Relationship Between Radiological Features of Primary Empty or Primary Partial Empty Sella and Pituitary Hormone Levels
Abstract
1. Introduction
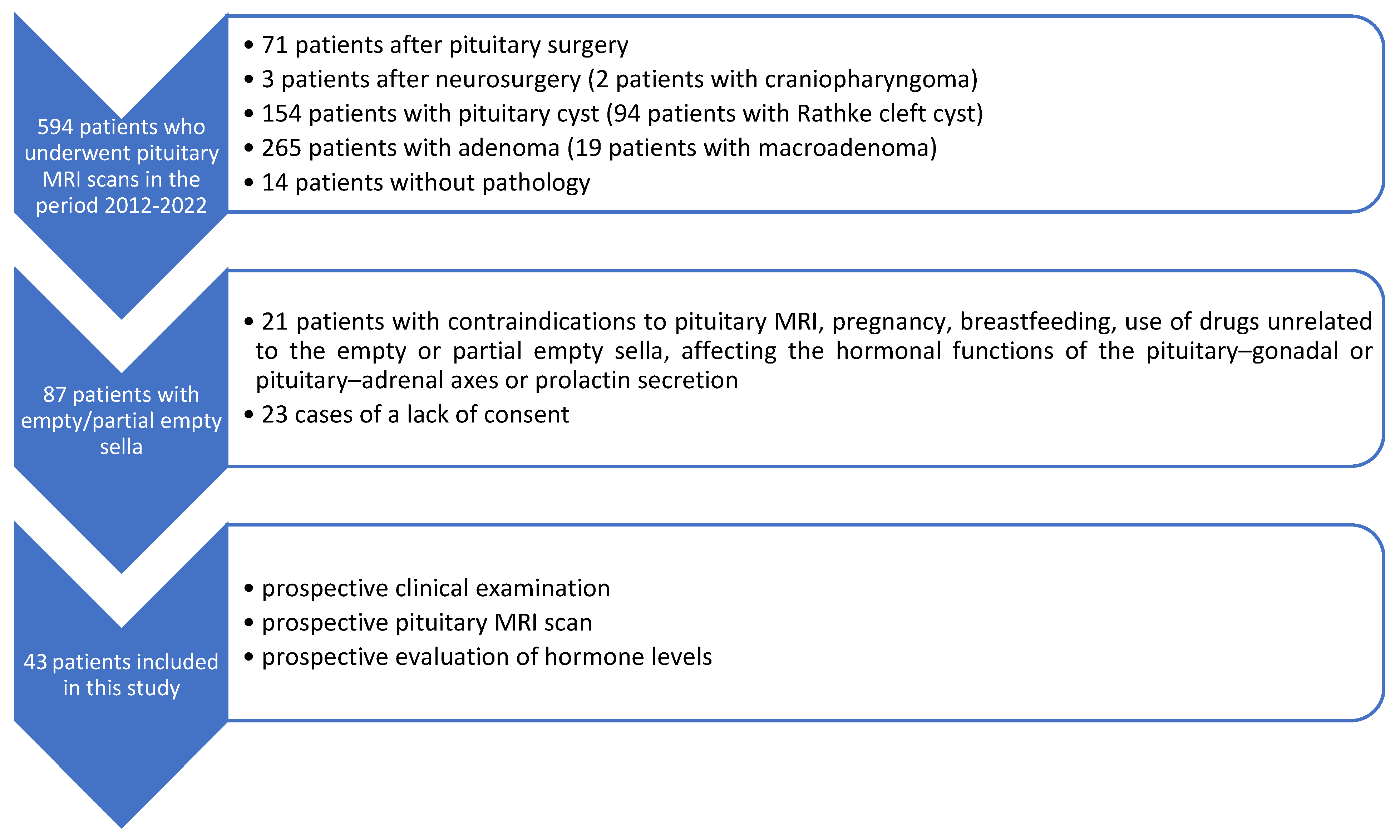
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iskra, T.; Stachera, B.; Możdżeń, K.; Murawska, A.; Ostrowski, P.; Bonczar, M.; Gregorczyk-Maga, I.; Walocha, J.; Koziej, M.; Wysiadecki, G.; et al. Morphology of the Sella Turcica: A Meta-Analysis Based on the Results of 18,364 Patients. Brain Sci. 2023, 13, 1208. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, T.; Jedliński, M.; Grocholewicz, K.; Janiszewska-Olszowska, J. Sella Turcica Morphology on Cephalometric Radiographs and Dental Abnormalities-Is There Any Association?—Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4456. [Google Scholar] [CrossRef] [PubMed]
- Bonczar, M.; Wysiadecki, G.; Ostrowski, P.; Michalczak, M.; Plutecki, D.; Wilk, J.; Michalik, W.; Walocha, J.; Balawender, K.; Iskra, T.; et al. The Morphology of the Pituitary Gland: A Meta-Analysis with Implications for Diagnostic Imaging. Brain Sci. 2023, 13, 89. [Google Scholar] [CrossRef]
- Rice-Canetto, T.E.; Carroll, P.; Reier, L.; Siddiqi, J. Asymptomatic Empty Sella: A Literature Review and Suggestions for Evaluation in Clinical Practice. Cureus 2024, 16, e75965. [Google Scholar] [CrossRef]
- Önal, V.; Evren, A.; Chatzioglou, G.O.N.; Tellioğlu, A.M. Anatomical features of sella turcica with comprehensive literature review. Rev. Assoc. Med. Bras. (1992) 2023, 69, e20230402. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Tartaglione, T.; Capobianco, A.; Anile, C.; De Marinis, L. Diagnosis of endocrine disease: Primary empty sella: A comprehensive review. Eur. J. Endocrinol. 2017, 177, 275–285. [Google Scholar] [CrossRef]
- Auer, M.K.; Stieg, M.R.; Crispin, A.; Sievers, C.; Stalla, G.K.; Kopczak, A. Primary Empty Sella Syndrome and the Prevalence of Hormonal Dysregulation. Dtsch. Arztebl. Int. 2018, 115, 99–105. [Google Scholar] [CrossRef]
- Guitelman, M.; Garcia Basavilbaso, N.; Vitale, M.; Chervin, A.; Katz, D.; Miragaya, K.; Herrera, J.; Cornalo, D.; Servidio, M.; Boero, L.; et al. Primary empty sella (PES): A review of 175 cases. Pituitary 2013, 16, 270–274. [Google Scholar] [CrossRef]
