Characterization of Sublingual Microvascular Tortuosity in Steady-State Physiology and Septic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Patients with Steady-State Physiology
2.3. Patients with Septic Shock
2.4. Measurements
2.4.1. Macrohemodynamics
2.4.2. Sublingual Microcirculation
2.4.3. Determination of Capillary Shear Stress
2.4.4. Oxygen Transport and Transitions of Metabolism
2.5. Statistical Analysis
3. Results
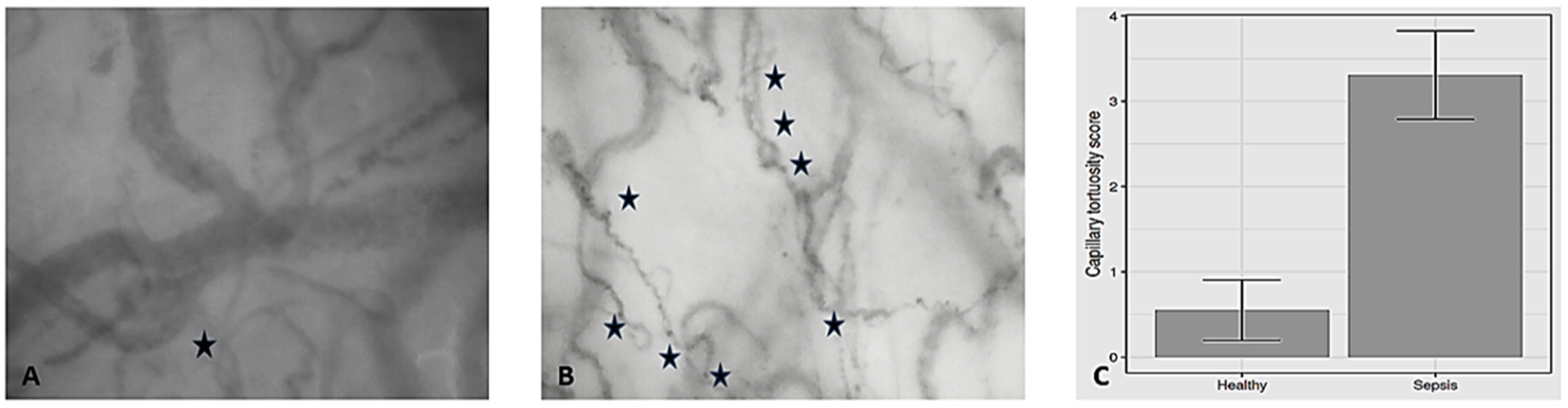
3.1. Capillary Tortuosity in Individuals with Steady-State Physiology
3.2. Capillary Tortuosity in Patients with Septic Shock
3.3. Association of Capillary Tortuosity with Hemodynamic and Oxygen Transport/Metabolic Variables in the Entire Study Sample
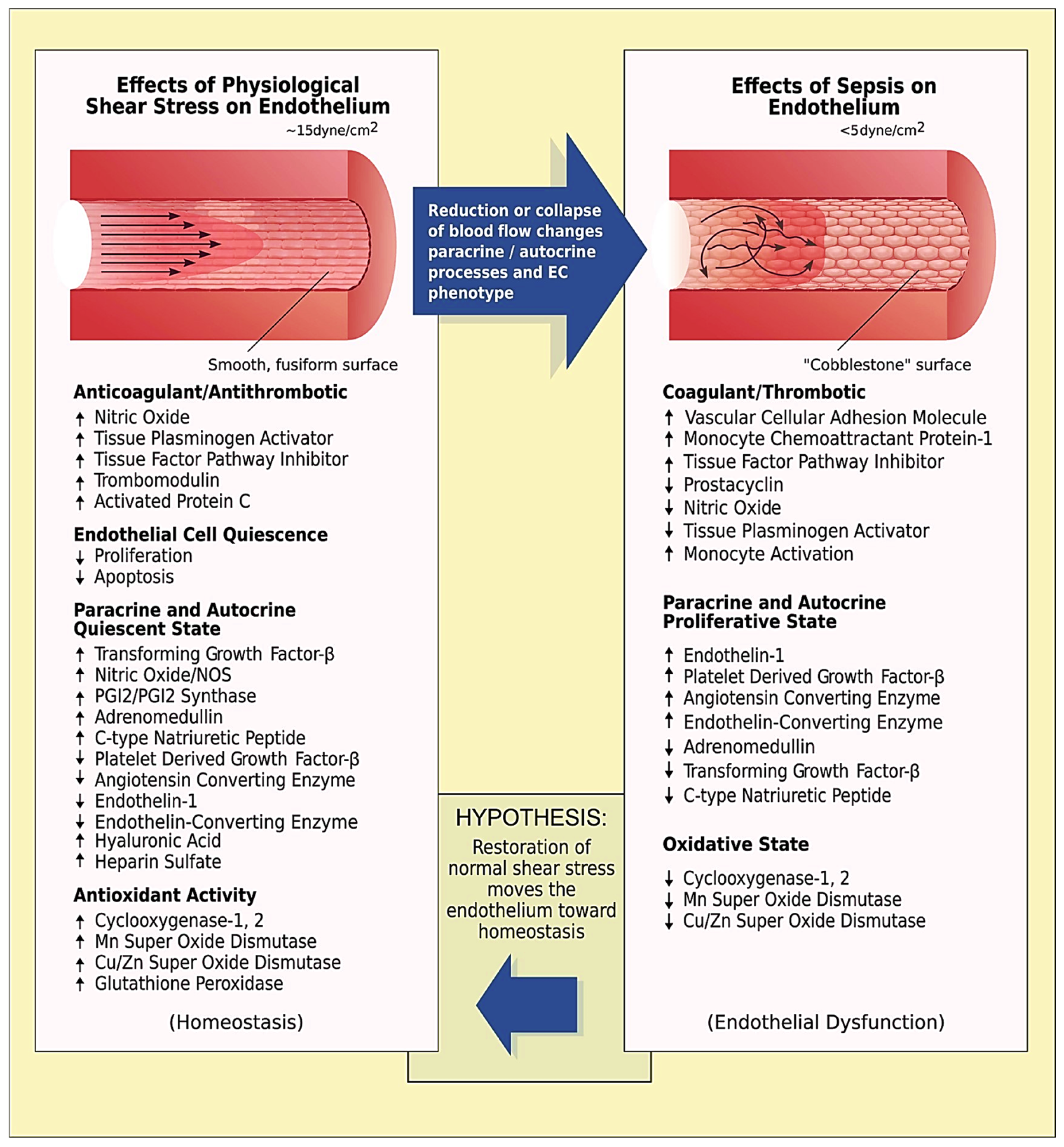
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duranteau, J.; De Backer, D.; Donadello, K.; Shapiro, N.I.; Hutchings, S.D.; Rovas, A.; Legrand, M.; Harrois, A.; Ince, C. The future of intensive care: The study of the microcirculation will help to guide our therapies. Crit. Care 2023, 27, 190. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Boerma, E.C.; Cecconi, M.; De Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, K.; Teboul, J.L.; Varin, L.; Vicaut, E.; Duranteau, J. Preload dependence is associated with reduced sublingual microcirculation during major abdominal surgery. Anesthesiology 2019, 130, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bansch, P.; Flisberg, P.; Bentzer, P. Changes in the sublingual microcirculation during major abdominal surgery and post-operative morbidity. Acta Anaesthesiol. Scand. 2014, 58, 89–97. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Chong, D.C.; Yu, Z.; Brighton, H.E.; Bear, J.E.; Bautch, V.L. Tortuous Microvessels Contribute to Wound Healing via Sprouting Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1903–1912. [Google Scholar] [CrossRef]
- Nichols, D.; Nielsen, N.D. Oxygen delivery and consumption: A macrocirculatory perspective. Crit. Care Clin. 2010, 26, 239–253. [Google Scholar] [CrossRef]
- Chalkias, A.; Xenos, M. Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. J. Clin. Med. 2022, 11, 4885. [Google Scholar] [CrossRef]
- Chalkias, A.; Laou, E.; Mermiri, M.; Michou, A.; Ntalarizou, N.; Koutsona, S.; Chasiotis, G.; Garoufalis, G.; Agorogiannis, V.; Kyriakaki, A.; et al. Microcirculation-guided treatment improves tissue perfusion and hemodynamic coherence in surgical patients with septic shock. Eur. J. Trauma Emerg. Surg. 2022, 48, 4699–4711. [Google Scholar] [CrossRef]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Frerk, C.; Mitchell, V.S.; McNarry, A.F.; Mendonca, C.; Bhagrath, R.; Patel, A.; O’Sullivan, E.P.; Woodall, N.M.; Ahmad, I. Difficult Airway Society intubation guidelines working group. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015, 115, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Higgs, A.; McGrath, B.A.; Goddard, C.; Rangasami, J.; Suntharalingam, G.; Gale, R.; Cook, T.M.; Difficult Airway Society; Intensive Care Society; Faculty of Intensive Care Medicine; et al. Guidelines for the management of tracheal intubation in critically ill adults. Br. J. Anaesth. 2018, 120, 323–352. [Google Scholar] [CrossRef] [PubMed]
- Russotto, V.; Myatra, S.N.; Laffey, J.G.; Tassistro, E.; Antolini, L.; Bauer, P.; Lascarrou, J.B.; Szuldrzynski, K.; Camporota, L.; Pelosi, P.; et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients from 29 Countries. JAMA 2021, 325, 1164–1172. [Google Scholar] [CrossRef]
