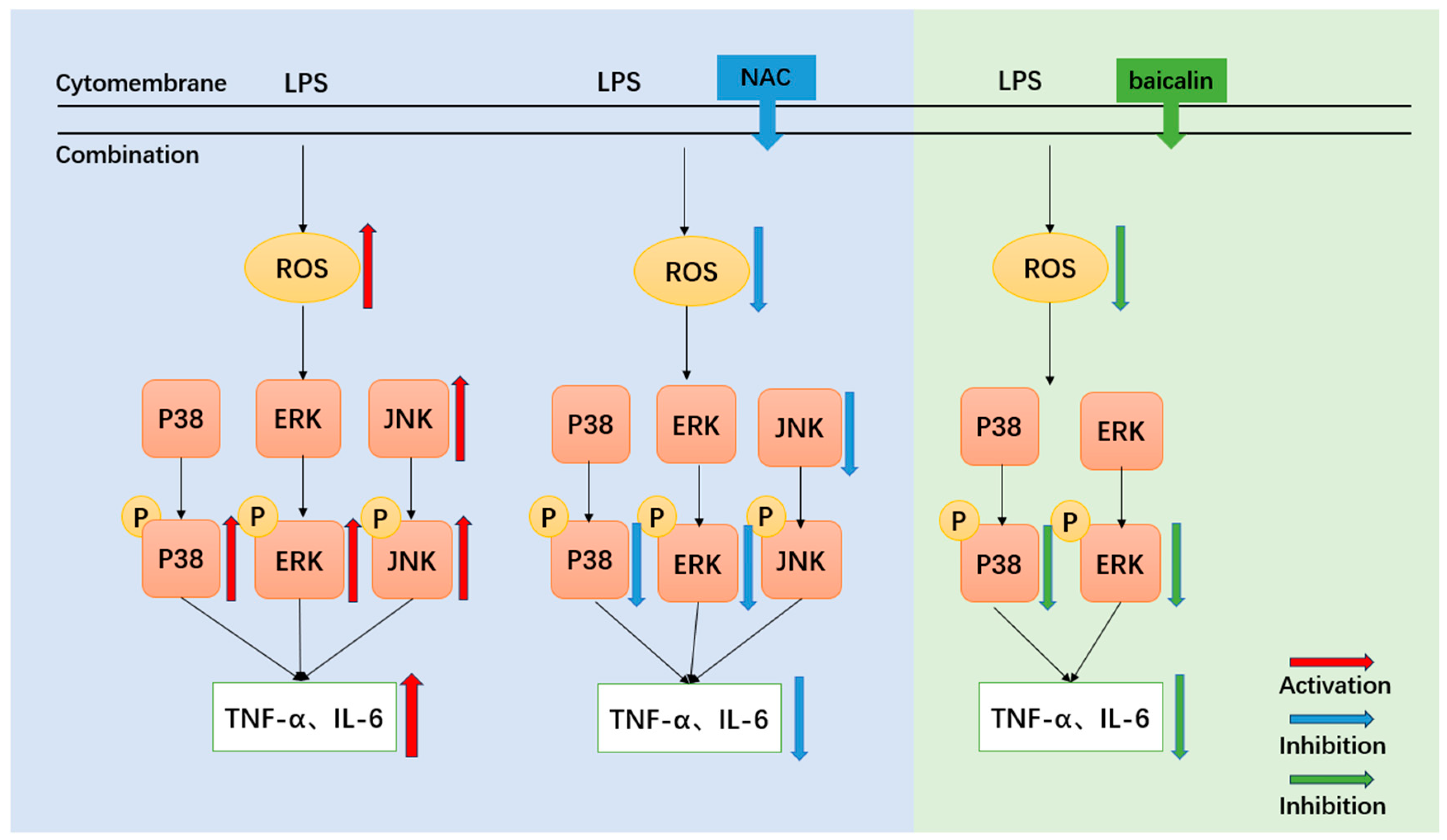
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| IPEC-J2 cells | porcine intestinal epithelial cells |
| LPS | Lipopolysaccharide |
| NAC | N-Acetyl-L-cysteine |
| BAI | Baicalin |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular regulated protein kinases |
| P38 | P38 MAPK |
| JNK | c-Jun N-terminal kinase |
| P-ERK | Phospho- extracellular regulated protein kinases |
| P-P38 | Phospho-P38 MAPK |
| P-JNK | Phospho- c-Jun N-terminal kinase |
| ROS | reactive oxygen species |
| LDH | Lactate dehydrogenase |
| TNF-α | tumor necrosis factor |
| IL-6 | Interleukin-6 |
| BCA | bicinchoninic acid |
| ECL | enhanced chemiluminescence |
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Buchet, A.; Belloc, C.; Leblanc-Maridor, M.; Merlot, E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE 2017, 12, e0178487. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Camba-Gómez, M.; Gualillo, O.; Conde-Aranda, J. New Perspectives in the Study of Intestinal Inflammation: Focus on the Resolution of Inflammation. Int. J. Mol. Sci. 2021, 22, 2605. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Buhrmann, C.; Pourbagher-Shahri, A.M.; Shakibaei, M.; Samarghandian, S. Organophosphorus Compounds and MAPK Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4258. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-kappaB/MAPK signaling pathway. J. Cell Physiol. 2020, 235, 5525–5540. [Google Scholar] [CrossRef]
- Chen, N.; Su, P.; Wang, M.; Li, Y.M. Ascorbic acid inhibits cadmium-induced disruption of the blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Environ. Sci. Pollut. Res. Int. 2018, 25, 21713–21720. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Z.; Liu, Y.; Du, Y.; Liu, X. Ozone oxidative postconditioning inhibits oxidative stress and apoptosis in renal ischemia and reperfusion injury through inhibition of MAPK signaling pathway. Drug Des. Devel Ther. 2018, 12, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xu, N.; Zhou, Y.; Li, B.; Li, M.; Wang, Q.; Yang, X.; Ge, X.; Zhang, F.; Ren, X. Anti-inflammatory effect of paeoniflorin combined with baicalin in oral inflammatory diseases. Oral Dis. 2019, 25, 1945–1953. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef] [PubMed]
- Olagaray, K.E.; Brouk, M.J.; Mamedova, L.K.; Sivinski, S.E.; Liu, H.; Robert, F.; Dupuis, E.; Zachut, M.; Bradford, B.J. Dietary supplementation of Scutellaria baicalensis extract during early lactation decreases milk somatic cells and increases whole lactation milk yield in dairy cattle. PLoS ONE 2019, 14, e0210744. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.-Y.; Lu, Q.-Y.; Liu, Q.-Z.; Guo, L.; Li, M.; Sun, Q.-Y. Baicalin relieves complement alternative pathway activation-induced lung inflammation through inhibition of NF-κB pathway. BMC Complement. Med. Ther. 2024, 24, 334. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, H.; Zhu, Z.; Ling, Z.; Zeng, F.; Wang, Y.; Wang, J. Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules 2023, 28, 1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chang, Y.; Liu, X.; Liu, L.; Hua, M.; Li, A. Protective effects of baicalin on diethyl nitrosamine-induced liver cirrhosis by suppressing oxidative stress and inflammation. Chem. Biol. Drug Des. 2023, 103, e14386. [Google Scholar] [CrossRef]