- Zuhur, S.S.; Kuzu, I.; Ozturk, F.Y.; Uysal, E.; Altuntas, Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: Do all cases need endocrinological evaluation? Turk. Neurosurg. 2014, 24, 374–379. [Google Scholar] [CrossRef]
- Padovano Sorrentino, F.; Chiloiro, S.; Giampietro, A.; Bianchi, A.; Pontecorvi, A.; De Marinis, L. Empty sella syndrome: An update. Pituitary 2024, 28, 13. [Google Scholar] [CrossRef]
- Foresti, M.; Guidali, A.; Susanna, P. Primary empty sella. Incidence in 500 asymptomatic subjects examined with magnetic resonance. Radiol. Med. 1991, 81, 803–807. [Google Scholar] [PubMed]
- Gallardo, E.; Schächter, D.; Cáceres, E.; Becker, P.; Colin, E.; Martínez, C.; Henríquez, C. The empty sella: Results of treatment in 76 successive cases and high frequency of endocrine and neurological disturbances. Clin. Endocrinol. 1992, 37, 529–533. [Google Scholar] [CrossRef]
- Lupi, I.; Manetti, L.; Raffaelli, V.; Grasso, L.; Sardella, C.; Cosottini, M.; Iannelli, A.; Gasperi, M.; Bogazzi, F.; Caturegli, P.; et al. Pituitary autoimmunity is associated with hypopituitarism in patients with primary empty sella. J. Endocrinol. Investig. 2011, 34, 240–244. [Google Scholar]
- Cannavò, S.; Curtò, L.; Venturino, M.; Squadrito, S.; Almoto, B.; Narbone, M.C.; Rao, R.; Trimarchi, F. Abnormalities of hypothalamic-pituitary-thyroid axis in patients with primary empty sella. J. Endocrinol. Investig. 2002, 25, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Cotta, O.R.; Ferone, D.; Torre, M.L.; Ferraù, F.; Di Somma, C.; Boschetti, M.; Teti, C.; Maria CSavanelli Alibrandi, A.; Trimarchi, F.; et al. Role of pituitary dysfunction on cardiovascular risk in primary empty sella patients. Clin. Endocrinol. 2013, 79, 211–216. [Google Scholar] [CrossRef]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; De Marinis, L. Empty sella syndrome: Multiple endocrine disorders. Handb. Clin. Neurol. 2021, 181, 29–40. [Google Scholar]
- Prabhat, N.; Kaur, K.; Takkar, A.; Ahuja, C.; Katoch, D.; Goyal, M.; Dutta, P.; Bhansali, A.; Lal, V. Pituitary Dysfunction in Idiopathic Intracranial Hypertension: An Analysis of 80 Patients. Can. J. Neurol. Sci. 2024, 51, 265–271. [Google Scholar] [CrossRef]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, H.M.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; He, H.; Chen, Y.; Wang, C.; Zhu, Y.; Ye, X. Osteoporotic fractures and persistent non-fusion of the hand epiphyses caused by empty sella syndrome in an adult: A case report. J. Int. Med. Res. 2013, 41, 1768–1772. [Google Scholar] [CrossRef]