- Laou, E.; Papagiannakis, N.; Sarchosi, S.; Kleisiaris, K.; Apostolopoulou, A.; Syngelou, V.; Kakagianni, M.; Christopoulos, A.; Ntalarizou, N.; Chalkias, A. The use of mean circulatory filling pressure analogue for monitoring hemodynamic coherence: A post-hoc analysis of the SPARSE data and proof-of-concept study. Clin. Hemorheol. Microcirc. 2023, 84, 19–32. [Google Scholar] [CrossRef]
- Fincke, R.; Hochman, J.S.; Lowe, A.M.; Menon, V.; Slater, J.N.; Webb, J.G.; LeJemtel, T.H.; Cotter, G.; SHOCK Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. J. Am. Coll. Cardiol. 2004, 44, 340–348. [Google Scholar] [CrossRef]
- Massey, M.J.; Larochelle, E.; Najarro, G.; Karmacharla, A.; Arnold, R.; Trzeciak, S.; Angus, D.C.; Shapiro, N.I. The microcirculation image quality score: Development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J. Crit. Care 2013, 28, 913–917. [Google Scholar] [CrossRef]
- Dobbe, J.G.; Streekstra, G.J.; Atasever, B.; van Zijderveld, R.; Ince, C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med. Biol. Eng. Comput. 2008, 46, 659–670. [Google Scholar] [CrossRef]
- Djaberi, R.; Schuijf, J.D.; de Koning, E.J.; Wijewickrama, D.C.; Pereira, A.M.; Smit, J.W.; Kroft, L.J.; Roos, A.; Bax, J.J.; Rabelink, T.J.; et al. Non-invasive assessment of microcirculation by sidestream dark field imaging as a marker of coronary artery disease in diabetes. Diab. Vasc. Dis. Res. 2013, 10, 123–134. [Google Scholar] [CrossRef]
- Yu, P.K.; Balaratnasingam, C.; Cringle, S.J.; McAllister, I.L.; Provis, J.; Yu, D.Y. Microstructure and network organization of the microvasculature in the human macula. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6735–6743. [Google Scholar] [CrossRef]
- Cho, Y.I.; Cho, D.J. Hemorheology and microvascular disorders. Korean Circ. J. 2011, 41, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Munson, B.R.; Young, D.F.; Okiishi, T.H.; Huebsch, W.W. Fundamentals of Fluid Mechanics, 6th ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Pittman, R.N. Oxygen gradients in the microcirculation. Acta Physiol. 2011, 202, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.P.; Pittman, R.N. Oxygen exchange in the microcirculation of hamster retractor muscle. Am. J. Physiol. 1989, 256, H247–H255. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Pittman, R.N. Effect of hemodilution on oxygen transport in arteriolar networks of hamster striated muscle. Am. J. Physiol. 1988, 254, H331–H339. [Google Scholar]
- Kuo, L.; Pittman, R.N. Influence of hemoconcentration on arteriolar oxygen transport in hamster striated muscle. Am. J. Physiol. 1990, 259, H1694–H1702. [Google Scholar] [CrossRef]
- Johnson, P.C.; Wayland, H. Regulation of blood flow in single capillaries. Am. J. Physiol. 1967, 212, 1405–1415. [Google Scholar] [CrossRef]
- Wayland, H.; Johnson, P.C. Erythrocyte velocity measurement in microvessels by a two-slit photometric method. J. Appl. Physiol. 1967, 22, 333–337. [Google Scholar] [CrossRef]
- Awan, Z.A.; Häggblad, E.; Wester, T.; Kvernebo, M.S.; Halvorsen, P.S.; Kvernebo, K. Diffuse reflectance spectroscopy: Systemic and microvascular oxygen saturation is linearly correlated and hypoxia leads to increased spatial heterogeneity of microvascular saturation. Microvasc. Res. 2011, 81, 245–251. [Google Scholar] [CrossRef]
- Torres Filho, I.; Nguyen, N.M.; Jivani, R.; Terner, J.; Romfh, P.; Vakhshoori, D.; Ward, K.R. Oxygen saturation monitoring using resonance Raman spectroscopy. J. Surg. Res. 2016, 201, 425–431. [Google Scholar] [CrossRef]
