HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease
Abstract
1. Introduction
2. An Overview of Iron Regulation
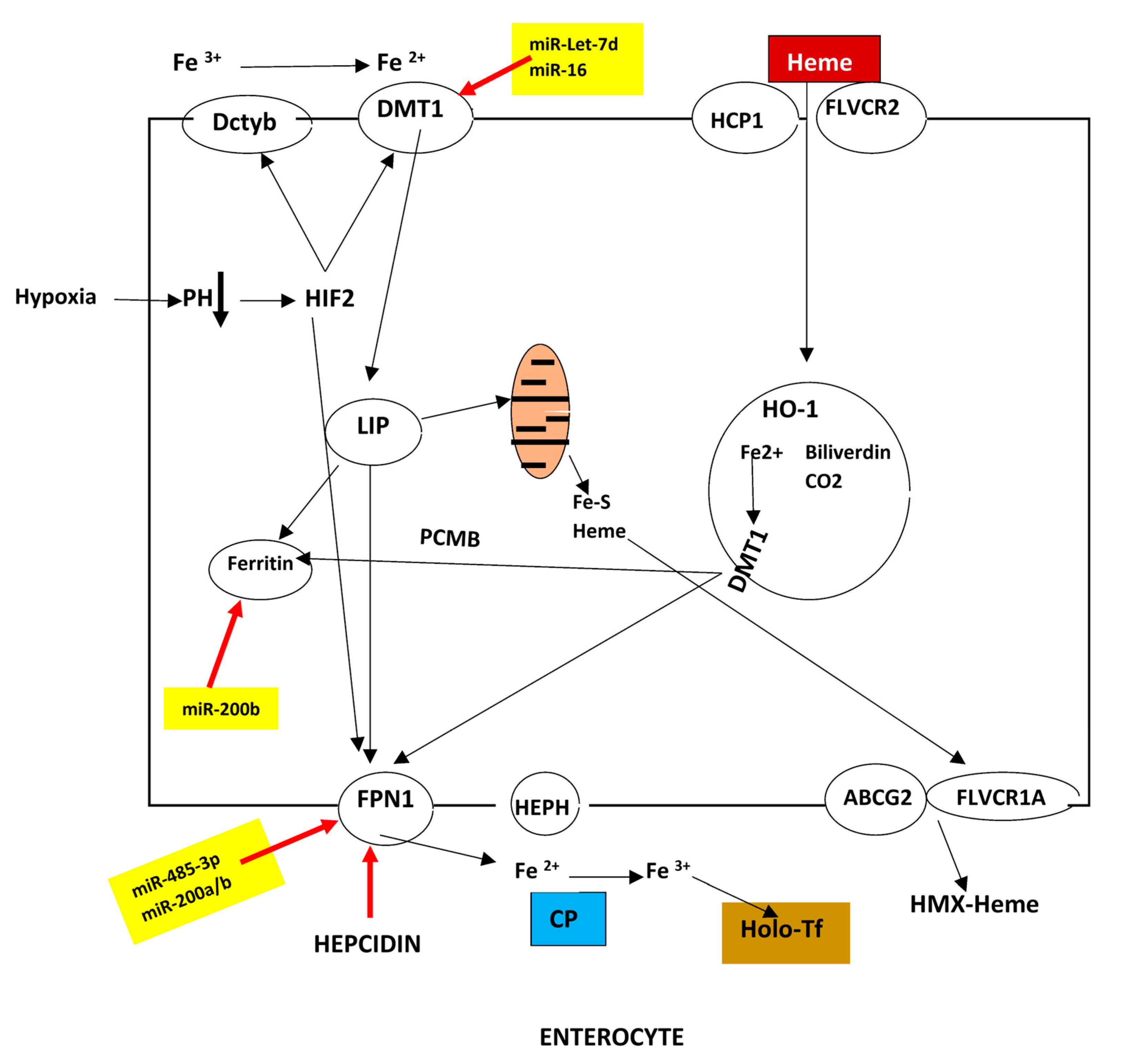
2.1. Duodenal Absorption
2.2. Storage
2.3. Cellular Uptake and Traffic
2.3.1. Transferrin (Tf) and Transferrin Receptor (TfR) in Iron Homeostasis

2.3.2. Macrophages
2.3.3. LSECs
2.3.4. Renal Interstitial Cells and Erythroferrone
2.4. Release
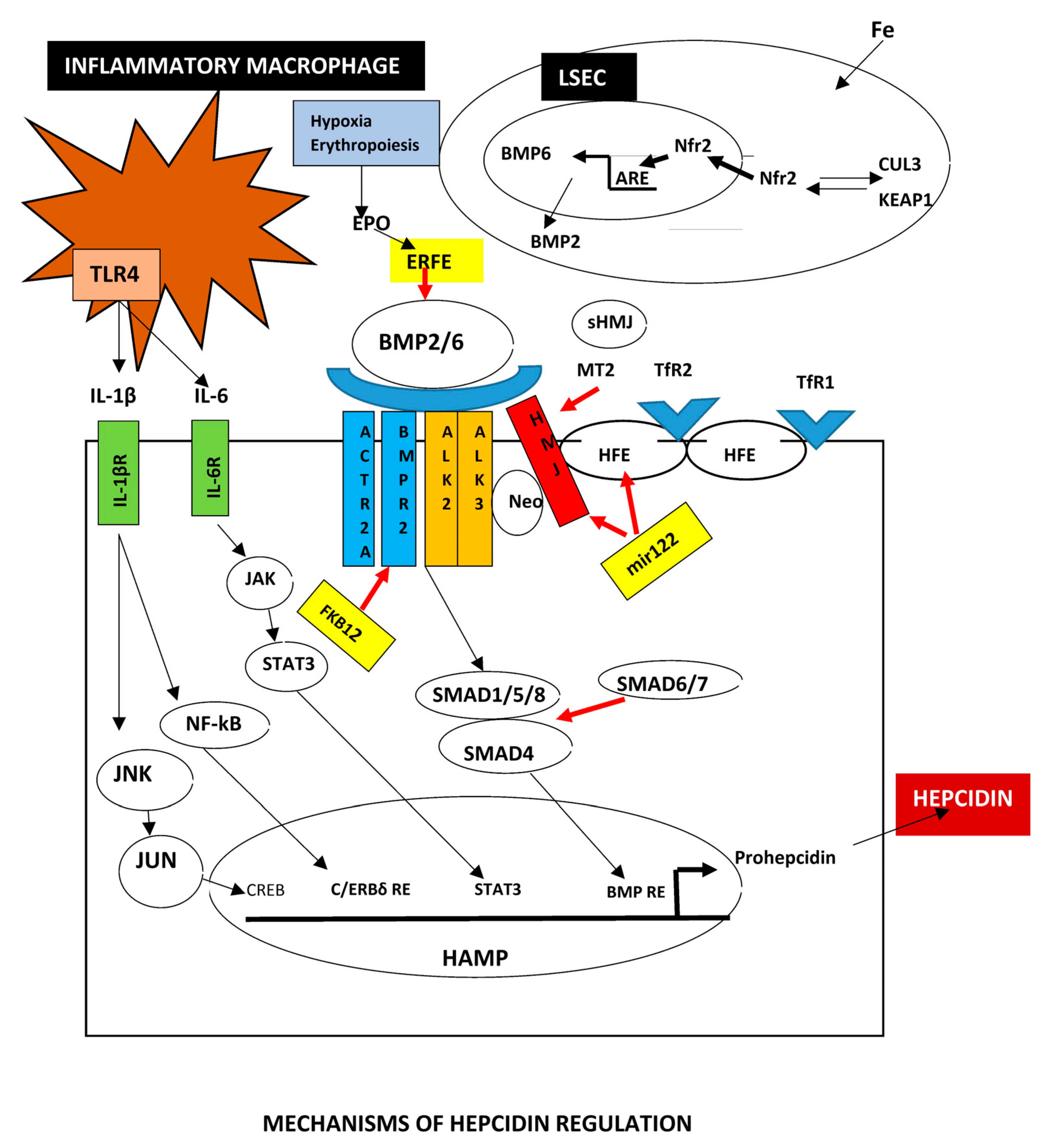
2.5. Major Regulators of Iron Metabolism
2.5.1. BMPs
2.5.2. Hepcidin
Posttranscriptional Hepcidin Modification
2.5.3. Ferroportin (FPN1)
2.5.4. Cellular Regulation of Iron by IRE/IRPs
2.5.5. Hypoxia
3. Hemochromatosis
3.1. Current Pathogenesis of Hemochromatosis
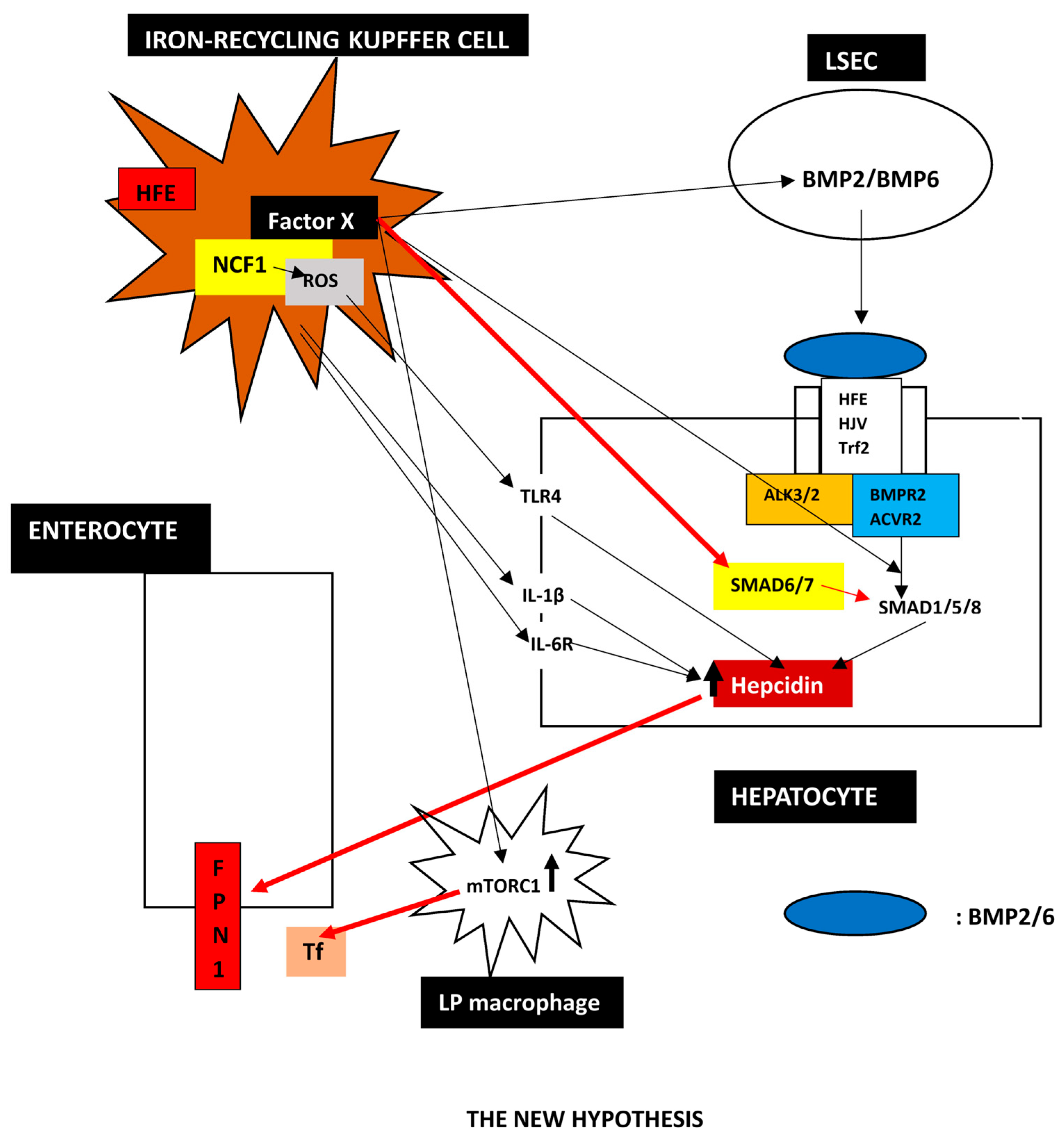
3.2. A Proposed New Hypothesis
- 1.