- Carosi, G.; Brunetti, A.; Mangone, A.; Baldelli, R.; Tresoldi, A.; Del Sindaco, G.; Lavezzi, E.; Sala, E.; Mungari, R.; Fatti, L.M.; et al. A multicenter Cohort Study in Patients with Primary Empty Sella: Hormonal and Neuroradiological Features Over a Long Follow-Up. Front. Endocrinol. 2022, 13, 925378. [Google Scholar] [CrossRef]
- Yuh, W.T.; Zhu, M.; Taoka, T.; Quets, J.P.; Maley, J.E.; Muhonen, M.G.; Schuster, M.E.; Kardon, R.H. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J. Magn. Reson. Imaging JMRI 2000, 12, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Barkatullah, A.F.; Leishangthem, L.; Moss, H.E. MRI findings as markers of idiopathic intracranial hypertension. Curr. Opin. Neurol. 2021, 34, 75–83. [Google Scholar] [CrossRef]
- Weber, K.T.; Singh, K.D.; Hey, J.C. Idiopathic intracranial hypertension with primary aldosteronism: Report of 2 cases. Am. J. Med. Sci. 2002, 324, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Khalid, H.; Salpietro, V.; Weber, K.T. Idiopathic intracranial hypertension associated with either primary or secondary aldosteronism. Am. J. Med. Sci. 2013, 346, 194–198. [Google Scholar] [CrossRef]
- Thaller, M.; Homer, V.; Sassani, M.; Mollan, S.P.; Sinclair, A.J. Longitudinal prospective cohort study evaluating prognosis in idiopathic intracranial hypertension patients with and without comorbid polycystic ovarian syndrome. Eye 2023, 37, 3621–3628. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, N.; Kunicki, J.; Pieniak, K.; Laskus, P.; Zabielska, B.; Smolarczyk, R.; Kunicki, M. A systematic review on idiopathic intracranial hypertension comorbid with polycystic ovarian syndrome and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 1–7. [Google Scholar] [CrossRef]
- Thaller M, Mytton J, Wakerley BR, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: Evaluation of births and fertility through the Hospital Episode Statistics dataset. BJOG 2022, 129, 2019–2027. [CrossRef] [PubMed] [PubMed Central]
- Leca, B.M.; Mytilinaiou, M.; Tsoli, M.; Epure, A.; Aylwin, S.J.B.; Kaltsas, G.; Randeva, H.S.; Dimitriadis, G.K. Identification of an optimal prolactin threshold to determine prolactinma size using receiver perating characteristic analysis. Sci. Rep. 2021, 11, 9801. [Google Scholar] [CrossRef]