- Lipowsky, H.H.; Usami, S.; Chien, S.; Pittman, R.N. Hematocrit determination in small bore tubes by differential spectrophotometry. Microvasc. Res. 1982, 24, 42–55. [Google Scholar] [CrossRef]
- Fåhræus, R. The suspension stability of blood. Physiol. Rev. 1929, 9, 241–274. [Google Scholar] [CrossRef]
- Farina, A.; Fasano, A.; Rosso, F. A theoretical model for the Fahraeus effect in medium-large microvessels. J. Theor. Biol. 2023, 558, 111355. [Google Scholar] [CrossRef] [PubMed]
- Archontakis-Barakakis, P.; Mavridis, T.; Chlorogiannis, D.-D.; Barakakis, G.; Laou, E.; Sessler, D.I.; Gkiokas, G.; Chalkias, A. Intestinal oxygen utilisation and cellular adaptation during intestinal ischaemia-reperfusion injury. Clin. Transl. Med. 2025, 15, e70136. [Google Scholar] [CrossRef] [PubMed]
- Dunham, C.M.; Siegel, J.H.; Weireter, L.; Fabian, M.; Goodarzi, S.; Guadalupi, P.; Gettings, L.; Linberg, S.E.; Vary, T.C. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit. Care Med. 1991, 19, 231–243. [Google Scholar] [CrossRef]
- Shoemaker, W.C.; Appel, P.L.; Kram, H.B. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit. Care Med. 1988, 16, 1117–1120. [Google Scholar] [CrossRef]
- Papagiannakis, N.; Ragias, D.; Ntalarizou, N.; Laou, E.; Kyriakaki, A.; Mavridis, T.; Vahedian-Azimi, A.; Sakellakis, M.; Chalkias, A. Transitions from Aerobic to Anaerobic Metabolism and Oxygen Debt during Elective Major and Emergency Non-Cardiac Surgery. Biomedicines 2024, 12, 1754. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Weibel, J.; Fields, W.S. Tortuosity, coiling, and kinking of the internal carotid artery. I. Etiology and radiographic anatomy. Neurology 1965, 15, 7–18. [Google Scholar] [CrossRef]
- Schep, G.; Kaandorp, D.W.; Bender, M.H.; Weerdenburg, H.; van Engeland, S.; Wijn, P.F. Magnetic resonance angiography used to detect kinking in the iliac arteries in endurance athletes with claudication. Physiol. Meas. 2001, 22, 475–487. [Google Scholar] [CrossRef]
- Helisch, A.; Schaper, W. Arteriogenesis: The development and growth of collateral arteries. Microcirculation 2003, 10, 83–97. [Google Scholar] [CrossRef]
- Batra, S.; Rakusan, K. Capillary length, tortuosity, and spacing in rat myocardium during cardiac cycle. Am. J. Physiol. 1992, 263, H1369–H1376. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Costello, O.; Potter, R.F.; Ellis, C.G.; Groom, A.C. Capillary configuration and fiber shortening in muscles of the rat hindlimb: Correlation between corrosion casts and stereological measurements. Microvasc. Res. 1988, 36, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W. Structural adaptation of microvascular networks and development of hypertension. Microcirculation 2002, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, P.M.; Marshburn, T.H.; Maultsby, S.J.; Lynch, C.D.; Smith, T.L.; Dusseau, J.W. Long-term microvascular response to hydralazine in spontaneously hypertensive rats. Hypertension 1988, 12, 74–79. [Google Scholar] [CrossRef]
- Miodoński, A.J.; Litwin, J.A. Microvascular architecture of the human urinary bladder wall: A corrosion casting study. Anat. Rec. 1999, 254, 375–381. [Google Scholar] [CrossRef]
- Owen, C.G.; Newsom, R.S.; Rudnicka, A.R.; Barman, S.A.; Woodward, E.G.; Ellis, T.J. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology 2008, 115, e27–e32. [Google Scholar] [CrossRef]
- Amemiya, T.; Bhutto, I.A. Retinal vascular changes and systemic diseases: Corrosion cast demonstration. Ital. J. Anat. Embryol. 2001, 106, 237–244. [Google Scholar]
- Zebic Mihic, P.; Saric, S.; Bilic Curcic, I.; Mihaljevic, I.; Juric, I. The Association of Severe Coronary Tortuosity and Non-Obstructive Coronary Artery Disease. Medicina 2023, 59, 1619. [Google Scholar] [CrossRef]