- Evidence that Kupffer cells are the critical cell in HH
- 2.
- Indications that low hepcidin in hepatocytes is a secondary phenomenon in HH
- 3.
- The Transplantation Experience
- 4.
- Is There an Indication that Kupffer Cells May Regulate Hepcidin?
- 5.
- The New Hypothesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36, 116–123. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. Iron as a therapeutic target in chronic liver disease. World J. Gastroenterol. 2023, 29, 616–655. [Google Scholar] [CrossRef]
- Ganz, T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012, 4, 446–453. [Google Scholar] [CrossRef]
- Higgins, J.M. Red blood cell population dynamics. Clin. Lab. Med. 2015, 35, 43–57. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 2010, 139, 393–408. [Google Scholar] [CrossRef]
- Sohal, A.; Kowdley, K.V. A Review of New Concepts in Iron Overload. Gastroenterol. Hepatol. 2024, 20, 98–107. [Google Scholar]
- Fisher, A.L.; Babitt, J.L. Coordination of iron homeostasis by bone morphogenetic proteins: Current understanding and unanswered questions. Dev. Dyn. 2022, 251, 26–46. [Google Scholar] [CrossRef]
- Andreini, C.; Putignano, V.; Rosato, A.; Banci, L. The human iron-proteome. Metallomics 2018, 10, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Cairo, G. Dual Role of ROS as Signal and Stress Agents: Iron Tips the Balance in favor of Toxic Effects. Oxid. Med. Cell. Longev. 2016, 2016, 8629024. [Google Scholar] [CrossRef] [PubMed]
- Leidgens, S.; Bullough, K.Z.; Shi, H.; Li, F.; Shakoury-Elizeh, M.; Yabe, T.; Subramanian, P.; Hsu, E.; Natarajan, N.; Nandal, A.; et al. Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J. Biol. Chem. 2013, 288, 17791–17802. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Jadhav, S. The ins and outs of iron: Escorting iron through the mammalian cytosol. Free. Radic. Biol. Med. 2019, 133, 112–117. [Google Scholar] [CrossRef]
- Wang, Y.; Protchenko, O.; Huber, K.D.; Shakoury-Elizeh, M.; Ghosh, M.C.; Philpott, C.C. The iron chaperone poly(rC)-binding protein 1 regulates iron efflux through intestinal ferroportin in mice. Blood 2023, 142, 1658–1671. [Google Scholar] [CrossRef]
- Jain, C.; Shah, Y.M. PCBP1 is essential for proper iron absorption. Blood 2023, 142, 1585–1587. [Google Scholar] [CrossRef]
- Shawki, A.; Engevik, M.A.; Kim, R.S.; Knight, P.B.; Baik, R.A.; Anthony, S.R.; Worrell, R.T.; Shull, G.E.; Mackenzie, B. Intestinal brush-border Na+/H+ exchanger-3 drives H+-coupled iron absorption in the mouse. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 311, G423–G430. [Google Scholar] [CrossRef]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Gochanour, E.M.; Sundaram, V.; Shah, R.A.; Handa, P. Hepcidin Signaling in Health and Disease: Ironing Out the Details. Hepatol. Commun. 2021, 5, 723–735. [Google Scholar] [CrossRef]
- Marro, S.; Chiabrando, D.; Messana, E.; Stolte, J.; Turco, E.; Tolosano, E.; Muckenthaler, M.U. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica 2010, 95, 1261–1268. [Google Scholar] [CrossRef]
- Iwasaki, K.; Mackenzie, E.L.; Hailemariam, K.; Sakamoto, K.; Tsuji, Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol. Cell Biol. 2006, 26, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Eggler, A.L.; Liu, G.; Pezzuto, J.M.; van Breemen, R.B.; Mesecar, A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 2005, 102, 10070–10075. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Yu, S.; Chang, P.P.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.N. Mechanism of action of sulforaphane: Inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006, 66, 8804–8813. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Theurl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflug. Arch. 2017, 469, 397–418. [Google Scholar] [CrossRef]
- Haldar, M.; Kohyama, M.; So, A.Y.; Kc, W.; Wu, X.; Briseño, C.G.; Satpathy, A.T.; Kretzer, N.M.; Arase, H.; Rajasekaran, N.S.; et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 2014, 156, 1223–1234. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation. Cells 2021, 10, 515. [Google Scholar] [CrossRef]
- Donegan, R.K.; Moore, C.M.; Hanna, D.A.; Reddi, A.R. Handling heme: The mechanisms underlying the movement of heme within and between cells. Free. Radic. Biol. Med. 2019, 133, 88–100. [Google Scholar] [CrossRef]
- Swenson, S.A.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells 2020, 9, 579. [Google Scholar] [CrossRef]
- Chambers, I.G.; Willoughby, M.M.; Hamza, I.; Reddi, A.R. One ring to bring them all and in the darkness bind them: The trafficking of heme without deliverers. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118881. [Google Scholar] [CrossRef]
- Duffy, S.P.; Shing, J.; Saraon, P.; Berger, L.C.; Eiden, M.V.; Wilde, A.; Tailor, C.S. The Fowler syn-drome-associated protein FLVCR2 is an importer of heme. Mol. Cell Biol. 2010, 30, 5318–5324. [Google Scholar] [CrossRef]
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.L.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010, 116, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.M.; Treffry, A.; Artymiuk, P.J.; Harrison, P.M.; Yewdall, S.J.; Luzzago, A.; Cesareni, G.; Levi, S.; Arosio, P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989, 254, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Mehlenbacher, M.; Poli, M.; Arosio, P.; Santambrogio, P.; Levi, S.; Chasteen, N.D.; Bou-Abdallah, F. Iron Oxidation and Core Formation in Recombinant Heteropolymeric Human Ferritins. Biochemistry 2017, 56, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10, 476. [Google Scholar] [CrossRef]
- Luck, A.N.; Mason, A.B. Transferrin-mediated cellular iron delivery. Curr. Top. Membr. 2012, 69, 3–35. [Google Scholar]
- Lambert, L.A.; Perri, H.; Meehan, T.J. Evolution of duplications in the transferrin family of proteins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 11–25. [Google Scholar] [CrossRef]
- Parrow, N.L.; Li, Y.; Feola, M.; Guerra, A.; Casu, C.; Prasad, P.; Mammen, L.; Ali, F.; Vaicikauskas, E.; Rivella, S.; et al. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood 2019, 134, 1373–1384. [Google Scholar] [CrossRef]
- Xiao, X.; Moschetta, G.A.; Xu, Y.; Fisher, A.L.; Alfaro-Magallanes, V.M.; Dev, S.; Wang, C.Y.; Babitt, J.L. Regulation of iron homeostasis by hepatocyte TfR1 requires HFE and contributes to hepcidin suppression in β-thalassemia. Blood 2023, 141, 422–432. [Google Scholar] [CrossRef]
- Scaramellini, N.; Fischer, D.; Agarvas, A.R.; Motta, I.; Muckenthaler, M.U.; Mertens, C. Interpreting Iron Homeostasis in Congenital and Acquired Disorders. Pharmaceuticals 2023, 16, 329. [Google Scholar] [CrossRef]
- Altamura, S.; Marques, O.; Colucci, S.; Mertens, C.; Alikhanyan, K.; Muckenthaler, M.U. Regulation of iron homeostasis: Lessons from mouse models. Mol. Asp. Med. 2020, 75, 100872. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Luscieti, S.; Gandini, V.; Maccarinelli, F.; Finazzi, D.; Silvestri, L.; Roetto, A.; Arosio, P. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica 2010, 95, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018, 38, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H. Transferrin and transferrin receptors update. Free. Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Vali, S.W.; Lindahl, P.A. Might nontransferrin-bound iron in blood plasma and sera be a nonproteinaceous high-molecular-mass FeIII aggregate? J. Biol. Chem. 2022, 298, 102667. [Google Scholar] [CrossRef]
- Knutson, M.D. Non-transferrin-bound iron transporters. Free. Radic. Biol. Med. 2019, 133, 101–111. [Google Scholar] [CrossRef]
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef]
- Li, J.Y.; Paragas, N.; Ned, R.M.; Qiu, A.; Viltard, M.; Leete, T.; Drexler, I.R.; Chen, X.; Sanna-Cherchi, S.; Mohammed, F.; et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev. Cell 2009, 16, 35–46. [Google Scholar] [CrossRef]
- Han, J.; Seaman, W.E.; Di, X.; Wang, W.; Willingham, M.; Torti, F.M.; Torti, S.V. Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLoS ONE 2011, 6, e23800. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Yanatori, I.; Richardson, D.R.; Imada, K.; Kishi, F. Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2. J. Biol. Chem. 2016, 291, 17303–17318. [Google Scholar] [CrossRef] [PubMed]
- Nandal, A.; Ruiz, J.C.; Subramanian, P.; Ghimire-Rijal, S.; Sinnamon, R.A.; Stemmler, T.L.; Bruick, R.K.; Philpott, C.C. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Holy, M.; Grado, S.; Fleming, R.; Kurita, R.; Nakamura, Y.; Goldfarb, A. A specialized pathway for erythroid iron delivery through lysosomal trafficking of transferrin receptor 2. Blood Adv. 2017, 1, 1181–1194. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Wang, J.; Huang, R.; Wan, D. Regulation of Iron Homeostasis and Related Diseases. Mediat. Inflamm. 2020, 2020, 6062094. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Yuan, X.; Schmidt, P.J.; Bresciani, E.; Samuel, T.K.; Campagna, D.; Hall, C.; Bishop, K.; Calicchio, M.L.; Lapierre, A.; et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013, 17, 261–270. [Google Scholar] [CrossRef]