- Alyami, N.; Alhenaki, G.; Al Atwah, S.; Alhenaki, N.; Smaisem, F.; Alotaibi, A.; Abu Risheh j Smaisem, M.; Alhenaki, A.; Alanazi, S.; Alshammeri, M.; et al. Correlation between MRI findings of pituitary gland and prolactin level among hyperprolactinemia adult female Saudi patients in rural areas: A retrospective multicentric study. Medicine 2025, 104, e40686. [Google Scholar] [CrossRef]
- Chen, H.C.; Sung, C.C. A young man with secondary adrenal insufficiency due to empty sella syndrome. BMC Nephrol. 2022, 23, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Monte, P.; Foppiani, L.; Cafferata, C.; Marugo, A.; Bernasconi, D. Primary “empty sella” in adults: Endocrine findings. Endocr. J. 2006, 53, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Terano, T.; Seya, A.; Tamura, Y.; Yoshida, S.; Hirayama, T. Characteristic of the pituitary gland in elderly subjects from magnetic resonance images: Relationship to pituitary hormone secretion. Clin. Endocrinol. 1996, 45, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Akkus, G.; Sözütok, S.; Odabaş, F.; Onan, B.; Evran, M.; Karagun, B.; Sert, M.; Tetiker, T. Pituitary Volume in Patients with Primary Empty Sella and Clinical Relevance to Pituitary Hormone Secretion: A Retrospective Single Center Study. Curr. Med. Imaging 2021, 17, 1018–1024. [Google Scholar] [CrossRef]
- Kaess, M.; Simmons, J.G.; Whittle, S.; Jovev, M.; Chanen, A.M.; Yücel, M.; Pantelis Ch Allen, N.B. Sex-specific prediction of hypothalamic-pituitary-adrenal axis activity by pituitary volume during adolescence: A longitudinal study from 12 to 17 years of age. Psychoneuroendocrinolog 2013, 38, 2694–2704. [Google Scholar] [CrossRef]
- Ertekin, T.; Acer, N.; Turgut, A.T.; Aycan, K.; Ozçelik, O.; Turgut, M. Comparison of three methods for the estimation of the pituitary gland volume using magnetic resonance imaging: A stereological study. Pituitary 2011, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, A.; Okuda, O.; Sato, K. MR Height of the Pituitary Gland as a Function of Age and Sex: Especially Physiological Hypertrophy in Adolescence and in Climacterium. AJNR Am. J. Neuroradiol. 1997, 18, 551–554. [Google Scholar] [PubMed]