- Pittman, R.N. Oxygen transport in the microcirculation and its regulation. Microcirculation 2013, 20, 117–137. [Google Scholar] [CrossRef]
- Han, H.C. Twisted blood vessels: Symptoms, etiology and biomechanical mechanisms. J. Vasc. Res. 2012, 49, 185–197. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Miner, M.M.; Bulkley, B.H. Tortuosity as an index of the age and diameter increase of coronary collateral vessels in patients after acute myocardial infarction. Am. J. Cardiol. 1978, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, U.; Scherillo, G.; Casaburi, C.; Di Martino, M.; Di Gianni, A.; Serpico, R.; Fazio, S.; Saccà, L. Prospective evaluation of hypertensive patients with carotid kinking and coiling: An ultrasonographic 7-year study. Angiology 2003, 54, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Ellis, C.G. Bench-to-bedside review: Microvascular dysfunction in sepsis–hemodynamics, oxygen transport, and nitric oxide. Crit. Care 2003, 7, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G. Sepsis impairs microvascular autoregulation and delays capillary response within hypoxic capillaries. Crit. Care 2015, 19, 389. [Google Scholar] [CrossRef]
- Singel, D.J.; Stamler, J.S. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005, 67, 99–145. [Google Scholar] [CrossRef]
- Khosravani-Rudpishi, M.; Joharimoghadam, A.; Rayzan, E. The significant coronary tortuosity and atherosclerotic coronary artery disease; What is the relation? J. Cardiovasc. Thorac. Res. 2018, 10, 209–213. [Google Scholar]
- Raia, L.; Zafrani, L. Endothelial Activation and Microcirculatory Disorders in Sepsis. Front. Med. 2022, 9, 907992. [Google Scholar] [CrossRef]
- Lundby, C.; Montero, D. CrossTalk opposing view: Diffusion limitation of O2 from microvessels into muscle does not contribute to the limitation of VO2max. J. Physiol. 2015, 593, 3759–3761. [Google Scholar] [CrossRef]
- Willi, C.E.; Abdelazim, H.; Chappell, J.C. Evaluating cell viability, capillary perfusion, and collateral tortuosity in an ex vivo mouse intestine fluidics model. Front. Bioeng. Biotechnol. 2022, 10, 1008481. [Google Scholar] [CrossRef]
- Chappell, J.C.; Song, J.; Klibanov, A.L.; Price, R.J. Ultrasonic microbubble destruction stimulates therapeutic arteriogenesis via the CD18-dependent recruitment of bone marrow-derived cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1117–1122. [Google Scholar] [CrossRef]
- Song, J.W.; Munn, L.L. Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 15342–15347. [Google Scholar] [CrossRef] [PubMed]
- Subramanyan, R.; Narayan, R.; Maskeri, S.A. Familial arterial tortuosity syndrome. Indian Heart J. 2007, 59, 178–180. [Google Scholar] [PubMed]
- Valeanu, L.; Bubenek-Turconi, S.I.; Ginghina, C.; Balan, C. Hemodynamic Monitoring in Sepsis—A Conceptual Framework of Macro- and Microcirculatory Alterations. Diagnostics 2021, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Sackarnd, J.; Rossaint, J.; Kampmeier, S.; Pavenstädt, H.; Vink, H.; Kümpers, P. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: The NOSTRADAMUS study. Crit. Care 2021, 25, 112. [Google Scholar] [CrossRef]
- Pan, F.; Mori, N.; Mugikura, S.; Ohta, M.; Anzai, H. The influence of blood velocity and vessel geometric parameters on wall shear stress. Med. Eng. Phys. 2024, 124, 104112. [Google Scholar] [CrossRef]
- Kahe, F.; Sharfaei, S.; Pitliya, A.; Jafarizade, M.; Seifirad, S.; Habibi, S.; Chi, G. Coronary artery tortuosity: A narrative review. Coron. Artery Dis. 2020, 31, 187–192. [Google Scholar] [CrossRef]