- Bao, M.; Sun, X.; Liang, M.; Ju, X.; Yong, Y.; Ma, X.; Yu, Z.; Guo, Y.; Liu, X. Baicalin alleviates lipopolysaccharide-induced jejunum inflammation and oxidative stress by inhibiting nuclear factor-κB and activating nuclear factor-e2-related factor-2 signaling pathways. Chin. J. Anim. Nutr. 2022, 34, 1217–1229. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X.; et al. Baicalin Alleviates LPS-Induced Oxidative Stress via NF-kappaB and Nrf2-HO1 Signaling Pathways in IPEC-J2 Cells. Front. Vet. Sci. 2021, 8, 808233. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yoshida, I.; Nakae, S.; Okita, K.; Gouda, M.; Matsubara, M.; Yokota, K.; Ishiguro, H.; Tada, T. Crystal structure of human mono-phosphorylated ERK1 at Tyr204. Biochem. Biophys. Res. Commun. 2008, 377, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Canagarajah, B.J.; Boehm, J.C.; Kassisà, S.; Cobb, M.H.; Young, P.R.; Abdel-Meguid, S.; Adams, J.L.; Goldsmith, E.J. Structural basis of inhibitor selectivity in MAP kinases. Structure 1998, 6, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Avila-Calderon, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velazquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodriguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, E.; Gryzinska, M.; Ognik, K.; Gil-Kulik, P.; Kocki, J. Oxidative Stress and Effect of Treatment on the Oxidation Product Decomposition Processes in IBD. Oxid. Med. Cell Longev. 2018, 2018, 7918261. [Google Scholar] [CrossRef]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Huang, Q.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (-)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Drent, M.; Cobben, N.A.; Henderson, R.F.; Wouters, E.F.; van Dieijen-Visser, M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 1996, 9, 1736–1742. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Ali, H.; Shah, K.U.; Ali, H.; Shehzad, O.; Onder, A.; Kim, Y.S. Anomalin attenuates LPS-induced acute lungs injury through inhibition of AP-1 signaling. Int. Immunopharmacol. 2019, 73, 451–460. [Google Scholar] [CrossRef]
- Sakurai, H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 2012, 33, 522–530. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Wu, F.; Shao, Q.Q.; Chen, G.; Xu, L.J.; Lu, F.E. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Xu, B.; Huang, S.W.; Luo, X.; Deng, X.L.; Luo, S.; Liu, C.; Wang, Q.; Chen, J.Y.; Zhou, L. Baicalin prevents LPS-induced activation of TLR4/NF-kappaB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol. Sin. 2021, 42, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Deng, X.; Lei, L.; Zheng, Y.; Ai, J.; Chen, L.; Xiong, H.; Mei, Z.; Cheng, Y.C.; Ren, Y. The Comparative Study of the Therapeutic Effects and Mechanism of Baicalin, Baicalein, and Their Combination on Ulcerative Colitis Rat. Front. Pharmacol. 2019, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, F.; Ma, Y.; Li, H.; Ju, X.; Xu, J. Effect of Puerarin, Baicalin and Berberine Hydrochloride on the Regulation of IPEC-J2 Cells Infected with Enterotoxigenic Escherichia coli. Evid. Based Complement. Altern. Med. 2019, 2019, 7438593. [Google Scholar] [CrossRef]