- Korolnek, T.; Hamza, I. Macrophages and iron trafficking at the birth and death of red cells. Blood 2015, 125, 2893–2897. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Corrons, J.L.V.; Casafont, L.B.; Frasnedo, E.F. Concise review: How do red blood cells born, live, and die? Ann. Hematol. 2021, 100, 2425–2433. [Google Scholar] [CrossRef]
- Klei, T.R.; Meinderts, S.M.; van den Berg, T.K.; van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Slusarczyk, P.; Mleczko-Sanecka, K. The Multiple Facets of Iron Recycling. Genes 2021, 12, 1364. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. Macrophages: Central regulators of iron balance. Metallomics 2014, 6, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Zhang, A.S.; Brown, C.; Shirihai, O.S.; Ponka, P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 2007, 110, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Marques, O.; Horvat, N.K.; Simonetti, M.; Muckenthaler, M.U.; Jung, M. The Macrophage Iron Signature in Health and Disease. Int. J. Mol. Sci. 2021, 22, 8457. [Google Scholar] [CrossRef]
- Alam, Z.; Devalaraja, S.; Li, M.; To, T.K.J.; Folkert, I.W.; Mitchell-Velasquez, E.; Dang, M.T.; Young, P.; Wilbur, C.J.; Silverman, M.A.; et al. Counter Regulation of Spic by NF-κB and STAT Signaling Controls Inflammation and Iron Metabolism in Macrophages. Cell Rep. 2020, 31, 107825. [Google Scholar] [CrossRef]
- Agoro, R.; Mura, C. Inflammation-induced up-regulation of hepcidin and down-regulation of ferroportin transcription are dependent on macrophage polarization. Blood Cells Mol. Dis. 2016, 61, 16–25. [Google Scholar] [CrossRef]
- Mesquita, G.; Silva, T.; Gomes, A.C.; Oliveira, P.F.; Alves, M.G.; Fernandes, R.; Almeida, A.A.; Moreira, A.C.; Gomes, M.S. H-Ferritin is essential for macrophages’ capacity to store or detoxify exogenously added iron. Sci. Rep. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Gan, Z.S.; Wang, Q.Q.; Li, J.H.; Wang, X.L.; Wang, Y.Z.; Du, H.H. Iron Reduces M1 Macrophage Polarization in RAW264.7 Macrophages Associated with Inhibition of STAT1. Mediat. Inflamm. 2017, 2017, 8570818. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Wu, X.; Zhang, Q.; Chen, S.; Ma, X.; Yu, M. Ironing Out the Details: How Iron Orchestrates Macrophage Polarization. Front. Immunol. 2021, 12, 669566. [Google Scholar] [CrossRef]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13, e0196921. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Roberts, E.R.; Stafford, A.R.; Banyard, K.L.; Matteucci, P.; Mace, K.A.; Hardman, M.J. Tissue Iron Promotes Wound Repair via M2 Macrophage Polarization and the Chemokine (C-C Motif) Ligands 17 and 22. Am. J. Pathol. 2019, 189, 2196–2208. [Google Scholar] [CrossRef] [PubMed]
- Handa, P.; Thomas, S.; Morgan-Stevenson, V.; Maliken, B.D.; Gochanour, E.; Boukhar, S.; Yeh, M.M.; Kowdley, K.V. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J. Leukoc. Biol. 2019, 105, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight 2018, 3, e120596. [Google Scholar] [CrossRef]
- Cai, C.; Zeng, D.; Gao, Q.; Ma, L.; Zeng, B.; Zhou, Y.; Wang, H. Decreased ferroportin in hepatocytes promotes macrophages polarize towards an M2-like phenotype and liver fibrosis. Sci. Rep. 2021, 11, 13386. [Google Scholar] [CrossRef]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef]
- Charlebois, E.; Fillebeen, C.; Presley, J.; Cagnone, G.; Lisi, V.; Lavallée, V.P.; Joyal, J.S.; Pantopoulos, K. Liver sinusoidal endothelial cells induce BMP6 expression in response to non-transferrin-bound iron. Blood 2023, 141, 271–284. [Google Scholar] [CrossRef]
- Fisher, A.L.; Wang, C.Y.; Xu, Y.; Joachim, K.; Xiao, X.; Phillips, S.; Moschetta, G.A.; Alfaro-Magallanes, V.M.; Babitt, J.L. Functional role of endothelial transferrin receptor 1 in iron sensing and homeostasis. Am. J. Hematol. 2022, 97, 1548–1559. [Google Scholar] [CrossRef]
- Jenkitkasemwong, S.; Wang, C.Y.; Coffey, R.; Zhang, W.; Chan, A.; Biel, T.; Kim, J.S.; Hojyo, S.; Fukada, T.; Knutson, M.D. SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. Cell Metab. 2015, 22, 138–150. [Google Scholar] [CrossRef]
- Knutson, M.D. Non-transferrin-bound iron takes the driver’s seat. Blood 2023, 141, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Viatte, L.; Bennoun, M.; Beaumont, C.; Kahn, A.; Vaulont, S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol. Dis. 2002, 29, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xu, Y.; Traeger, L.; Dogan, D.Y.; Xiao, X.; Steinbicker, A.U.; Babitt, J.L. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood 2020, 135, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Srole, D.N.; Ganz, T. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Arezes, J.; Foy, N.; McHugh, K.; Quinkert, D.; Benard, S.; Sawant, A.; Frost, J.N.; Armitage, A.E.; Pasricha, S.R.; Lim, P.J.; et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood 2020, 135, 547–557. [Google Scholar] [CrossRef]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; LaVallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef]
- Zhang, A.S.; Enns, C.A. A long sought after “receptor” for ERFE? Blood 2018, 132, 1463–1464. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, Y.; Moschetta, G.A.; Yu, Y.; Fisher, A.L.; Alfaro-Magallanes, V.M.; McMillen, S.; Phillips, S.; Wang, C.Y.; Christian, J.; et al. BMP5 contributes to hepcidin regulation and systemic iron homeostasis in mice. Blood 2023, 142, 1312–1322. [Google Scholar] [CrossRef]
- Ganz, T. Erythropoietic regulators of iron metabolism. Free. Radic. Biol. Med. 2019, 133, 69–74. [Google Scholar] [CrossRef]
- Zhao, N.; Nizzi, C.P.; Anderson, S.A.; Wang, J.; Ueno, A.; Tsukamoto, H.; Eisenstein, R.S.; Enns, C.A.; Zhang, A.S. Low intracellular iron increases the stability of matriptase-2. J. Biol. Chem. 2015, 290, 4432–4446. [Google Scholar] [CrossRef]
- Nai, A.; Rubio, A.; Campanella, A.; Gourbeyre, O.; Artuso, I.; Bordini, J.; Gineste, A.; Latour, C.; Besson-Fournier, C.; Lin, H.Y.; et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood 2016, 127, 2327–2336. [Google Scholar] [CrossRef]
- Wallace, D.F.; Secondes, E.S.; Rishi, G.; Ostini, L.; McDonald, C.J.; Lane, S.W.; Vu, T.; Hooper, J.D.; Velasco, G.; Ramsay, A.J.; et al. A critical role for murine transferrin receptor 2 in erythropoiesis during iron restriction. Br. J. Haematol. 2015, 168, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Aschemeyer, S.; Gabayan, V.; Ganz, T.; Nemeth, E.; Kautz, L. Erythroferrone and matriptase-2 independently regulate hepcidin expression. Am. J. Hematol. 2017, 92, E61–E63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ukomadu, C. Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem. Biophys. Res. Commun. 2008, 365, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Sardo, U.; Perrier, P.; Cormier, K.; Sotin, M.; Personnaz, J.; Medjbeur, T.; Desquesnes, A.; Cannizzo, L.; Ruiz-Martinez, M.; Thevenin, J.; et al. The hepatokine FGL1 regulates hepcidin and iron metabolism during anemia in mice by antagonizing BMP signaling. Blood 2024, 143, 1282–1292. [Google Scholar] [CrossRef]
- Personnaz, J.; Guillou, H.; Kautz, L. Fibrinogen-like 1: A hepatokine linking liver physiology to hematology. Hemasphere 2024, 8, e115. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F.; Toyokuni, S. New iron export pathways acting via holo-ferritin secretion. Arch. Biochem. Biophys. 2023, 746, 109737. [Google Scholar] [CrossRef]
- Vogt, A.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Truman-Rosentsvit, M.; Berenbaum, D.; Spektor, L.; Cohen, L.A.; Belizowsky-Moshe, S.; Lifshitz, L.; Ma, J.; Li, W.; Kesselman, E.; Abutbul-Ionita, I.; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433–1461. [Google Scholar] [PubMed]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, I.; Russo, R. Novel Insights and Future Perspective in Iron Metabolism and Anemia. Metabolites 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G.; et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Gikandi, A.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Ferroptosis. Adv. Exp. Med. Biol. 2021, 1301, 41–57. [Google Scholar]