- Kaplan, A.D.; Jaffa, A.J.; Timor, I.E.; Elad, D. Hemodynamic analysis of arterial blood flow in the coiled umbilical cord. Reprod. Sci. 2010, 17, 258–268. [Google Scholar] [CrossRef]
- Wood, N.B.; Zhao, S.Z.; Zambanini, A.; Jackson, M.; Gedroyc, W.; Thom, S.A.; Hughes, A.D.; Xu, X.Y. Curvature and tortuosity of the superficial femoral artery: A possible risk factor for peripheral arterial disease. J. Appl. Physiol. 2006, 101, 1412–1418. [Google Scholar] [CrossRef]
- Qiao, A.K.; Guo, X.L.; Wu, S.G.; Zeng, Y.J.; Xu, X.H. Numerical study of nonlinear pulsatile flow in S-shaped curved arteries. Med. Eng. Phys. 2004, 26, 545–552. [Google Scholar] [CrossRef]
- Datir, P.; Lee, A.Y.; Lamm, S.D.; Han, H.C. Effects of geometric variations on the buckling of arteries. Int. J. Appl. Mech. 2011, 3, 385–406. [Google Scholar] [CrossRef]
- Lupu, F.; Kinasewitz, G.; Dormer, K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J. Cell. Mol. Med. 2020, 24, 12258–12271. [Google Scholar] [CrossRef] [PubMed]
- Pereira Duque Estrada, A.; de Oliveira Lopes, R.; Villacorta Junior, H. Coronary tortuosity and its role in myocardial ischemia in patients with no coronary obstructions. Int. J. Cardiovasc. Sci. 2017, 30, 163–170. [Google Scholar]
- Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Jagger, J.E.; Sharpe, M.D.; Ellsworth, M.L.; Mehta, S.; Ellis, C.G. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2848–H2856. [Google Scholar] [CrossRef]
- Condon, M.R.; Kim, J.E.; Deitch, E.A.; Machiedo, G.W.; Spolarics, Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2177–H2184. [Google Scholar] [CrossRef]
- Eichelbrönner, O.; Sielenkämper, A.; Cepinskas, G.; Sibbald, W.J.; Chin-Yee, I.H. Endotoxin promotes adhesion of human erythrocytes to human vascular endothelial cells under conditions of flow. Crit. Care Med. 2000, 28, 1865–1870. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef]
- Schödel, J.; Ratcliffe, P.J. Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 2019, 15, 641–659. [Google Scholar] [CrossRef]
- Milstein, D.M.; Helmers, R.; Hackmann, S.; Belterman, C.N.; van Hulst, R.A.; de Lange, J. Sublingual microvascular perfusion is altered during normobaric and hyperbaric hyperoxia. Microvasc. Res. 2016, 105, 93–102. [Google Scholar] [CrossRef]
- Schwarte, L.A.; Schober, P.; Loer, S.A. Benefits and harms of increased inspiratory oxygen concentrations. Curr. Opin. Anaesthesiol. 2019, 32, 783–791. [Google Scholar] [CrossRef]
- Orbegozo Cortés, D.; Puflea, F.; Donadello, K.; Taccone, F.S.; Gottin, L.; Creteur, J.; Vincent, J.L.; De Backer, D. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc. Res. 2015, 98, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef] [PubMed]
| Microvessel Diameter (μm) | Blood Volume | Average Velocity of RBCs * | |
|---|---|---|---|
| Microvessel Hct (%) | Plasma (%) (1—Microvessel Hct) | ||
| 1100 | 40.5 | 59.5 | 100 |
| 750 | 40.1 | 59.9 | 101 |
| 450 | 39.8 | 60.2 | 103 |
| 250 | 39.2 | 60.8 | 106 |
| 95 | 33.6 | 66.4 | 135 |
| 50 | 28.0 | 72.0 | 175 |
| Steady-State (n = 20) | Septic Shock (n = 13) | p-Value | |
|---|---|---|---|
| Age (years) | 39.7 (7.06) | 70.5 (8.97) | <0.001 |
| Sex (male), n (%) | 12 (60%) | 9 (69.2%) | 0.009 |
| ASA class, n (%) | |||
| 1 | 20 (100) | 0 (0) | <0.001 |
| 2 | 0 (0) | 0 (0) | NA |
| 3 | 0 (0) | 0 (0) | NA |
| 4 | 0 (0) | 0 (0) | NA |
| 4E | 0 (0) | 7 (54) | <0.001 |
| 5E | 0 (0) | 6 (46) | <0.001 |
| Height (cm) | 176.4 (5.83) | 173.85 (5.67) | 0.178 |
| Weight (kg) | 76.4 (7.78) | 81.92 (6.82) | 0.06 |