- Destrieux, C.; Kakou, M.K.; Velut, S.; Lefrancq, T.; Jan, M. Microanatomy of the hypophyseal fossa boundaries. J. Neurosurg. 1998, 88, 743–752. [Google Scholar] [CrossRef]
- Peker, S.; Kurtkaya-Yapicier, O.; Kiliç, T.; Pamir, M.N. Microsurgical anatomy of the lateral walls of the pituitary fossa. Acta Neurochir. 2005, 147, 641–648. [Google Scholar] [CrossRef]
- Yasuda, A.; Campero, A.; Martins, C.; Rhoton, A.L.; Ribas, G.C. The medial wall of the cavernous sinus: Microsurgical anatomy. Neurosurgery 2004, 55, 179–189. [Google Scholar] [CrossRef]
- Dos Santos Silva, J.; Schreiner, C.A.; de Lima, L.; Brigido, C.E.P.L.; Wilson, C.D.; McVeigh, L.; Acchiardo, J.; Landeiro, J.A.; Acioly, M.A.; Cohen-Gadol, A. Volumetric measurement of intracranial meningiomas: A comparison between linear, planimetric, and machine learning with multiparametric voxel-based morphometry methods. J. Neurooncol. 2023, 161, 235–243. [Google Scholar] [CrossRef]
| Variable | Patients Without Hormonal Disorders n = 37 | Patients with Hormonal Disorders n = 6 | p-Value |
|---|---|---|---|
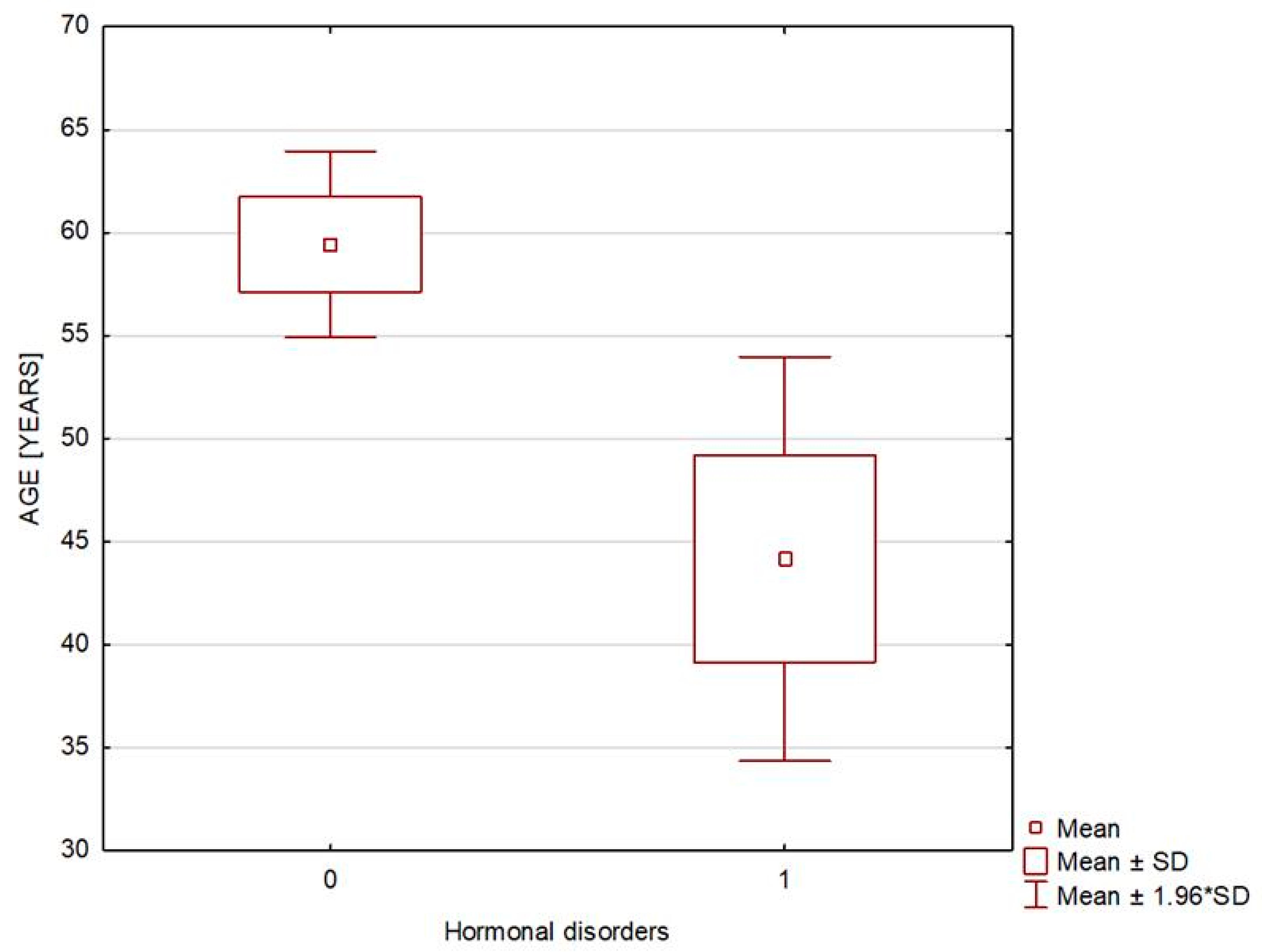
| Age [years] | 59.432 ± 14.041 | 44.167 ± 12.254 | 0.021 |
| Sex [M] | 9 (2.324%) | 0 (0%) | 0.187 |
| Body weight [kg] | 79.697 ± 13.462 | 67.583 ± 10.414 | 0.034 |
| BMI [kg/m2] | 28.096 ± 4.567 | 25.45 ± 3.169 | 0.045 |
| Overweight and obesity | 27 (72.973%) | 3 (50%) | 0.271 |
| Waist circumference [cm] | 95.125 ± 11.284 | 79.833 ± 15.943 | 0.022 |
| Arterial hypertension | 16 (43.243%) | 1 (16.667%) | 0.16 |
| Variable | Patients Without Hormonal Disorders n = 37 | Patients with Hormonal Disorders n = 6 | p-Value | ||
|---|---|---|---|---|---|
| Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | ||
| Specific gravity of urine [g/mL] | 1.016 (1.007–1.04) | 1.017 ± 0.007 | 1.013 (1.006–1.028) | 1.016 ± 0.009 | 0.663 |
| Serum sodium [mmol/L] | 142 (128–145) | 141 ± 3.04 | 137.50 (136–141) | 138 ± 1.79 | 0.005 |
| Serum potassium [mmol/L] | 4.55 (3.78–5.39) | 4.54 ± 0.39 | 4.13 (3.69–4.49) | 4.12 ± 0.29 | 0.016 |
| Serum osmolality [mOsm/kg] | 291 (278–313) | 290.87 ± 6.79 | 287 (282–293) | 287.33 ± 3.67 | 0.171 |
| Urine osmolality [mOsm/kg] | 539.50 (251–956) | 571.75 ± 203.21 | 440.5 (258–957) | 558.33 ± 304.79 | 0.787 |
| TSH [µIU/mL] | 1.95 (0.27–4.08) | 1.959 ± 0.97 | 2.02 (0.95–2.82) | 1.90 ± 0.74 | 0.899 |
| fT3 [pg/mL] | 3.06 (2.16–3.82) | 3.10 ± 0.39 | 2.75 (2.34–3.58) | 2.83 ± 0.43 | 0.109 |