- Das, N.K.; Jain, C.; Sankar, A.; Schwartz, A.J.; Santana-Codina, N.; Solanki, S.; Zhang, Z.; Ma, X.; Parimi, S.; Rui, L.; et al. Modulation of the HIF2α-NCOA4 axis in enterocytes attenuates iron loading in a mouse model of hemochromatosis. Blood 2022, 139, 2547–2552. [Google Scholar] [CrossRef]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell Signal 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Koch, P.S.; Olsavszky, V.; Ulbrich, F.; Sticht, C.; Demory, A.; Leibing, T.; Henzler, T.; Meyer, M.; Zierow, J.; Schneider, S.; et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 2017, 129, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Canali, S.; Wang, C.Y.; Zumbrennen-Bullough, K.B.; Bayer, A.; Babitt, J.L. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am. J. Hematol. 2017, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Manco, M.; Altruda, F.; Fagoonee, S.; Tolosano, E. Liver Sinusoidal Endothelial Cells at the Crossroad of Iron Overload and Liver Fibrosis. Antioxid. Redox Signal. 2021, 35, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.J.; Duarte, T.L.; Arezes, J.; Garcia-Santos, D.; Hamdi, A.; Pasricha, S.R.; Armitage, A.E.; Mehta, H.; Wideman, S.; Santos, A.G.; et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat. Metab. 2019, 1, 519–531. [Google Scholar] [CrossRef]
- Antebi, Y.E.; Linton, J.M.; Klumpe, H.; Bintu, B.; Gong, M.; Su, C.; McCardell, R.; Elowitz, M.B. Combinatorial Signal Perception in the BMP Pathway. Cell 2017, 170, 1184–1196.e24. [Google Scholar] [CrossRef]
- Xiao, X.; Alfaro-Magallanes, V.M.; Babitt, J.L. Bone morphogenic proteins in iron homeostasis. Bone 2020, 138, 115495. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef]
- Colucci, S.; Pagani, A.; Pettinato, M.; Artuso, I.; Nai, A.; Camaschella, C.; Silvestri, L. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood 2017, 130, 2111–2120. [Google Scholar] [CrossRef]
- Pagani, A.; Pettinato, M.; Colucci, S.; Dulja, A.; Rauner, M.; Nai, A.; Camaschella, C.; Altamura, S.; Muckenthaler, M.U.; Silvestri, L. Hemochromatosis proteins are dispensable for the acute hepcidin response to BMP2. Haematologica 2020, 105, e493. [Google Scholar] [CrossRef]
- Dogan, D.Y.; Urzica, E.I.; Hornung, I.; Kastl, P.; Oguama, D.; Fette, F.M.; Nguyen, L.H.; Rosenbauer, F.; Zacharowski, K.; Klingmüller, U.; et al. Hemojuvelin-mediated hepcidin induction requires both bone morphogenetic protein type I receptors ALK2 and ALK3. Blood Adv. 2024, 8, 2870–2879. [Google Scholar] [CrossRef]
- Traeger, L.; Gallitz, I.; Sekhri, R.; Bäumer, N.; Kuhlmann, T.; Kemming, C.; Holtkamp, M.; Müller, J.C.; Karst, U.; Canonne-Hergaux, F.; et al. ALK3 undergoes ligand-independent homodimerization and BMP-induced heterodimerization with ALK2. Free. Radic. Biol. Med. 2018, 129, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Dev, S.; Canali, S.; Bayer, A.; Xu, Y.; Agarwal, A.; Wang, C.Y.; Babitt, J.L. Endothelial Bone Morphogenetic Protein 2 (Bmp2) Knockout Exacerbates Hemochromatosis in Homeostatic Iron Regulator (Hfe) Knockout Mice but not Bmp6 Knockout Mice. Hepatology 2020, 72, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Wang, C.Y.; Xu, Y.; Phillips, S.; Paulo, J.A.; Małachowska, B.; Xiao, X.; Fendler, W.; Mancias, J.D.; Babitt, J.L. Quantitative proteomics and RNA-sequencing of mouse liver endothelial cells identify novel regulators of BMP6 by iron. iScience 2023, 26, 108555. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Li, Y.; Xu, A.; Zhang, N.; Chen, S.; Zhou, D.; Zhang, B.; Ou, X.; Jia, J.; Huang, J.; et al. Recurrent BMP4 variants in exon 4 cause non-HFE-associated hemochromatosis via the BMP/SMAD signaling pathway. Orphanet. J. Rare Dis. 2024, 19, 429. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Kim, Y.H.; Jung, Y.S.; Kim, J.; Kim, D.K.; Cho, S.J.; Lee, I.K.; Dooley, S.; Lee, C.H.; Choi, H.S. Orphan Nuclear Receptor ERRγ Is a Novel Transcriptional Regulator of IL-6 Mediated Hepatic BMP6 Gene Expression in Mice. Int. J. Mol. Sci. 2020, 21, 7148. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, P.; Li, Y.; Ouyang, Q.; Hou, F.; Zhu, G.; Zhang, B.; Huang, J.; Jia, J.; Xu, A. Serum Iron Overload Activates the SMAD Pathway and Hepcidin Expression of Hepatocytes via SMURF1. J. Clin. Transl. Hepatol. 2024, 12, 227–235. [Google Scholar] [CrossRef]
- Beneduce, E.; Matte, A.; De Falco, L.; Mbiandjeu, S.; Chiabrando, D.; Tolosano, E.; Federti, E.; Petrillo, S.; Mohandas, N.; Siciliano, A.; et al. Fyn kinase is a novel modulator of erythropoietin signaling and stress erythropoiesis. Am. J. Hematol. 2019, 94, 10–20. [Google Scholar] [CrossRef]
- Ruart, M.; Chavarria, L.; Campreciós, G.; Suárez-Herrera, N.; Montironi, C.; Guixé-Muntet, S.; Bosch, J.; Friedman, S.L.; Garcia-Pagán, J.C.; Hernández-Gea, V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 2019, 70, 458–469. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.A.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 2006, 38, 531–539. [Google Scholar] [CrossRef]
- Meynard, D.; Kautz, L.; Darnaud, V.; Canonne-Hergaux, F.; Coppin, H.; Roth, M.P. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 2009, 41, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Yee, J. Hepcidin. Adv. Chronic. Kidney Dis. 2019, 26, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Hollerer, I.; Bachmann, A.; Muckenthaler, M.U. Pathophysiological consequences and benefits of HFE mutations: 20 years of research. Haematologica 2017, 102, 809–817. [Google Scholar] [CrossRef]
- Brasse-Lagnel, C.; Karim, Z.; Letteron, P.; Bekri, S.; Bado, A.; Beaumont, C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 2011, 140, 1261–1271.e1. [Google Scholar] [CrossRef]
- Canali, S.; Vecchi, C.; Garuti, C.; Montosi, G.; Babitt, J.L.; Pietrangelo, A. The SMAD Pathway Is Required for Hepcidin Response During Endoplasmic Reticulum Stress. Endocrinology 2016, 157, 3935–3945. [Google Scholar] [CrossRef]
- Gao, J.; Chen, J.; Kramer, M.; Tsukamoto, H.; Zhang, A.S.; Enns, C.A. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009, 9, 217–227. [Google Scholar] [CrossRef]
- Casanovas, G.; Mleczko-Sanecka, K.; Altamura, S.; Hentze, M.W.; Muckenthaler, M.U. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J. Mol. Med. 2009, 87, 471–480. [Google Scholar] [CrossRef]
- Fillebeen, C.; Charlebois, E.; Wagner, J.; Katsarou, A.; Mui, J.; Vali, H.; Garcia-Santos, D.; Ponka, P.; Presley, J.; Pantopoulos, K. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood 2019, 133, 344–355. [Google Scholar] [CrossRef]
- Lee, D.H.; Zhou, L.J.; Zhou, Z.; Xie, J.X.; Jung, J.U.; Liu, Y.; Xi, C.X.; Mei, L.; Xiong, W.C. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood 2010, 115, 3136–3145. [Google Scholar] [CrossRef]
- Zhao, N.; Maxson, J.E.; Zhang, R.H.; Wahedi, M.; Enns, C.A.; Zhang, A.S. Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver. J. Biol. Chem. 2016, 291, 12322–12335. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Endo, M.; Hata, K.; Higuchi, C.; Takaoka, K.; Yoshikawa, H.; Yamashita, T. Neogenin, a receptor for bone morphogenetic proteins. J. Biol. Chem. 2011, 286, 5157–5165. [Google Scholar] [CrossRef] [PubMed]
- Enns, C.A.; Ahmed, R.; Zhang, A.S. Neogenin interacts with matriptase-2 to facilitate hemojuvelin cleavage. J. Biol. Chem. 2012, 287, 35104–35117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Xiao, X.; Bayer, A.; Xu, Y.; Dev, S.; Canali, S.; Nair, A.V.; Masia, R.; Babitt, J.L. Ablation of Hepatocyte Smad1, Smad5, and Smad8 Causes Severe Tissue Iron Loading and Liver Fibrosis in Mice. Hepatology 2019, 70, 1986–2002. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Teng, F.; Hammad, S.; Werle, J.; Maas, T.; Teufel, A.; Muckenthaler, M.U.; Dooley, S.; Vujić Spasić, M. Hepatic Smad7 overexpression causes severe iron overload in mice. Blood 2018, 131, 581–585. [Google Scholar] [CrossRef]
- An, P.; Wang, H.; Wu, Q.; Wang, J.; Xia, Z.; He, X.; Wang, X.; Chen, Y.; Min, J.; Wang, F. Smad7 deficiency decreases iron and haemoglobin through hepcidin up-regulation by multilayer compensatory mechanisms. J. Cell Mol. Med. 2018, 22, 3035–3044. [Google Scholar] [CrossRef]
- Corradini, E.; Garuti, C.; Montosi, G.; Ventura, P.; Andriopoulos, B., Jr.; Lin, H.Y.; Pietrangelo, A.; Babitt, J.L. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology 2009, 137, 1489–1497. [Google Scholar] [CrossRef]
- Wallace, D.F.; Summerville, L.; Crampton, E.M.; Frazer, D.M.; Anderson, G.J.; Subramaniam, V.N. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology 2009, 50, 1992–2000. [Google Scholar] [CrossRef]
- Traeger, L.; Enns, C.A.; Krijt, J.; Steinbicker, A.U. The hemochromatosis protein HFE signals predominantly via the BMP type I receptor ALK3 in vivo. Commun. Biol. 2018, 1, 65. [Google Scholar] [CrossRef]
- Traeger, L.; Schnittker, J.; Dogan, D.Y.; Oguama, D.; Kuhlmann, T.; Muckenthaler, M.U.; Krijt, J.; Urzica, E.I.; Steinbicker, A.U. HFE and ALK3 act in the same signaling pathway. Free. Radic. Biol. Med. 2020, 160, 501–505. [Google Scholar] [CrossRef]
- Latour, C.; Besson-Fournier, C.; Meynard, D.; Silvestri, L.; Gourbeyre, O.; Aguilar-Martinez, P.; Schmidt, P.J.; Fleming, M.D.; Roth, M.P.; Coppin, H. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology 2016, 63, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.; Rivella, S. New potential players in hepcidin regulation. Haematologica 2019, 104, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Enns, C.A.; Jue, S.; Zhang, A.S. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood 2020, 136, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, T.B. Cutting not the key to TMPRSS6 activity? Blood 2020, 136, 922–923. [Google Scholar] [CrossRef]