| Body Surface Area (m2) | 1.93 (0.13) | 1.96 (0.1) | 0.396 |
| BMI (kg m−2) | 24.5 (1.52) | 27.15 (2.22) | 0.002 |
| History, n (%) | |||
| Ischemic heart disease | 0 (0) | 5 (38.5) | NA |
| Hypertension | 0 (0) | 10 (76.9) | NA |
| Hypercholesterolemia | 0 (0) | 8 (61.5) | NA |
| Diabetes | 0 (0) | 6 (46.2) | NA |
| Stroke | 0 (0) | 3 (23.1) | NA |
| COPD | 0 (0) | 4 (30.8) | NA |
| Temperature (°C) | 36.72 (0.12) | 36.72 (0.49) | 0.823 |
| Bispectral index | 42.45 (1.47) | 50 (5.12) | 0.001 |
| Fraction of inspired oxygen | 0.31 (0.03) | 0.49 (0.16) | <0.001 |
| Tidal volume (ml kg−1 IBW) | 560 (52.21) | 464.62 (55.92) | <0.001 |
| Respiratory rate (min−1) | 13.7 (1.08) | 17.92 (3.52) | <0.001 |
| PEEP (cmH2O) | 5 (0) | 5.23 (1.92) | 0.081 |
| ETCO2 (mmHg) | 36.9 (0.85) | 31 (6.11) | 0.002 |
| PIP (cmH2O) | 17.75 (1.29) | 20.38 (5.04) | 0.217 |
| Plateau pressure (cmH2O) | 15.95 (1.39) | 19.15 (4.41) | 0.044 |
| Steady-State (n = 20) | Septic Shock (n = 13) | p-Value | |
|---|---|---|---|
| Heart rate (bpm) | 67.5 (6.98) | 96.92 (23.5) | <0.001 |
| Systolic arterial pressure (mmHg) | 120 (7.43) | 116.92 (22.13) | 0.863 |
| Diastolic arterial pressure (mmHg) | 71.25 (7.41) | 66.54 (14.91) | 0.595 |
| Mean arterial pressure (mmHg) | 88.13 (6.97) | 83.62 (16.76) | 0.956 |
| Cardiac output (L min−1) | 5.04 (0.68) | 5.48 (1.01) | 0.16 |
| Cardiac index (L min−1 m−2) | 2.6 (0.3) | 2.75 (0.54) | 0.554 |
| Stroke volume (mL beat−1) | 74.7 (9.57) | 61.54 (26.04) | 0.001 |
| Stroke volume variation (%) | 5.9 (1.83) | 12.69 (5.14) | <0.001 |
| Systemic vascular resistance (dynes s cm−5) | 1306.3 (176.32) | 896.31 (247.49) | <0.001 |
| Central venous pressure (mmHg) | 7.05 (0.69) | 11.31 (4.29) | <0.001 |
| Mean circulatory filling pressure analog (mmHg) | 13.06 (0.86) | 18.62 (4.61) | <0.001 |
| Cardiac Power Output (W) | 0.99 (0.17) | 1.01 (0.27) | 0.54 |
| Power (W) | 0.9 (0.16) | 0.87 (0.24) | 0.696 |
| De Backer score (mm−1) | 3.7 (1.17) | 3.62 (1.19) | 0.754 |
| Consensus PPV (%) | 94.15 (5.66) | 60.2 (11.3) | <0.001 |
| Consensus PPV (small) (%) | 122.89 (146.74) | 50.57 (12.64) | <0.001 |
| Microvascular Flow Index (AU) | 2.76 (0.25) | 1.83 (0.61) | <0.001 |
| Vessel diameter (μm) | 10.07 (5.02) | 4.35 (1.83) | <0.001 |
| Vessel length (μm) | 141 (154.25) | 42.54 (15.98) | <0.001 |
| Red blood cell velocity (μm s−1) | 15.69 (15.02) | 13.46 (12.45) | 0.519 |
| Wall shear stress (dyne cm−2) | 3.86 (2.68) | 0.72 (0.36) | <0.001 |
| Capillary tortuosity score | 0.55 (0.76) | 3.31 (0.86) | <0.001 |
| Steady-State (n = 20) | Septic Shock (n = 13) | p-Value | |
|---|---|---|---|
| Venous-arterial carbon dioxide difference (mmHg) | 2.8 (0.89) | 9 (1.87) | <0.001 |
| pH | 7.39 (0.02) | 7.32 (0.11) | 0.052 |
| Arterial partial pressure of oxygen (mmHg) | 92.5 (5.12) | 104.31 (38.73) | 0.971 |
| Arterial partial pressure of carbon dioxide (mmHg) | 39.2 (1.28) | 36.85 (6.44) | 0.64 |
| Bicarbonate (mmol L−1) | 25.6 (0.99) | 21.19 (6.85) | 0.003 |
| Base deficit (mmol L−1) | 2.08 (0.19) | −0.19 (7.59) | 0.183 |
| Hemoglobin (g dL−1) | 14.06 (0.94) | 9.73 (1.83) | <0.001 |
| Glucose (mg dL−1) | 113.6 (6.21) | 119.62 (39.65) | 0.338 |
| Lactate (mmol L−1) | 0.81 (0.15) | 3.45 (2.78) | <0.001 |
| A-a O2 Gradient (mmHg) | 80.33 (25.43) | 198.46 (126.17) | <0.001 |
| Expected A-a O2 Gradient for age (mmHg) | 13.95 (1.77) | 21.65 (2.24) | <0.001 |
| Peripheral oxygen saturation (%) | 99.6 (0.5) | 95.31 (4.01) | 0.001 |
| Arterial oxygen saturation (%) | 100 (0) | 96.77 (3.11) | <0.001 |
| Central venous oxygen saturation (%) | 74.15 (2.3) | 77.92 (6.12) | 0.015 |