| fT4 [ng/dL] | 1.26 (1.02–1.70) | 1.27 ± 0.162 | 1.10 (0.90–1.33) | 1.11 ± 0.168 | 0.081 |
| Prolactin [ng/mL] | 11.00 (4.9–20.8) | 11.86 ± 5.21 | 15.15 (3.20–52.60) | 22.08 ± 18.69 | 0.326 |
| Cortisol at 8 a.m. [µg/dL] | 14.30 (6.1–24.5) | 13.68 ± 3.63 | 11.6 (8.90–24.30) | 14.73 ± 6.56 | 0.746 |
| ACTH at 8 a.m. [pg/mL] | 22.5 (7.3–87.2) | 25.82 ± 16.29 | 15.8 (2.30–28.90) | 16.08 ± 9.04 | 0.136 |
| Growth hormone [ng/mL] | 0.44 (0.05–17.10) | 1.32 ± 2.99 | 0.45 (0.17–8.96) | 2.17 ± 3.47 | 0.472 |
| IGF-1 [ng/mL] | 135.50 (61.33–253.50) | 140.84 ± 46.41 | 188.70 (82.65–350.80) | 195.09 ± 94.43 | 0.166 |
| Variable | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| ACTH [pg/mL] (n = 6) | 16.067 | 9.041 | 15.800 | 2.300 | 28.900 |
| ACTH [pg/mL] in patients with secondary adrenocortical insufficiency on glucocorticoid replacement with Hydrocortisonum (n = 3) | 10.667 | 7.786 | 12.000 | 2.300 | 17.700 |
| Serum cortisol [µg/dL] | 14.733 | 6.563 | 11.600 | 8.900 | 24.300 |
| Serum cortisol [µg/dL] in patients with secondary adrenocortical insufficiency on glucocorticoid replacement with Hydrocortisonum (n = 3) | 18.300 | 8.24 | 21.700 | 8.900 | 24.300 |
| Serum prolactin [ng/mL] (n = 6) | 22.083 | 18.691 | 15.150 | 3.200 | 52.600 |
| Serum prolactin [ng/mL] in patients with hyperprolactinemia (n = 4) | 26.700 | 22.154 | 25.500 | 3.200 | 52.600 |
| Serum prolactin [ng/mL] in patients with hyperprolactinemia receiving dopamine receptor agonists (n = 2) | 8.800 | 7.919 | 8.800 | 3.200 | 14.400 |
| Serum prolactin [ng/mL] in patients with hyperprolactinemia receiving no dopamine receptor agonists (n = 2) | 44.600 | 11.314 | 44.600 | 36.600 | 52.600 |
| TSH [µIU/mL] | 1.904 | 0.741 | 2.020 | 0.952 | 2.820 |
| fT3 [pg/mL] | 2.833 | 0.426 | 2.745 | 2.340 | 3.580 |
| fT4 [ng/dL] | 1.110 | 0.168 | 1.100 | 0.900 | 1.330 |
| Growth hormone [ng/mL] | 2.168 | 3.465 | 0.450 | 0.170 | 8.960 |
| IGF-1 [ng/mL] | 195.092 | 94.434 | 188.700 | 82.650 | 350.800 |
| Variable | Patients Without Hormonal Disorders n = 37 | Patients with Hormonal Disorders n = 6 | p-Value | ||
|---|---|---|---|---|---|
| Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | ||
| Pituitary AP diameter [mm] | 12.00 (7.50–17.10) | 12.40 ± 2.03 | 12.00(10.00–13.30) | 12.00 ± 1.38 | 0.659 |