- Wahedi, M.; Wortham, A.M.; Kleven, M.D.; Zhao, N.; Jue, S.; Enns, C.A.; Zhang, A.S. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J. Biol. Chem. 2017, 292, 18354–18371. [Google Scholar] [CrossRef]
- Yu, L.N.; Wang, S.J.; Chen, C.; Rausch, V.; Elshaarawy, O.; Mueller, S. Direct modulation of hepatocyte hepcidin signaling by iron. World J. Hepatol. 2021, 13, 1378–1393. [Google Scholar] [CrossRef]
- Pettinato, M.; Dulja, A.; Colucci, S.; Furiosi, V.; Fette, F.; Steinbicker, A.U.; Muckenthaler, M.U.; Nai, A.; Pagani, A.; Silvestri, L. FKBP12 inhibits hepcidin expression by modulating BMP receptors interaction and ligand responsiveness in hepatocytes. Am. J. Hematol. 2023, 98, 1223–1235. [Google Scholar] [CrossRef]
- Xu, Y.; Alfaro-Magallanes, V.M.; Babitt, J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: Clinical implications for iron disorders. Br. J. Haematol. 2021, 193, 882–893. [Google Scholar] [CrossRef]
- Ma, S.; Dubin, A.E.; Zhang, Y.; Mousavi, S.A.R.; Wang, Y.; Coombs, A.M.; Loud, M.; Andolfo, I.; Patapoutian, A. A role of PIEZO1 in iron metabolism in mice and humans. Cell 2021, 184, 969–982.e13. [Google Scholar] [CrossRef]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis-Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Pantopoulos, K. Inherited Disorders of Iron Overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/EBPδ) expression in hepatocytes. J. Biol. Chem. 2017, 292, 10275–10287. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Murakami, M.; Matsui, T.; Funaba, M. JNK facilitates IL-1β-induced hepcidin transcription via JunB activation. Cytokine 2018, 111, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Arvedson, T.L.; Cooke, K.S.; Dickmann, L.J.; Forte, C.; Li, H.; Merriam, K.L.; Perry, V.K.; Tran, L.; Rottman, J.B.; et al. IL-22 regulates iron availability in vivo through the induction of hepcidin. J. Immunol. 2013, 191, 1845–1855. [Google Scholar] [CrossRef]
- Kanda, J.; Uchiyama, T.; Tomosugi, N.; Higuchi, M.; Uchiyama, T.; Kawabata, H. Oncostatin M and leukemia inhibitory factor increase hepcidin expression in hepatoma cell lines. Int. J. Hematol. 2009, 90, 545–552. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Lazrak, M.; Bellitir, S.; Mir, N.E.; Hamdouchi, A.E.; Barkat, A.; Zeder, C.; Moretti, D.; Aguenaou, H.; Zimmermann, M.B. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica 2019, 104, 1143–1149. [Google Scholar] [CrossRef]
- Constante, M.; Jiang, W.; Wang, D.; Raymond, V.A.; Bilodeau, M.; Santos, M.M. Distinct requirements for Hfe in basal and induced hepcidin levels in iron overload and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G229–G237. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhao, G.P.; Wang, X.F.; Liu, X.X.; Li, Y.X.; Qiu, L.L.; Wang, X.Y.; Ren, F.Z. Glycochenodeoxycholate Affects Iron Homeostasis via Up-Regulating Hepcidin Expression. Nutrients 2022, 14, 3176. [Google Scholar] [CrossRef]
- Latour, C.; Kautz, L.; Besson-Fournier, C.; Island, M.L.; Canonne-Hergaux, F.; Loréal, O.; Ganz, T.; Coppin, H.; Roth, M.P. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014, 59, 683–694. [Google Scholar] [CrossRef]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E.; Shelton, G.; Kramer, Y.; McCulloh, D.; Keefe, D.; et al. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Investig. 2016, 126, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, Y.; Tanaka, K.; Matsui, T.; Sakaguchi, T.; Yamagishi, S.I.; Motomiya, Y. Fibroblast Growth Factor 23 Contributes to Regulation of Hepcidin/Ferroportin Axis. Austin J. Pharmacol. Ther. 2020, 8, 1118. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Foka, P.; Kyratzopoulou, E.; Karamichali, E.; Petroulia, S.; Tsitoura, P.; Kakkanas, A.; Eliadis, P.; Georgopoulou, U.; Mamalaki, A. The Hepatitis C virus NS5A and core proteins exert antagonistic effects on HAMP gene expression: The hidden interplay with the MTF-1/MRE pathway. FEBS Open Bio. 2021, 11, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Balesaria, S.; Ramesh, B.; McArdle, H.; Bayele, H.K.; Srai, S.K. Divalent metal-dependent regulation of hepcidin expression by MTF-1. FEBS Lett. 2010, 584, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Ito, M.; Chida, T.; Nakashima, K.; Sakai, S.; Kanegae, Y.; Kawasaki, H.; Aoshima, T.; Takabayashi, S.; Takahashi, H.; et al. Role of hepcidin upregulation and proteolytic cleavage of ferroportin 1 in hepatitis C virus-induced iron accumulation. PLoS Pathog. 2023, 19, e1011591. [Google Scholar] [CrossRef]
- Vela, D. Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol. Med. 2018, 24, 5. [Google Scholar] [CrossRef]
- Zmijewski, E.; Lu, S.; Harrison-Findik, D.D. TLR4 signaling and the inhibition of liver hepcidin expression by alcohol. World J. Gastroenterol. 2014, 20, 12161–12170. [Google Scholar] [CrossRef]
- Harrison-Findik, D.D.; Klein, E.; Crist, C.; Evans, J.; Timchenko, N.; Gollan, J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology 2007, 46, 1979–1985. [Google Scholar] [CrossRef]
- Castoldi, M.; Vujic Spasic, M.; Altamura, S.; Elmén, J.; Lindow, M.; Kiss, J.; Stolte, J.; Sparla, R.; D’Alessandro, L.A.; Klingmüller, U.; et al. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J. Clin. Investig. 2011, 121, 1386–1396. [Google Scholar] [CrossRef]
- Zumbrennen-Bullough, K.B.; Wu, Q.; Core, A.B.; Canali, S.; Chen, W.; Theurl, I.; Meynard, D.; Babitt, J.L. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J. Biol. Chem. 2014, 289, 23796–23808. [Google Scholar] [CrossRef]
- Wang, R.H.; Li, C.; Xu, X.; Zheng, Y.; Xiao, C.; Zerfas, P.; Cooperman, S.; Eckhaus, M.; Rouault, T.; Mishra, L.; et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005, 2, 399–409. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Lim, P.J.; Duarte, T.L.; Casu, C.; Oosterhuis, D.; Mleczko-Sanecka, K.; Suciu, M.; Da Silva, A.R.; Al-Hourani, K.; Arezes, J.; et al. Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat. Commun. 2017, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Sugianto, P.; Fung, E.; Del-Castillo-Rueda, A.; Moran-Jimenez, M.J.; Ganz, T.; Nemeth, E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012, 15, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 2020, 586, 807–811. [Google Scholar] [CrossRef]
- Canonne-Hergaux, F.; Donovan, A.; Delaby, C.; Wang, H.J.; Gros, P. Comparative studies of duodenal and macrophage ferroportin proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G156–G163. [Google Scholar] [CrossRef]
- Sangokoya, C.; Doss, J.F.; Chi, J.T. Iron-responsive miR-485-3p regulates cellular iron homeostasis by targeting ferroportin. PLoS Genet. 2013, 9, e1003408. [Google Scholar] [CrossRef]
- Guida, C.; Altamura, S.; Klein, F.A.; Galy, B.; Boutros, M.; Ulmer, A.J.; Hentze, M.W.; Muckenthaler, M.U. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 2015, 125, 2265–2275. [Google Scholar] [CrossRef]
- Bayele, H.K.; Srai, S.K.S. A disease-causing mutation K240E disrupts ferroportin trafficking by SUMO (ferroportin SUMOylation). Biochem. Biophys. Rep. 2021, 25, 100873. [Google Scholar] [CrossRef]
- Gammella, E.; Correnti, M.; Cairo, G.; Recalcati, S. Iron Availability in Tissue Microenvironment: The Key Role of Ferroportin. Int. J. Mol. Sci. 2021, 22, 2986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Xu, Y.; Wu, R.; Chen, X.; Song, X.; Zeh, H.; Kang, R.; Klionsky, D.J.; Wang, X.; et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy 2021, 17, 3361–3374. [Google Scholar] [CrossRef] [PubMed]
- Cairo, G.; Recalcati, S. Iron-regulatory proteins: Molecular biology and pathophysiological implications. Expert Rev. Mol. Med. 2007, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Gammella, E.; Cairo, G. New perspectives on the molecular basis of the interaction between oxygen homeostasis and iron metabolism. Hypoxia 2015, 3, 93–103. [Google Scholar]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Recalcati, S.; Minotti, G.; Cairo, G. Iron regulatory proteins: From molecular mechanisms to drug development. Antioxid. Redox Signal 2010, 13, 1593–1616. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Woolf, C.J.; Weiss, G.; Penninger, J.M. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front. Mol. Biosci. 2019, 6, 116. [Google Scholar] [CrossRef]
- Salahudeen, A.A.; Thompson, J.W.; Ruiz, J.C.; Ma, H.W.; Kinch, L.N.; Li, Q.; Grishin, N.V.; Bruick, R.K. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 2009, 326, 722–726. [Google Scholar] [CrossRef]
- Thompson, J.W.; Salahudeen, A.A.; Chollangi, S.; Ruiz, J.C.; Brautigam, C.A.; Makris, T.M.; Lipscomb, J.D.; Tomchick, D.R.; Bruick, R.K. Structural and molecular characterization of iron-sensing hemerythrin-like domain within F-box and leucine-rich repeat protein 5 (FBXL5). J. Biol. Chem. 2012, 287, 7357–7365. [Google Scholar] [CrossRef]