| Oxygen extraction ratio (%) | 25.85 (2.3) | 19.31 (5.59) | 0.001 |
| Arterial oxygen content (vol%) | 19.7 (1.3) | 13.3 (2.41) | <0.001 |
| Venous oxygen content (vol%) | 14.73 (1.4) | 10.76 (2.27) | <0.001 |
| Venous-arterial oxygen content difference (vol%) | 4.96 (0.78) | 2.54 (0.74) | <0.001 |
| Oxygen delivery (mL min−1) | 973.88 (116.23) | 724.19 (160.4) | <0.001 |
| Oxygen consumption (mL min−1) | 247.43 (35.64) | 136.45 (41.54) | <0.001 |
| Convective oxygen flow (μm2 sec−1 kg−1) | 26.44 (39.96) | 1.1 (1.41) | <0.001 |
| Oxygen debt | −8.62 (1.13) | 13.23 (29.62) | 0.002 |
| Rho | p-Value | Adjusted for Multiple Comparisons | |
|---|---|---|---|
| Heart rate (bpm) | 0.114 | 0.633 | 0.951 |
| Systolic arterial pressure (mmHg) | 0.014 | 0.955 | 0.998 |
| Diastolic arterial pressure (mmHg) | −0.471 | 0.036 | 0.262 |
| Mean arterial pressure (mmHg) | −0.384 | 0.095 | 0.449 |
| Cardiac output (L min−1) | 0.059 | 0.803 | 0.992 |
| Cardiac index (L min−1 m−2) | 0.27 | 0.25 | 0.618 |
| Stroke volume (mL beat−1) | 0.031 | 0.895 | 0.992 |
| Stroke volume variation (%) | 0.274 | 0.242 | 0.618 |
| Systemic vascular resistance (dynes s cm−5) | −0.266 | 0.257 | 0.618 |
| Central venous pressure (mmHg) | 0.065 | 0.785 | 0.992 |
| Pmca (mmHg) | 0.04 | 0.869 | 0.992 |
| Cardiac Power Output (W) | 0.03 | 0.9 | 0.992 |
| Power (W) | −0.006 | 0.979 | 0.998 |
| De Backer score (mm−1) | 0.202 | 0.393 | 0.771 |
| Consensus PPV (%) | −0.369 | 0.11 | 0.449 |
| Consensus PPV (small) (%) | −0.458 | 0.042 | 0.262 |
| Microvascular Flow Index (AU) | 0.083 | 0.726 | 0.992 |
| Vessel diameter (μm) | 0.137 | 0.565 | 0.936 |
| Vessel length (μm) | −0.042 | 0.86 | 0.992 |
| Red blood cell velocity (μm s−1) | −0.031 | 0.898 | 0.992 |
| Wall shear stress (dyne cm−2) | 0.115 | 0.628 | 0.942 |
| Rho | p-Value | Adjusted for Multiple Comparisons | |
|---|---|---|---|
| Venous-arterial carbon dioxide difference (mmHg) | 0.109 | 0.646 | 0.951 |
| pH | 0.252 | 0.285 | 0.629 |
| Arterial partial pressure of oxygen (mmHg) | −0.309 | 0.184 | 0.618 |
| Arterial partial pressure of carbon dioxide (mmHg) | 0.512 | 0.021 | 0.262 |
| Bicarbonate (mmol L−1) | 0.338 | 0.145 | 0.55 |
| Base deficit (mmol L−1) | −0.463 | 0.04 | 0.262 |
| Hemoglobin (g dL−1) | −0.459 | 0.042 | 0.262 |
| Glucose (mg dL−1) | 0.195 | 0.41 | 0.775 |
| Lactate (mmol L−1) | −0.042 | 0.86 | 0.992 |
| A-a O2 Gradient (mmHg) | 0.011 | 0.963 | 0.998 |
| Expected A-a O2 Gradient for age (mmHg) | −0.685 | 0.001 | 0.046 |
| Peripheral oxygen saturation (%) | −0.283 | 0.227 | 0.618 |
| Central venous oxygen saturation (%) | 0.025 | 0.918 | 0.992 |
| Oxygen extraction ratio (%) | −0.025 | 0.918 | 0.992 |
| Arterial oxygen content (vol%) | −0.474 | 0.035 | 0.262 |
| Venous oxygen content (vol%) | −0.413 | 0.07 | 0.373 |
| Venous-arterial oxygen content difference (vol%) | −0.165 | 0.486 | 0.859 |
| Oxygen delivery (mL min−1) | −0.146 | 0.538 | 0.92 |
| Oxygen consumption (mL min−1) | −0.173 | 0.467 | 0.853 |
| Convective oxygen flow (μm2 sec−1 kg−1) | 0.112 | 0.637 | 0.951 |
| Oxygen debt | 0.125 | 0.599 | 0.951 |
| Rho | p-Value | Adjusted for Multiple Comparisons | |
|---|---|---|---|
| Heart rate (bpm) | 0.153 | 0.618 | 0.978 |
| Systolic arterial pressure (mmHg) | −0.11 | 0.722 | 0.978 |
| Diastolic arterial pressure (mmHg) | −0.127 | 0.679 | 0.978 |
| Mean arterial pressure (mmHg) | −0.195 | 0.523 | 0.978 |
| Cardiac output (L min−1) | 0.228 | 0.455 | 0.978 |
| Cardiac index (L min−1 m−2) | 0.098 | 0.75 | 0.978 |
| Stroke volume (mL beat−1) | 0.203 | 0.506 | 0.978 |