- Chen, L.-L.; Song, C.; Zhang, Y.; Li, Y.; Zhao, Y.-H.; Lin, F.-Y.; Han, D.-D.; Dai, M.-H.; Li, W.; Pan, P.-H. Quercetin protects against LPS-induced lung injury in mice via SIRT1-mediated suppression of PKM2 nuclear accumulation. Eur. J. Pharmacol. 2022, 936, 175352. [Google Scholar] [CrossRef]
- Dicarlo, M.; Teti, G.; Verna, G.; Liso, M.; Cavalcanti, E.; Sila, A.; Raveenthiraraj, S.; Mastronardi, M.; Santino, A.; Serino, G.; et al. Quercetin Exposure Suppresses the Inflammatory Pathway in Intestinal Organoids from Winnie Mice. Int. J. Mol. Sci. 2019, 20, 5771. [Google Scholar] [CrossRef]
- Lv, W.; Jin, W.; Lin, J.; Wang, Z.; Ma, Y.; Zhang, W.; Zhu, Y.; Hu, Y.; Qu, Q.; Guo, S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine 2024, 125, 155336. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Xiang, D.; Yang, J.; Liu, D. Pharmacokinetic Characteristics of Baicalin in Rats with 17α-ethynyl-estradiol-induced Intrahepatic Cholestasis. Curr. Med. Sci. 2018, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]






| Grouping | Dispose |
|---|---|
| control group (CON) | 1–7 d 200 μL PBS by gavage |
| LPS group (LPS) | 1–7 d 200 μL PBS by gavage, 7 d 200 μL LPS intraperitoneal (3.5 mg kg−1) |
| Baicalin (BAI) | 1–7d, BAI gavage for 7d (200 mg kg−1), 7 d 200 μL LPS intraperitoneal (3.5 mg kg−1) |
| Grouping | Dispose |
|---|---|
| control group (CON) | No processing |
| LPS group (LPS) | LPS processing 1 h (40 μg/mL) |
| N-Acetyl-L-cysteine (NAC) | 1 mL LPS treatment for 1 h (40 μg/mL) after pretreatment with 1 mL NAC (diluted 1:500) |
| Grouping | Dispose |
|---|---|
| control group (CON) | No processing |
| LPS group (LPS) | LPS processing 1 h (40 μg/mL) |
| Low-dose baicalin group (LBA) | 1 mL baicalin pretreatment for 24 h (10 μM), 1 mL LPS treatment for 1 h (40 μg/mL) |
| Medium-dose baicalin group (MBA) | 1 mL baicalin pretreatment for 24 h (20 μM), 1 mL LPS treatment for 1 h (40 μg/mL) |
| High-dose baicalin group (HBA) | 1 mL baicalin pretreatment for 24 h (40 μM), 1 mL LPS treatment for 1 h (40 μg/mL) |
| Protein (PDB ID) | Center Coordinate (XYZ) | Inhibitor (Binding Energy) | Baicalin Binding Energy |
|---|---|---|---|
| ERK (2ZOQ) | 28.290769, 7.829962, 13.876654 | 5IOD (−8.03 kal/mol) | −10.18 kal/mol |
| P38 (1BL7) | 2.279160, 12.465800, 29.790320 | SB220025 (−7.9 kal/mol) | −10.25 kal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Guo, M.; Su, H.; Liang, M.; Wu, H.; Zhao, L.; Zhang, J.; He, J.; Yong, Y.; Yu, Z.; et al. Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro. Biomedicines 2025, 13, 251. https://doi.org/10.3390/biomedicines13020251
Sun X, Guo M, Su H, Liang M, Wu H, Zhao L, Zhang J, He J, Yong Y, Yu Z, et al. Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro. Biomedicines. 2025; 13(2):251. https://doi.org/10.3390/biomedicines13020251
Chicago/Turabian StyleSun, Xinyi, Mengru Guo, He Su, Mei Liang, Huining Wu, Linlu Zhao, Jin Zhang, Jieyi He, Yanhong Yong, Zhichao Yu, and et al. 2025. "Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro" Biomedicines 13, no. 2: 251. https://doi.org/10.3390/biomedicines13020251
APA StyleSun, X., Guo, M., Su, H., Liang, M., Wu, H., Zhao, L., Zhang, J., He, J., Yong, Y., Yu, Z., Ma, X., Ju, X., & Liu, X. (2025). Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro. Biomedicines, 13(2), 251. https://doi.org/10.3390/biomedicines13020251