- Iwai, K. Regulation of cellular iron metabolism: Iron-dependent degradation of IRP by SCFFBXL5 ubiquitin ligase. Free. Radic. Biol. Med. 2019, 133, 64–68. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hughes, R.M.; Ollivierre-Wilson, H.; Ghosh, M.C.; Rouault, T.A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009, 9, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Ferring-Appel, D.; Becker, C.; Gretz, N.; Gröne, H.J.; Schümann, K.; Hentze, M.W. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep. 2013, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Pappalardi, M.B.; McNulty, D.E.; Martin, J.D.; Fisher, K.E.; Jiang, Y.; Burns, M.C.; Zhao, H.; Ho, T.; Sweitzer, S.; Schwartz, B.; et al. Biochemical characterization of human HIF hydroxylases using HIF protein substrates that contain all three hydroxylation sites. Biochem. J. 2011, 436, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Place, T.L.; Domann, F.E. Prolyl-hydroxylase 3: Evolving Roles for an Ancient Signaling Protein. Hypoxia 2013, 2013, 13–17. [Google Scholar]
- Peyssonnaux, C.; Zinkernagel, A.S.; Schuepbach, R.A.; Rankin, E.; Vaulont, S.; Haase, V.H.; Nizet, V.; Johnson, R.S. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J. Clin. Investig. 2007, 117, 1926–1932. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Investig. 2019, 129, 336–348. [Google Scholar] [CrossRef]
- Lee, F.S. At the crossroads of oxygen and iron sensing: Hepcidin control of HIF-2α. J. Clin. Investig. 2019, 129, 72–74. [Google Scholar] [CrossRef]
- Pelucchi, S.; Ravasi, G.; Arosio, C.; Mauri, M.; Piazza, R.; Mariani, R.; Piperno, A. HIF1A: A Putative Modifier of Hemochromatosis. Int. J. Mol. Sci. 2021, 22, 1245. [Google Scholar] [CrossRef]
- Mathieu, J.R.; Heinis, M.; Zumerle, S.; Delga, S.; Le Bon, A.; Peyssonnaux, C. Investigating the real role of HIF-1 and HIF-2 in iron recycling by macrophages. Haematologica 2014, 99, e112–e114. [Google Scholar] [CrossRef]
- Renassia, C.; Peyssonnaux, C. New insights into the links between hypoxia and iron homeostasis. Curr. Opin. Hematol. 2019, 26, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannaki, M.; Matak, P.; Delga, S.; Deschemin, J.C.; Vaulont, S.; Peyssonnaux, C. Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood 2012, 119, 587–590. [Google Scholar] [CrossRef]
- Krzywoszyńska, K.; Witkowska, D.; Swiatek-Kozlowska, J.; Szebesczyk, A.; Kozłowski, H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Conrad, M.; Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2024, 25, 133–155. [Google Scholar] [CrossRef]
- Brissot, P.; Pietrangelo, A.; Adams, P.C.; de Graaff, B.; McLaren, C.E.; Loréal, O. Haemochromatosis. Nat. Rev. Dis. Primers 2018, 4, 18016. [Google Scholar] [CrossRef]
- Valberg, L.S.; Simon, J.B.; Manley, P.N.; Corbett, W.E.; Ludwig, J. Distribution of storage iron as body stores expand in patients with hemochromatosis. J. Lab. Clin. Med. 1975, 86, 479–489. [Google Scholar]
- Brink, B.; Disler, P.; Lynch, S.; Jacobs, P.; Charlton, R.; Bothwell, T. Patterns of iron storage in dietary iron overload and idiopathic hemochromatosis. J. Lab. Clin. Med. 1976, 88, 725–731. [Google Scholar]
- Trousseau, A. Glycosurie, diabète sucre. Clin. Médicale L’hôtel-Dieu Paris 1865, 2, 663–698. [Google Scholar]
- von Recklinghausen, F.D. Hämochromatose. Tageblatt Naturforschenden Versamml. 1890, 1889, 324. [Google Scholar]
- Simon, M.; Alexandre, J.L.; Bourel, M.; Le Marec, B.; Scordia, C. Heredity of idiopathic haemochromatosis: A study of 106 families. Clin. Genet. 1977, 11, 327–341. [Google Scholar] [CrossRef]
- Simon, M.; Bourel, M.; Genetet, B.; Fauchet, R. Heredity of idiopathic haemochromatosis. Lancet 1977, 1, 706. [Google Scholar] [CrossRef]
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R., Jr.; Ellis, M.C.; Fullan, A.; et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 1996, 13, 399–408. [Google Scholar] [CrossRef]
- Ryan, E.; O’keane, C.; Crowe, J. Hemochromatosis in Ireland and HFE. Blood Cells Mol. Dis. 1998, 24, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Chung, J.W.; Lapointe, R.; Santos, M.M. The hemochromatosis protein HFE 20 years later: An emerging role in antigen presentation and in the immune system. Immun. Inflamm. Dis. 2017, 5, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Distante, S.; Robson, K.J.; Graham-Campbell, J.; Arnaiz-Villena, A.; Brissot, P.; Worwood, M. The origin and spread of the HFE-C282Y haemochromatosis mutation. Hum. Genet. 2004, 115, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Survival advantage of the hemochromatosis C282Y mutation. Perspect. Biol. Med. 2008, 51, 98–102. [Google Scholar] [CrossRef]
- Bomford, A. Genetics of haemochromatosis. Lancet 2002, 360, 1673–1681. [Google Scholar] [CrossRef]
- Adams, P.C.; Reboussin, D.M.; Barton, J.C.; McLaren, C.E.; Eckfeldt, J.H.; McLaren, G.D.; Dawkins, F.W.; Acton, R.T.; Harris, E.L.; Gordeuk, V.R.; et al. Hemochromatosis and iron-overload screening in a racially diverse population. N. Engl. J. Med. 2005, 352, 1769–1778. [Google Scholar] [CrossRef]
- Merryweather-Clarke, A.T.; Pointon, J.J.; Shearman, J.D.; Robson, K.J. Global prevalence of putative haemochromatosis mutations. J. Med. Genet. 1997, 34, 275–278. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Politou, M.; Terpos, E.; Fourlemadis, S.; Sakellaropoulos, N.; Loukopoulos, D. Hereditary hemochromatosis: HFE mutation analysis in Greeks reveals genetic heterogeneity. Blood Cells Mol. Dis. 2000, 26, 163–168. [Google Scholar] [CrossRef]
- Steinberg, K.K.; Cogswell, M.E.; Chang, J.C.; Caudill, S.P.; McQuillan, G.M.; Bowman, B.A.; Grummer-Strawn, L.M.; Sampson, E.J.; Khoury, M.J.; Gallagher, M.L. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. JAMA 2001, 285, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- McLaren, C.E.; Barton, J.C.; Adams, P.C.; Harris, E.L.; Acton, R.T.; Press, N.; Reboussin, D.M.; McLaren, G.D.; Sholinsky, P.; Walker, A.P.; et al. Hemochromatosis and Iron Overload Screening (HEIRS) study design for an evaluation of 100,000 primary care-based adults. Am. J. Med. Sci. 2003, 325, 53–62. [Google Scholar] [CrossRef]
- Hanson, E.H.; Imperatore, G.; Burke, W. HFE gene and hereditary hemochromatosis: A HuGE review. Hum. Genome Epidemiol. Am. J. Epidemiol. 2001, 154, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Pilling, L.C.; Tamosauskaite, J.; Jones, G.; Wood, A.R.; Jones, L.; Kuo, C.L.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Common conditions associated with hereditary haemochromatosis genetic variants: Cohort study in UK Biobank. BMJ 2019, 364, k5222. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Bardou-Jacquet, E. Revisiting hemochromatosis: Genetic vs. phenotypic manifestations. Ann. Transl. Med. 2021, 9, 731. [Google Scholar] [CrossRef]
- Whitlock, E.P.; Garlitz, B.A.; Harris, E.L.; Beil, T.L.; Smith, P.R. Screening for hereditary hemochromatosis: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2006, 145, 209–223. [Google Scholar] [CrossRef]
- Powell, E.E.; Ali, A.; Clouston, A.D.; Dixon, J.L.; Lincoln, D.J.; Purdie, D.M.; Fletcher, L.M.; Powell, L.W.; Jonsson, J.R. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology 2005, 129, 1937–1943. [Google Scholar] [CrossRef]
- Allen, K.J.; Gurrin, L.C.; Constantine, C.C.; Osborne, N.J.; Delatycki, M.B.; Nicoll, A.J.; McLaren, C.E.; Bahlo, M.; Nisselle, A.E.; Vulpe, C.D.; et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med. 2008, 358, 221–230. [Google Scholar] [CrossRef]
- Sandhu, K.; Flintoff, K.; Chatfield, M.D.; Dixon, J.L.; Ramm, L.E.; Ramm, G.A.; Powell, L.W.; Subramaniam, V.N.; Wallace, D.F. Phenotypic analysis of hemochromatosis subtypes reveals variations in severity of iron overload and clinical disease. Blood 2018, 132, 101–110. [Google Scholar] [CrossRef]
- Brissot, P.; Loréal, O. Hemochromatoses. J. Hepatol. 2021, 75, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Turshudzhyan, A.; Wu, D.C.; Wu, G.Y. Primary Non-HFE Hemochromatosis: A Review. J. Clin. Transl. Hepatol. 2023, 11, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Samuels, M.E.; Ludwig, E.H.; MacDonald, M.L.; Franchini, P.L.; Dubé, M.P.; Andres, L.; MacFarlane, J.; Sakellaropoulos, N.; Politou, M.; et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 2004, 36, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef]
- Valenti, L.; Fracanzani, A.L.; Bugianesi, E.; Dongiovanni, P.; Galmozzi, E.; Vanni, E.; Canavesi, E.; Lattuada, E.; Roviaro, G.; Marchesini, G.; et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2010, 138, 905–912. [Google Scholar] [CrossRef]
- Piperno, A.; Pelucchi, S.; Mariani, R. Inherited iron overload disorders. Transl. Gastroenterol. Hepatol. 2020, 5, 25. [Google Scholar] [CrossRef]
- Daher, R.; Kannengiesser, C.; Houamel, D.; Lefebvre, T.; Bardou-Jacquet, E.; Ducrot, N.; de Kerguenec, C.; Jouanolle, A.M.; Robreau, A.M.; Oudin, C.; et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology 2016, 150, 672–683.e4. [Google Scholar] [CrossRef]