| Stroke volume variation (%) | −0.373 | 0.209 | 0.978 |
| Systemic vascular resistance (dynes s cm−5) | −0.048 | 0.875 | 0.978 |
| Central venous pressure (mmHg) | −0.027 | 0.929 | 0.978 |
| Pmca (mmHg) | 0.07 | 0.82 | 0.978 |
| Cardiac Power Output (W) | −0.025 | 0.937 | 0.978 |
| Power (W) | −0.13 | 0.671 | 0.978 |
| De Backer score (mm−1) | 0.341 | 0.254 | 0.978 |
| Consensus PPV (%) | 0.357 | 0.231 | 0.978 |
| Consensus PPV (small) (%) | 0.355 | 0.235 | 0.978 |
| Microvascular Flow Index (AU) | 0.08 | 0.796 | 0.978 |
| Vessel diameter (μm) | 0.091 | 0.768 | 0.978 |
| Vessel length (μm) | −0.03 | 0.922 | 0.978 |
| Red blood cell velocity (μm s−1) | −0.201 | 0.509 | 0.978 |
| Wall shear stress (dyne cm−2) | 0.286 | 0.343 | 0.978 |
| Rho | p-Value | Adjusted for Multiple Comparisons | |
|---|---|---|---|
| Venous-arterial carbon dioxide difference (mmHg) | −0.14 | 0.648 | 0.978 |
| Fraction of inspired oxygen | 0.234 | 0.442 | 0.978 |
| pH | 0.116 | 0.706 | 0.978 |
| Arterial partial pressure of oxygen (mmHg) | −0.46 | 0.114 | 0.893 |
| Arterial partial pressure of carbon dioxide (mmHg) | 0.031 | 0.921 | 0.978 |
| Bicarbonate (mmol L−1) | 0.176 | 0.566 | 0.978 |
| Base deficit (mmol L−1) | 0.701 | 0.008 | 0.209 |
| Hemoglobin (g dL−1) | 0.021 | 0.945 | 0.978 |
| Glucose (mg dL−1) | −0.218 | 0.474 | 0.978 |
| Lactate (mmol L−1) | −0.467 | 0.107 | 0.893 |
| A-a O2 Gradient (mmHg) | 0.658 | 0.015 | 0.267 |
| Expected A-a O2 Gradient for age (mmHg) | −0.079 | 0.798 | 0.978 |
| Peripheral oxygen saturation (%) | 0.168 | 0.584 | 0.978 |
| Arterial oxygen saturation (%) | −0.015 | 0.96 | 0.978 |
| Central venous oxygen saturation (%) | 0.098 | 0.75 | 0.978 |
| Oxygen extraction ratio (%) | 0.058 | 0.851 | 0.978 |
| Arterial oxygen content (vol%) | −0.03 | 0.922 | 0.978 |
| Venous oxygen content (vol%) | −0.085 | 0.782 | 0.978 |
| Venous-arterial oxygen content difference (vol%) | 0.215 | 0.48 | 0.978 |
| Oxygen delivery (mL min−1) | 0.03 | 0.922 | 0.978 |
| Oxygen consumption (mL min−1) | 0.169 | 0.58 | 0.978 |
| Convective oxygen flow (μm2 sec−1 kg−1) | 0.197 | 0.519 | 0.978 |
| Oxygen debt | −0.769 | 0.002 | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A.; Papagiannakis, N.; Katsifa, K.; Destounis, A.; Gravos, A.; Kanakaki, S.; Karapiperis, G.; Koufaki, F.; Prekates, A.; Tselioti, P. Characterization of Sublingual Microvascular Tortuosity in Steady-State Physiology and Septic Shock. Biomedicines 2025, 13, 691. https://doi.org/10.3390/biomedicines13030691
Chalkias A, Papagiannakis N, Katsifa K, Destounis A, Gravos A, Kanakaki S, Karapiperis G, Koufaki F, Prekates A, Tselioti P. Characterization of Sublingual Microvascular Tortuosity in Steady-State Physiology and Septic Shock. Biomedicines. 2025; 13(3):691. https://doi.org/10.3390/biomedicines13030691
Chicago/Turabian StyleChalkias, Athanasios, Nikolaos Papagiannakis, Konstantina Katsifa, Antonios Destounis, Athanasios Gravos, Sofia Kanakaki, Georgios Karapiperis, Faidra Koufaki, Athanasios Prekates, and Paraskevi Tselioti. 2025. "Characterization of Sublingual Microvascular Tortuosity in Steady-State Physiology and Septic Shock" Biomedicines 13, no. 3: 691. https://doi.org/10.3390/biomedicines13030691
APA StyleChalkias, A., Papagiannakis, N., Katsifa, K., Destounis, A., Gravos, A., Kanakaki, S., Karapiperis, G., Koufaki, F., Prekates, A., & Tselioti, P. (2025). Characterization of Sublingual Microvascular Tortuosity in Steady-State Physiology and Septic Shock. Biomedicines, 13(3), 691. https://doi.org/10.3390/biomedicines13030691