| Pituitary RL diameter [mm] | 16.00 (5.00–25.00) | 15.95 ± 3.27 | 16.25 (14.40–20.00) | 16.80 ± 2.39 | 0.516 |
| Pituitary CC diameter [mm] | 2.80 (0.80–5.00) | 2.97 ± 1.06 | 2.90 (1.40–4.50) | 3.05 ± 1.093 | 0.752 |
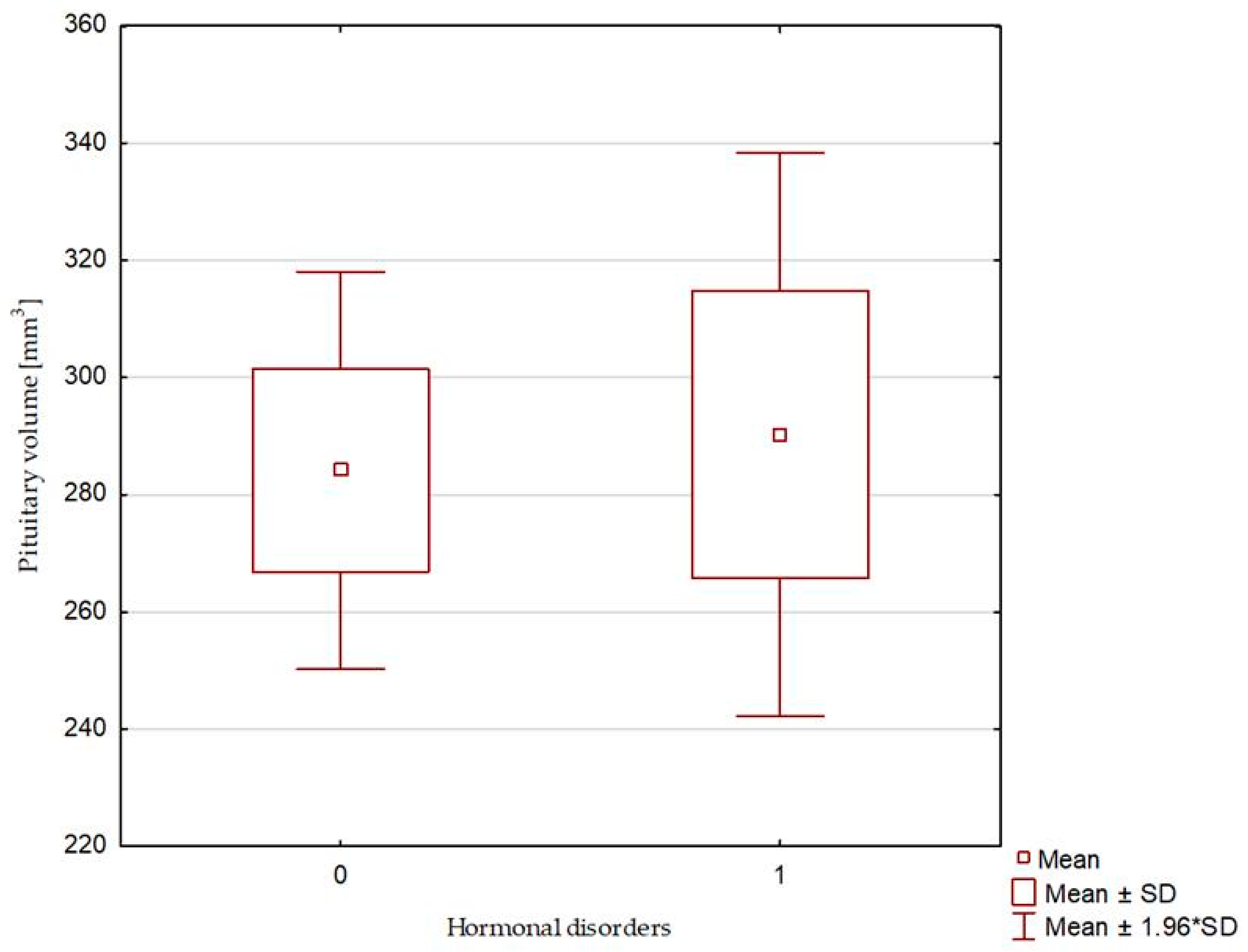
| Pituitary volume [mm3] | 276.12 (62.40–536.30) | 284.14 ± 105.12 | 299.55 (194.194–368.00) | 290.29 ± 60.05 | 0.739 |
| Sella turcica—AP diameter [mm] | 12.00 (7.50–17.10) | 12.51 ± 2.08 | 12.50 (10.00–14.30) | 12.38 ± 1.15 | 0.902 |
| Sella turcica—RL diameter [mm] | 16.00 (5.00–25.00) | 15.95 ± 3.27 | 16.25 (14.40–20.00) | 16.80 ± 2.39 | 0.516 |
| Sella turcica—bony—CC diameter [mm] | 10.40 (7.30–17.40) | 11.25 ± 2.53 | 10.40 (9.50–13.30) | 10.68 ± 1.43 | 0.833 |
| Sella turcica—diaphragma sellae—CC diameter [mm] | 7.40 (5.50–15.70) | 8.20 ± 2.46 | 7.90 (6.50–9.70) | 7.97 ± 1.26 | 0.699 |
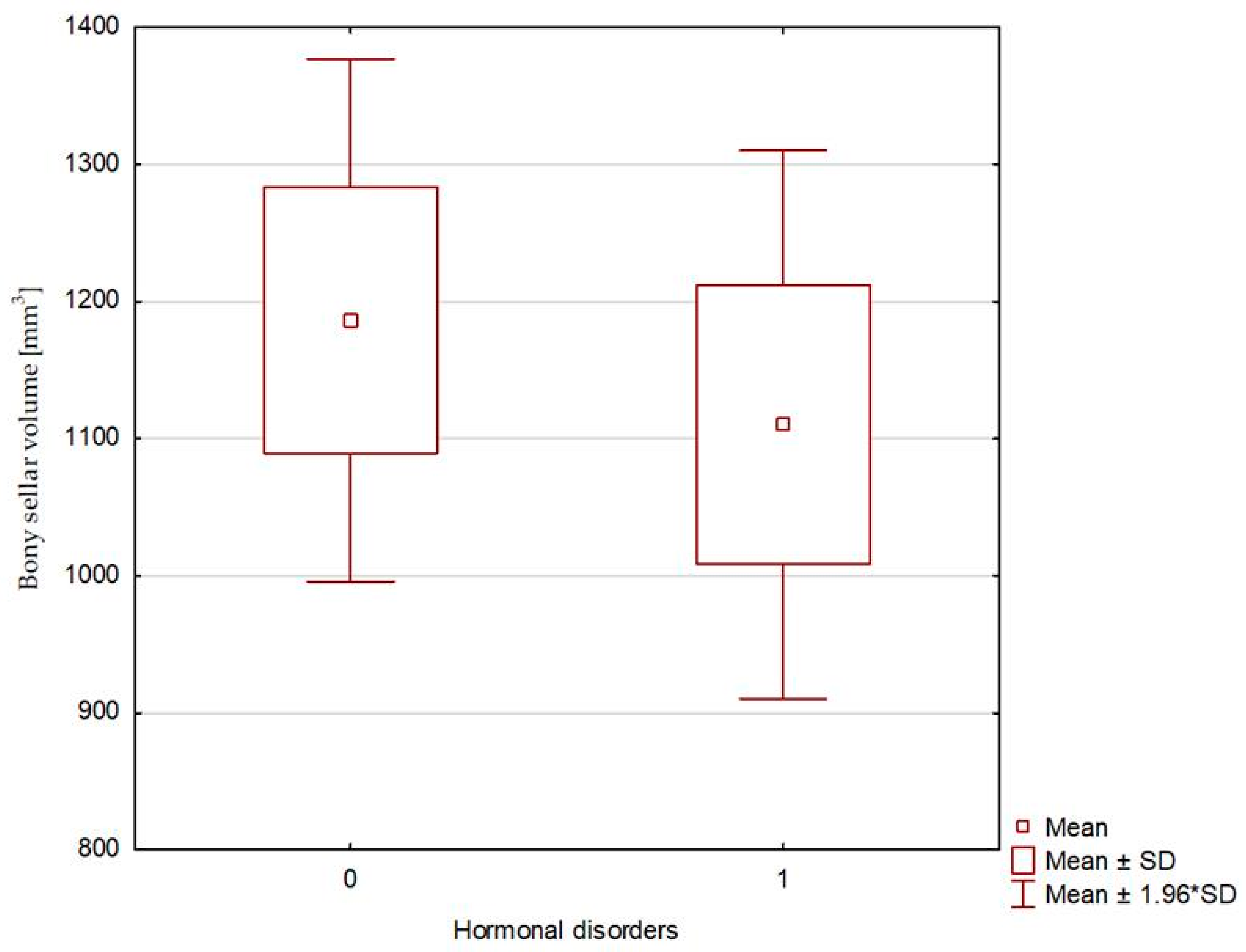
| Bony sellar volume [mm3] | 976.19 (140.63–1683.71) | 1186.41 ± 591.43 | 1099.03 (792.00–1498.07) | 1110.28 ± 249,81 | 0.661 |
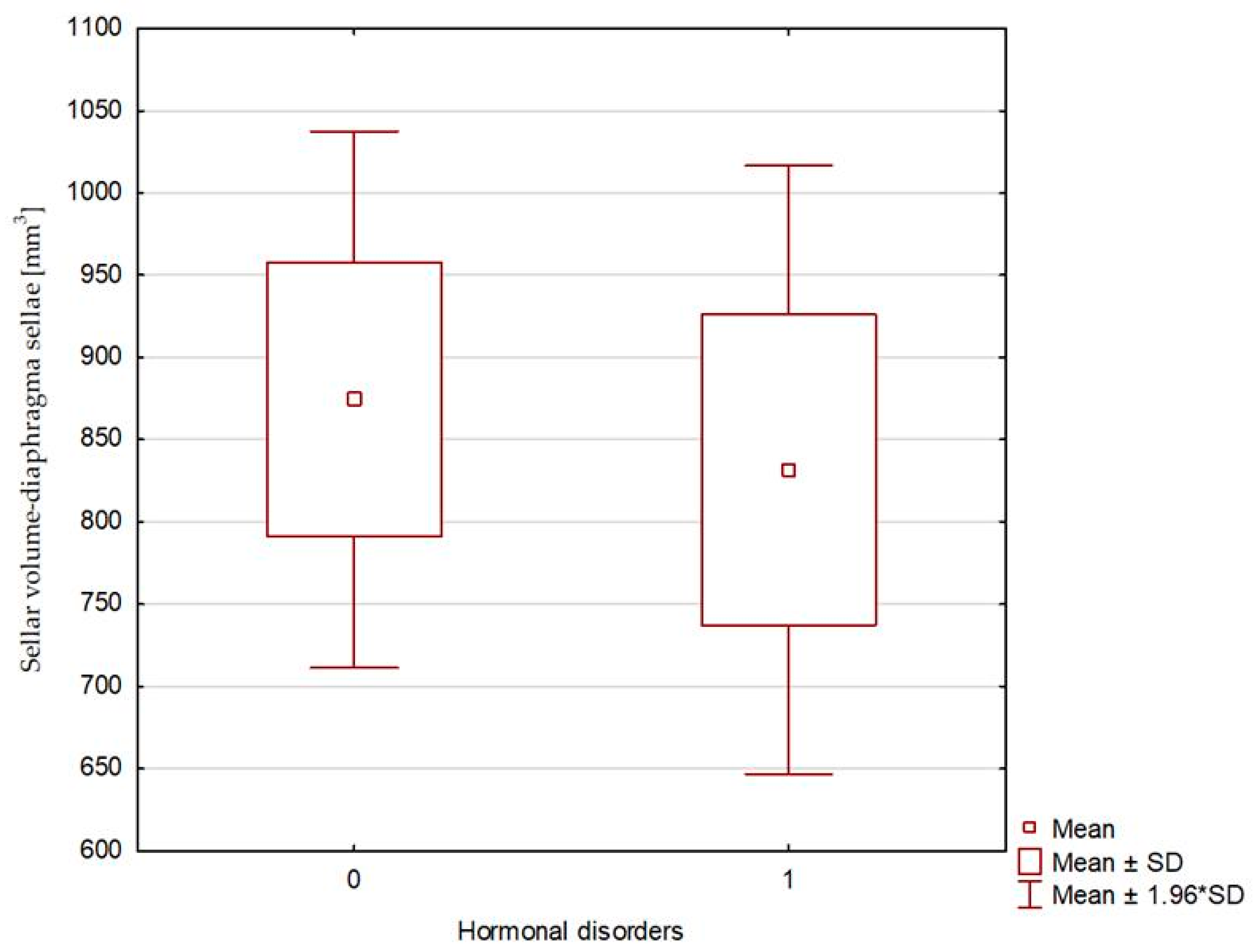
| Sellar volume—diaphragma sellae [mm3] | 665.63 (116.25–2316.99) | 874.48 ± 506.25 | 769.97 (597.60–1248.39) | 831.69 ± 231.16 | 0.451 |
| Pituitary volume expressed as a percentage of sellar volume [%] | 28.79 (6.02–66.67) | 28.15 ± 12.93 | 27.25 (12.96–40.91) | 28.14 ± 10.91 | 0.986 |
| Pituitary height expressed as a percentage of bony sella height [%] | 28.79 (6.02–66.67) | 28.35 ± 12.94 | 28.95 (12.96–40.91) | 28.93 ± 10.74 | 0.875 |
| Pituitary height expressed as a percentage of sella height—diaphragma sellae [%] | 39.79 (9.09–80.65) | 40.08 ± 19.25 | 41.43 (15.56–54.22) | 39.31 ± 14.84 | 0.986 |
| Right optic nerve sheath diameter [mm] | 4.65 (3.80–5.80) | 4.71 ± 0.44 | 4.75 (4.40–5.30) | 4.77 ± 0.33 | 0.793 |
| Left optic nerve sheath diameter [mm] | 4.60 (3.70–6.30) | 4.56 ± 0.51 | 4.5 (3.90–5.30) | 4.57 ± 0.47 | 0.930 |
| VARIABLE | N (%) | N (%) | p-value | ||
| Empty sella—with the pituitary CC diameter less than 2 mm | 8 (21.62%) | 1 (16.67%) | 0.804 | ||
| Empty sella—with the pituitary CC diameter less than 3 mm | 19 (51.35%) | 4 (66.67%) | 0.503 | ||
| Pituitary stalk displacement | 21 (56.76%) | 3 (50.00%) | 0.757 | ||
| Enlarged cerebrospinal fluid spaces | 10 (27.03%) | 1 (16.67%) | 0.589 | ||
| Variable | Hormonal Disorders OR (95% CI) p-Value | |