- Piubelli, C.; Castagna, A.; Marchi, G.; Rizzi, M.; Busti, F.; Badar, S.; Marchetti, M.; De Gobbi, M.; Roetto, A.; Xumerle, L.; et al. Identification of new BMP6 pro-peptide mutations in patients with iron overload. Am. J. Hematol. 2017, 92, 562–568. [Google Scholar] [CrossRef]
- Bell, S.; Rigas, A.S.; Magnusson, M.K.; Ferkingstad, E.; Allara, E.; Bjornsdottir, G.; Ramond, A.; Sørensen, E.; Halldorsson, G.H.; Paul, D.S.; et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun. Biol. 2021, 4, 156. [Google Scholar] [CrossRef]
- Alvarenga, A.M.; Brissot, P.; Santos, P.C.J.L. Haemochromatosis revisited. World J. Hepatol. 2022, 14, 1931–1939. [Google Scholar] [CrossRef]
- Girelli, D.; Busti, F.; Brissot, P.; Cabantchik, I.; Muckenthaler, M.U.; Porto, G. Hemochromatosis classification: Update and recommendations by the BIOIRON Society. Blood 2022, 139, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Ferrer-Cortès, X.; Venturi, V.; Musri, M.; Pilquil, M.F.; Torres, P.M.M.; Rodríguez, I.H.; Mínguez, M.À.R.; Kelleher, N.J.; Pelucchi, S.; et al. New Mutations in HFE2 and TFR2 Genes Causing Non HFE-Related Hereditary Hemochromatosis. Genes 2021, 12, 1980. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, V.; Ryan, E.; O’Keane, C.; Crowe, J. Immunohistochemistry of the Hfe protein in patients with hereditary hemochromatosis, iron deficiency anemia, and normal controls. Blood Cells Mol. Dis. 2000, 26, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Haemochromatosis. Gut 2003, 52, ii23–ii30. [Google Scholar] [CrossRef]
- Fleming, R.E.; Britton, R.S.; Waheed, A.; Sly, W.S.; Bacon, B.R. Pathogenesis of hereditary hemochromatosis. Clin. Liver. Dis. 2004, 8, 755–773. [Google Scholar] [CrossRef]
- Girelli, D.; Trombini, P.; Busti, F.; Campostrini, N.; Sandri, M.; Pelucchi, S.; Westerman, M.; Ganz, T.; Nemeth, E.; Piperno, A.; et al. A time course of hepcidin response to iron challenge in patients with HFE and TFR2 hemochromatosis. Haematologica 2011, 96, 500–506. [Google Scholar] [CrossRef][Green Version]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef]
- Ryan, J.D.; Ryan, E.; Fabre, A.; Lawless, M.W.; Crowe, J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology 2010, 52, 1266–1273. [Google Scholar] [CrossRef]
- Lebeau, A.; Frank, J.; Biesalski, H.K.; Weiss, G.; Srai, S.K.; Simpson, R.J.; McKie, A.T.; Bahram, S.; Gilfillan, S.; Schümann, K. Long-term sequelae of HFE deletion in C57BL/6 x 129/O1a mice, an animal model for hereditary haemochromatosis. Eur. J. Clin. Investig. 2002, 32, 603–612. [Google Scholar] [CrossRef]
- Delima, R.D.; Chua, A.C.; Tirnitz-Parker, J.E.; Gan, E.K.; Croft, K.D.; Graham, R.M.; Olynyk, J.K.; Trinder, D. Disruption of hemochromatosis protein and transferrin receptor 2 causes iron-induced liver injury in mice. Hepatology 2012, 56, 585–593. [Google Scholar] [CrossRef]
- Stål, P.; Glaumann, H.; Hultcrantz, R. Liver cell damage and lysosomal iron storage in patients with idiopathic hemochromatosis. A light and electron microscopic study. J. Hepatol. 1990, 11, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Iancu, T.C.; Deugnier, Y.; Halliday, J.W.; Powell, L.W.; Brissot, P. Ultrastructural sequences during liver iron overload in genetic hemochromatosis. J. Hepatol. 1997, 27, 628–638. [Google Scholar] [CrossRef]
- Lunova, M.; Goehring, C.; Kuscuoglu, D.; Mueller, K.; Chen, Y.; Walther, P.; Deschemin, J.C.; Vaulont, S.; Haybaeck, J.; Lackner, C.; et al. Hepcidin knockout mice fed with iron-rich diet develop chronic liver injury and liver fibrosis due to lysosomal iron overload. J. Hepatol. 2014, 61, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Koo, J.H.; Kim, S.H.; Gardenghi, S.; Rivella, S.; Strnad, P.; Hwang, S.J.; Kim, S.G. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat. Commun. 2016, 7, 13817. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017, 3, 17025. [Google Scholar] [CrossRef]
- Pietrangelo, A. Ferroportin disease: Pathogenesis, diagnosis and treatment. Haematologica 2017, 102, 1972–1984. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, A.; Li, Y.; Zhao, S.; Zhou, D.; Wu, L.; Zhang, B.; Zhao, X.; Wang, Y.; Wang, X.; et al. A novel SLC40A1 p.Y333H mutation with gain of function of ferroportin: A recurrent cause of haemochromatosis in China. Liver. Int. 2019, 39, 1120–1127. [Google Scholar] [CrossRef]
- Vlasveld, L.T.; Janssen, R.; Bardou-Jacquet, E.; Venselaar, H.; Hamdi-Roze, H.; Drakesmith, H.; Swinkels, D.W. Twenty Years of Ferroportin Disease: A Review or An Update of Published Clinical, Biochemical, Molecular, and Functional Features. Pharmaceuticals 2019, 12, 132. [Google Scholar] [CrossRef]
- Cunat, S.; Giansily-Blaizot, M.; Bismuth, M.; Blanc, F.; Dereure, O.; Larrey, D.; Quellec, A.L.; Pouderoux, P.; Rose, C.; Raingeard, I.; et al. Global sequencing approach for characterizing the molecular background of hereditary iron disorders. Clin. Chem. 2007, 53, 2060–2069. [Google Scholar] [CrossRef]
- Speletas, M.; Kioumi, A.; Loules, G.; Hytiroglou, P.; Tsitouridis, J.; Christakis, J.; Germenis, A.E. Analysis of SLC40A1 gene at the mRNA level reveals rapidly the causative mutations in patients with hereditary hemochromatosis type IV. Blood Cells Mol. Dis. 2008, 40, 353–359. [Google Scholar] [CrossRef]
- Speletas, M.; Onoufriadis, E.; Kioumi, A.; Germenis, A.E. SLC40A1-R178G mutation and ferroportin disease. J. Hepatol. 2011, 55, 730–731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ka, C.; Guellec, J.; Pepermans, X.; Kannengiesser, C.; Ged, C.; Wuyts, W.; Cassiman, D.; de Ledinghen, V.; Varet, B.; de Kerguenec, C.; et al. The SLC40A1 R178Q mutation is a recurrent cause of hemochromatosis and is associated with a novel pathogenic mechanism. Haematologica 2018, 103, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, M.; Montosi, G.; Garuti, C.; Caleffi, A.; Oliveto, S.; Biffo, S.; Pietrangelo, A. Human macrophage ferroportin biology and the basis for the ferroportin disease. Hepatology 2017, 65, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, A.; Panzer, M.; Baumgartner, N.; Schaefer, B.; Finkenstedt, A.; Henninger, B.; Theurl, I.; Nachbaur, K.; Weiss, G.; Haubner, R.; et al. Reduced iron export associated with hepcidin resistance can explain the iron overload spectrum in ferroportin disease. Liver. Int. 2020, 40, 1941–1951. [Google Scholar] [CrossRef]
- Cairo, G.; Recalcati, S.; Montosi, G.; Castrusini, E.; Conte, D.; Pietrangelo, A. Inappropriately high iron regulatory protein activity in monocytes of patients with genetic hemochromatosis. Blood 1997, 89, 2546–2553. [Google Scholar] [CrossRef]
- Zoller, H.; Koch, R.O.; Theurl, I.; Obrist, P.; Pietrangelo, A.; Montosi, G.; Haile, D.J.; Vogel, W.; Weiss, G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001, 120, 1412–1419. [Google Scholar] [CrossRef]
- Parrow, N.L.; Gardenghi, S.; Ramos, P.; Casu, C.; Grady, R.W.; Anderson, E.R.; Shah, Y.M.; Li, H.; Ginzburg, Y.Z.; Fleming, R.E.; et al. Decreased hepcidin expression in murine β-thalassemia is associated with suppression of Bmp/Smad signaling. Blood 2012, 119, 3187–3189. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Berezovsky, B.; Frýdlová, J.; Gurieva, I.; Rogalsky, D.W.; Vokurka, M.; Krijt, J. Heart Ferroportin Protein Content Is Regulated by Heart Iron Concentration and Systemic Hepcidin Expression. Int. J. Mol. Sci. 2022, 23, 5899. [Google Scholar] [CrossRef]
- Bastin, J.M.; Jones, M.; O’Callaghan, C.A.; Schimanski, L.; Mason, D.Y.; Townsend, A.R. Kupffer cell staining by an HFE-specific monoclonal antibody: Implications for hereditary haemochromatosis. Br. J. Haematol. 1998, 103, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Montosi, G.; Paglia, P.; Garuti, C.; Guzman, C.A.; Bastin, J.M.; Colombo, M.P.; Pietrangelo, A. Wild-type HFE protein normalizes transferrin iron accumulation in macrophages from subjects with hereditary hemochromatosis. Blood 2000, 96, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Grubb, J.H.; Zhou, X.Y.; Tomatsu, S.; Fleming, R.E.; Costaldi, M.E.; Britton, R.S.; Bacon, B.R.; Sly, W.S. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc. Natl. Acad. Sci. USA 2002, 99, 3117–3122. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Sweetland, E.; Schimanski, L.; Edwards, J.; Cowley, D.; Ashraf, M.; Bastin, J.; Townsend, A.R. The hemochromatosis protein HFE inhibits iron export from macrophages. Proc. Natl. Acad. Sci. USA 2002, 99, 15602–15607. [Google Scholar] [CrossRef] [PubMed]
- Tangudu, N.K.; Yilmaz, D.; Wörle, K.; Gruber, A.; Colucci, S.; Leopold, K.; Muckenthaler, M.U.; Vujic Spasic, M. Macrophage-HFE controls iron metabolism and immune responses in aged mice. Haematologica 2021, 106, 259–263. [Google Scholar] [CrossRef]
- Wang, L.; Johnson, E.E.; Shi, H.N.; Walker, W.A.; Wessling-Resnick, M.; Cherayil, B.J. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 2008, 181, 2723–2731. [Google Scholar] [CrossRef]
- Adams, P.C.; McAlister, V.; Chakrabarti, S.; Levstik, M.; Marotta, P. Is serum hepcidin causative in hemochromatosis? Novel analysis from a liver transplant with hemochromatosis. Can. J. Gastroenterol. 2008, 22, 851–853. [Google Scholar] [CrossRef]
- Charlebois, E.; Pantopoulos, K. Iron overload inhibits BMP/SMAD and IL-6/STAT3 signaling to hepcidin in cultured hepatocytes. PLoS ONE 2021, 16, e0253475. [Google Scholar] [CrossRef]
- Mehta, K.J.; Busbridge, M.; Patel, V.B.; Farnaud, S.J. Hepcidin secretion was not directly proportional to intracellular iron-loading in recombinant-TfR1 HepG2 cells: Short communication. Mol. Cell Biochem. 2020, 468, 121–128. [Google Scholar] [CrossRef]
- Adams, P.C.; Ghent, C.N.; Grant, D.R.; Frei, J.V.; Wall, W.J. Transplantation of a donor liver with haemochromatosis: Evidence against an inherited intrahepatic defect. Gut 1991, 32, 1082–1083. [Google Scholar] [CrossRef]
- Koskinas, J.; Portmann, B.; Lombard, M.; Smith, T.; Williams, R. Persistent iron overload 4 years after inadvertent transplantation of a haemochromatotic liver in a patient with primary biliary cirrhosis. J. Hepatol. 1992, 16, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.K.; Martinez-Hernandez, A.; Schichman, S.; Chaudhry, S.; Waters, B. Transplantation of a liver with the C282Y mutation into a recipient heterozygous for H63D results in iron overload. Am. J. Med. Sci. 2009, 337, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.P.; Sarwar, S.; Egan, B.; Nolan, N.; Hegarty, J. Hepatic iron overload following liver transplantation of a C282y homozygous allograft: A case report and literature review. Liver. Int. 2011, 31, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Veitsman, E.; Pras, E.; Pappo, O.; Arish, A.; Eshkenazi, R.; Feray, C.; Calderaro, J.; Azoulay, D.; Ari, Z.B. Hepatic Iron Overload following Liver Transplantation from a C282Y/H63D Compound Heterozygous Donor. Case Rep. Hepatol. 2018, 2018, 4298649. [Google Scholar] [CrossRef]
- Crawford, D.H.; Fletcher, L.M.; Hubscher, S.G.; Stuart, K.A.; Gane, E.; Angus, P.W.; Jeffrey, G.P.; McCaughan, G.W.; Kerlin, P.; Powell, L.W.; et al. Patient and graft survival after liver transplantation for hereditary hemochromatosis: Implications for pathogenesis. Hepatology 2004, 39, 1655–1662. [Google Scholar] [CrossRef]
- Adams, P.C. Lessons from liver transplantation: Flip, flop, and why? Gut 2003, 52, 318. [Google Scholar] [CrossRef][Green Version]
- Bardou-Jacquet, E.; Philip, J.; Lorho, R.; Ropert, M.; Latournerie, M.; Houssel-Debry, P.; Guyader, D.; Loréal, O.; Boudjema, K.; Brissot, P. Liver transplantation normalizes serum hepcidin level and cures iron metabolism alterations in HFE hemochromatosis. Hepatology 2014, 59, 839–847. [Google Scholar] [CrossRef]
- Dobrindt, E.M.; Keshi, E.; Neulichedl, J.; Schöning, W.; Öllinger, R.; Pratschke, J.; Eurich, D. Long-term Outcome of Orthotopic Liver Transplantation in Patients with Hemochromatosis: A Summary of a 30-year Transplant Program. Transpl. Direct 2020, 6, e560. [Google Scholar] [CrossRef]
- Ng, I.O.; Chan, K.L.; Shek, W.H.; Lee, J.M.; Fong, D.Y.; Lo, C.M.; Fan, S.T. High frequency of chimerism in transplanted livers. Hepatology 2003, 38, 989–998. [Google Scholar] [CrossRef]
- Garuti, C.; Tian, Y.; Montosi, G.; Sabelli, M.; Corradini, E.; Graf, R.; Ventura, P.; Vegetti, A.; Clavien, P.A.; Pietrangelo, A. Hepcidin expression does not rescue the iron-poor phenotype of Kupffer cells in Hfe-null mice after liver transplantation. Gastroenterology 2010, 139, 315–322.e1. [Google Scholar] [CrossRef]
- De Domenico, I.; Kushner, J.P. Reconstitution of normal hepcidin expression in Hfe-deficient mice after liver transplantation: A new role of HFE in Kupffer cells? Gastroenterology 2010, 139, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Makui, H.; Soares, R.J.; Jiang, W.; Constante, M.; Santos, M.M. Contribution of Hfe expression in macrophages to the regulation of hepatic hepcidin levels and iron loading. Blood 2005, 106, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Theurl, M.; Theurl, I.; Hochegger, K.; Obrist, P.; Subramaniam, N.; van Rooijen, N.; Schuemann, K.; Weiss, G. Kupffer cells modulate iron homeostasis in mice via regulation of hepcidin expression. J. Mol. Med. 2008, 86, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Shimonaka, Y.; Ikuta, K.; Hosoki, T.; Sasaki, K.; Torimoto, Y.; Kanada, H.; Moriguchi, Y.; Kohgo, Y. Hepcidin production in response to iron is controlled by monocyte-derived humoral factors. Int. J. Hematol. 2014, 99, 12–20. [Google Scholar] [CrossRef]
- Colucci, S.; Müdder, K.; Muckenthaler, M.U.; Altamura, S. Hfe Is Highly Expressed in Liver Sinusoidal Endothelial Cells But Is Not Needed to Maintain Systemic Iron Homeostasis In Vivo. Hemasphere 2021, 6, e667. [Google Scholar] [CrossRef]
- Fisher, A.L.; Phillips, S.; Wang, C.Y.; Paulo, J.A.; Xiao, X.; Moschetta, G.A.; Sridhar, A.; Mancias, J.D.; Babitt, J.L. Endothelial ZIP8 plays a minor role in BMP6 regulation by iron in mice. Blood 2024, 143, 2433–2437. [Google Scholar] [CrossRef]
- Noguchi, T.; Ikeda, M.; Murakami, M.; Masuzawa, M.; Imamura, T.; Hashimoto, O.; Matsui, T.; Funaba, M. Regulatory expression of bone morphogenetic protein 6 by 2,2′-dipyridyl. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129610. [Google Scholar] [CrossRef]
- Colucci, S.; Altamura, S.; Marques, O.; Müdder, K.; Agarvas, A.R.; Hentze, M.W.; Muckenthaler, M.U. Iron-dependent BMP6 Regulation in Liver Sinusoidal Endothelial Cells Is Instructed by Hepatocyte-derived Secretory Signals. Hemasphere 2022, 6, e773. [Google Scholar] [CrossRef]
- Nixon, A.M.; Neely, E.; Simpson, I.A.; Connor, J.R. The role of HFE genotype in macrophage phenotype. J. Neuroinflamm. 2018, 15, 30. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Peccerella, T.; Mueller, S.; Rausch, V. IL-1 beta-mediated macrophage-hepatocyte crosstalk upregulates hepcidin under physiological low oxygen levels. Redox Biol. 2019, 24, 101209. [Google Scholar] [CrossRef] [PubMed]
- Bessman, N.J.; Mathieu, J.R.R.; Renassia, C.; Zhou, L.; Fung, T.C.; Fernandez, K.C.; Austin, C.; Moeller, J.B.; Zumerle, S.; Louis, S.; et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science 2020, 368, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.A.; Seu, P.; Gao, F.Q.; Wyllie, S. Ischemia-reperfusion of rat liver modulates hepcidin in vivo expression. Liver. Transpl. 2005, 11, 800–806. [Google Scholar] [CrossRef]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron Regulator Hepcidin Impairs Macrophage-Dependent Cardiac Repair After Injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Z.; Bandyopadhyay, G.; Wang, G.; Zhang, D.; Rocha, K.C.E.; Liu, X.; Zhao, H.; Kisseleva, T.; Brenner, D.A.; et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. 2022, 34, 1201–1213.e5. [Google Scholar] [CrossRef]
- Pantopoulos, K. Macrophage checkpoint for iron absorption. Blood 2023, 141, 2791–2793. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Schöller, M.; Fritsch, S.D.; Linke, M.; Horer, S.; Träger, M.; Mazić, M.; Forisch, S.; Gonzales, K.; Kahler, J.P.; et al. Duodenal macrophages control dietary iron absorption via local degradation of transferrin. Blood 2023, 141, 2878–2890. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Fan, M.; Guan, Y.; Zhang, W.; Huang, F.; Zhang, Z.; Li, X.; Yuan, B.; Liu, W.; et al. Reactive oxygen species regulation by NCF1 governs ferroptosis susceptibility of Kupffer cells to MASH. Cell Metab. 2024, 36, 1745–1763.e6. [Google Scholar] [CrossRef]

| HFE-related | p.Cys282Tyr homozygosity or compound heterozygosity of p.Cys282Tyr with other rare HFE pathogenic variants or HFE deletion. |
| Non-HFE-related | Rare pathogenic variants in non-HFE genes: -HJV-related; -HAMP-related; -TfR2-related; -SLC40A1 (very rare gain-of-function variant)-related. |
| Digenic | Double heterozygosity and/or double homozygosity/heterozygosity for variants in two different genes involved in iron metabolism (HFE and/or non-HFE). |
| Molecularly undefined | Molecular characterization still not available after sequencing of known genes (provisional diagnosis). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease. Biomedicines 2025, 13, 683. https://doi.org/10.3390/biomedicines13030683
Kouroumalis E, Tsomidis I, Voumvouraki A. HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease. Biomedicines. 2025; 13(3):683. https://doi.org/10.3390/biomedicines13030683
Chicago/Turabian StyleKouroumalis, Elias, Ioannis Tsomidis, and Argyro Voumvouraki. 2025. "HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease" Biomedicines 13, no. 3: 683. https://doi.org/10.3390/biomedicines13030683
APA StyleKouroumalis, E., Tsomidis, I., & Voumvouraki, A. (2025). HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease. Biomedicines, 13(3), 683. https://doi.org/10.3390/biomedicines13030683