|---|---|---|
| Multivariate Logistic Regression | Univariate Logistic Regression | |
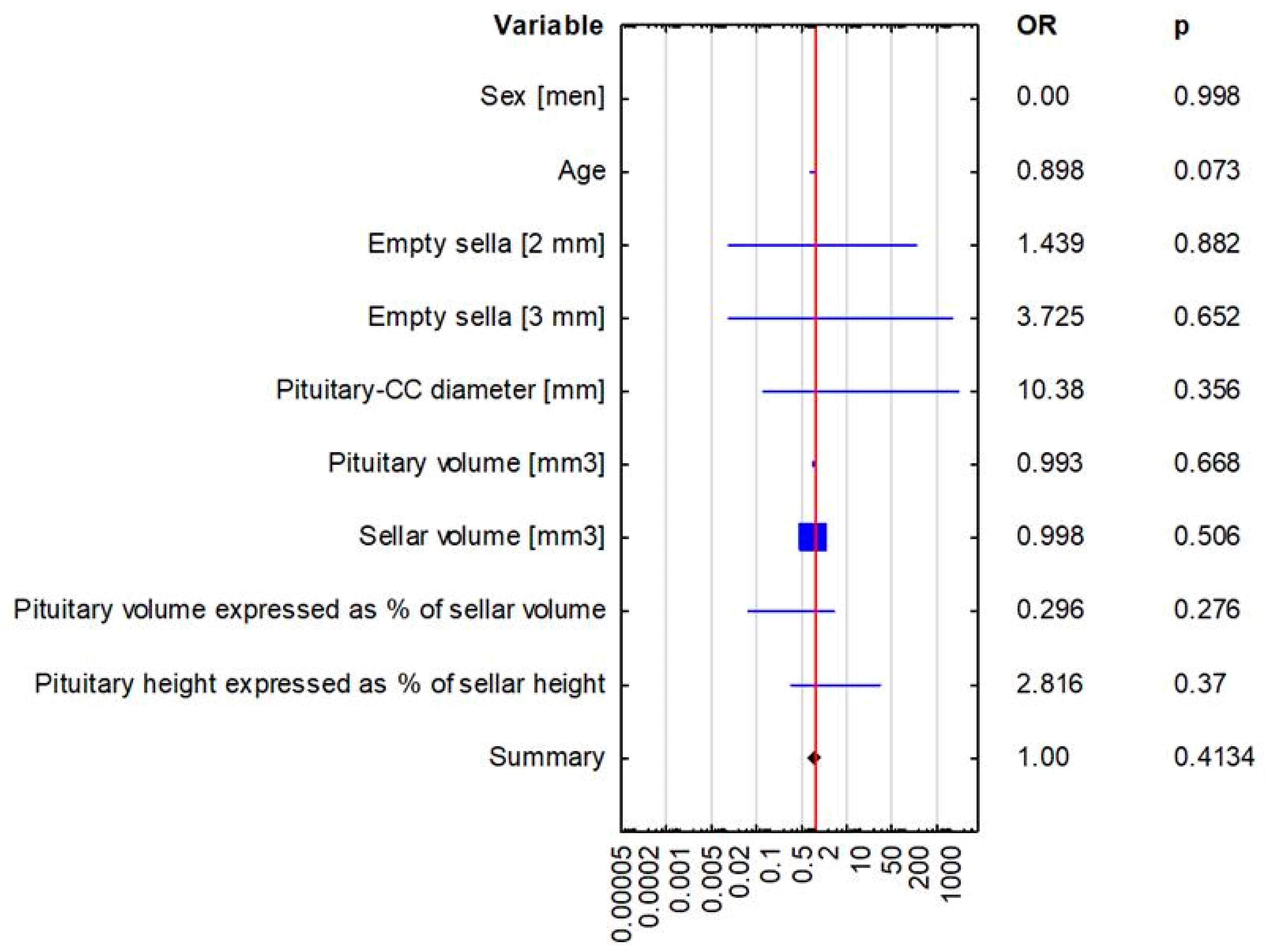
| Sex | 0.00 (0.00–0.00), p = 0.998 | 0.00 (0.00–0.00), p = 0.997 |
| Age | 0.898 (0.798–1.01), p = 0.073 | 0.916 (0.844–0.993), p = 0.034 |
| Empty sella [2 mm] | 1.439 (0.008–297.121), p = 0.882 | 0.725 (0.074–7.126), p = 0.783 |
| Empty sella [3 mm] | 3.725 (0.012–1131.502), p = 0.652 | 1.895 (0.308–11.644), p = 0.49 |
| Pituitary—craniocaudal diameter [mm] | 10.380 (0.072–1495.711), p = 0.356 | 1.073 (0.469–2.454), p = 0.8668 |
| Pituitary volume [mm3] | 0.993 (0.964–1.024), p = 0.668 | 1.001 (0.992–1.009), p = 0.887 |
| Sellar volume [mm3] | 0.998 (0.992–1.004), p = 0.506 | 1 (0.998–1.001), p = 0.7531 |
| Pituitary volume expressed as percentage of sellar volume(bony sella) | 0.296 (0.033–2.639), p = 0.276 | 1 (0.933–1.072), p = 0.999 |
| Pituitary height expressed as percentage of sellar height (bony sella) | 2.816 (0.293–27.105), p = 0.37 | 1.004 (0.937–1.076), p = 0.916 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałuża, B.; Furmanek, M.; Domański, J.; Żuk-Łapan, A.; Babula, E.; Poprawa, I.; Walecki, J.; Franek, E. Relationship Between Radiological Features of Primary Empty or Primary Partial Empty Sella and Pituitary Hormone Levels. Biomedicines 2025, 13, 722. https://doi.org/10.3390/biomedicines13030722
Kałuża B, Furmanek M, Domański J, Żuk-Łapan A, Babula E, Poprawa I, Walecki J, Franek E. Relationship Between Radiological Features of Primary Empty or Primary Partial Empty Sella and Pituitary Hormone Levels. Biomedicines. 2025; 13(3):722. https://doi.org/10.3390/biomedicines13030722
Chicago/Turabian StyleKałuża, Bernadetta, Mariusz Furmanek, Jan Domański, Aleksandra Żuk-Łapan, Emilia Babula, Iga Poprawa, Jerzy Walecki, and Edward Franek. 2025. "Relationship Between Radiological Features of Primary Empty or Primary Partial Empty Sella and Pituitary Hormone Levels" Biomedicines 13, no. 3: 722. https://doi.org/10.3390/biomedicines13030722
APA StyleKałuża, B., Furmanek, M., Domański, J., Żuk-Łapan, A., Babula, E., Poprawa, I., Walecki, J., & Franek, E. (2025). Relationship Between Radiological Features of Primary Empty or Primary Partial Empty Sella and Pituitary Hormone Levels. Biomedicines, 13(3), 722. https://doi.org/10.3390/biomedicines13030722





