Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Plant Extracts Preparation
2.2. Silver Nanoparticle Biosynthesis
2.3. Physical–Chemical Characterization Methods of Nanoparticles
2.4. Cytotoxicity Evaluation of Cell Cultures and Treatments—In Vitro Antitumoral Tests
- MTS-Based Cytotoxicity Assay
2.5. Preparation of In-Vitro-Tested Samples
2.6. Statistical Analysis
2.7. IC 50, Selectivity Index
2.8. Chou–Talalay Method
3. Results and Discussions
3.1. Physical–Chemical Nanoparticles Characterization
- UV-VIS Spectrometry
- Dynamic Light Scattering (DLS), Zeta Potential, and Scanning Electron Microscopy (SEM)
3.2. In Vitro Antitumoral Studies
- Taraxaci extractum (TE)-based samples
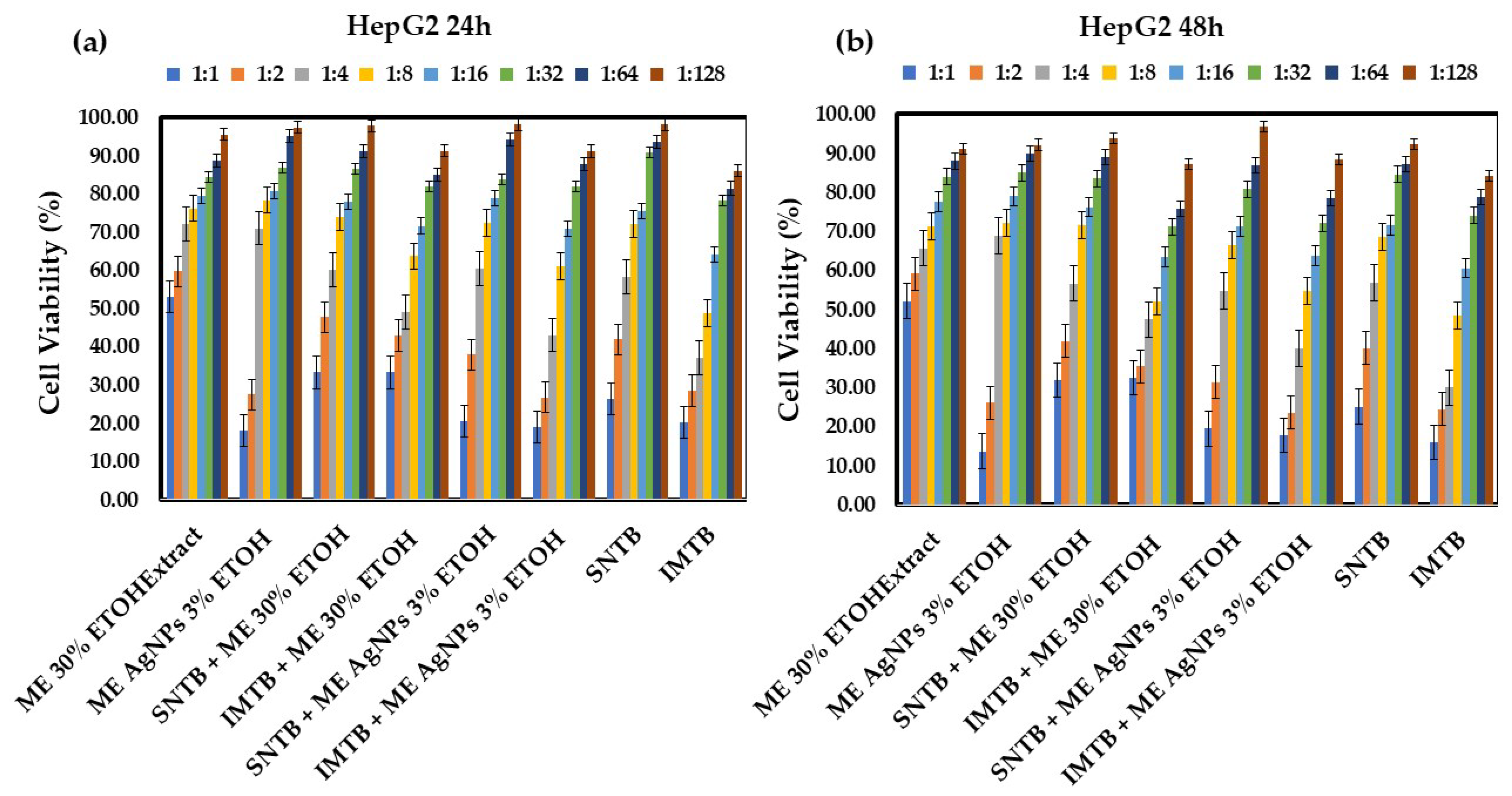
- Melissae extractum (ME)-based samples
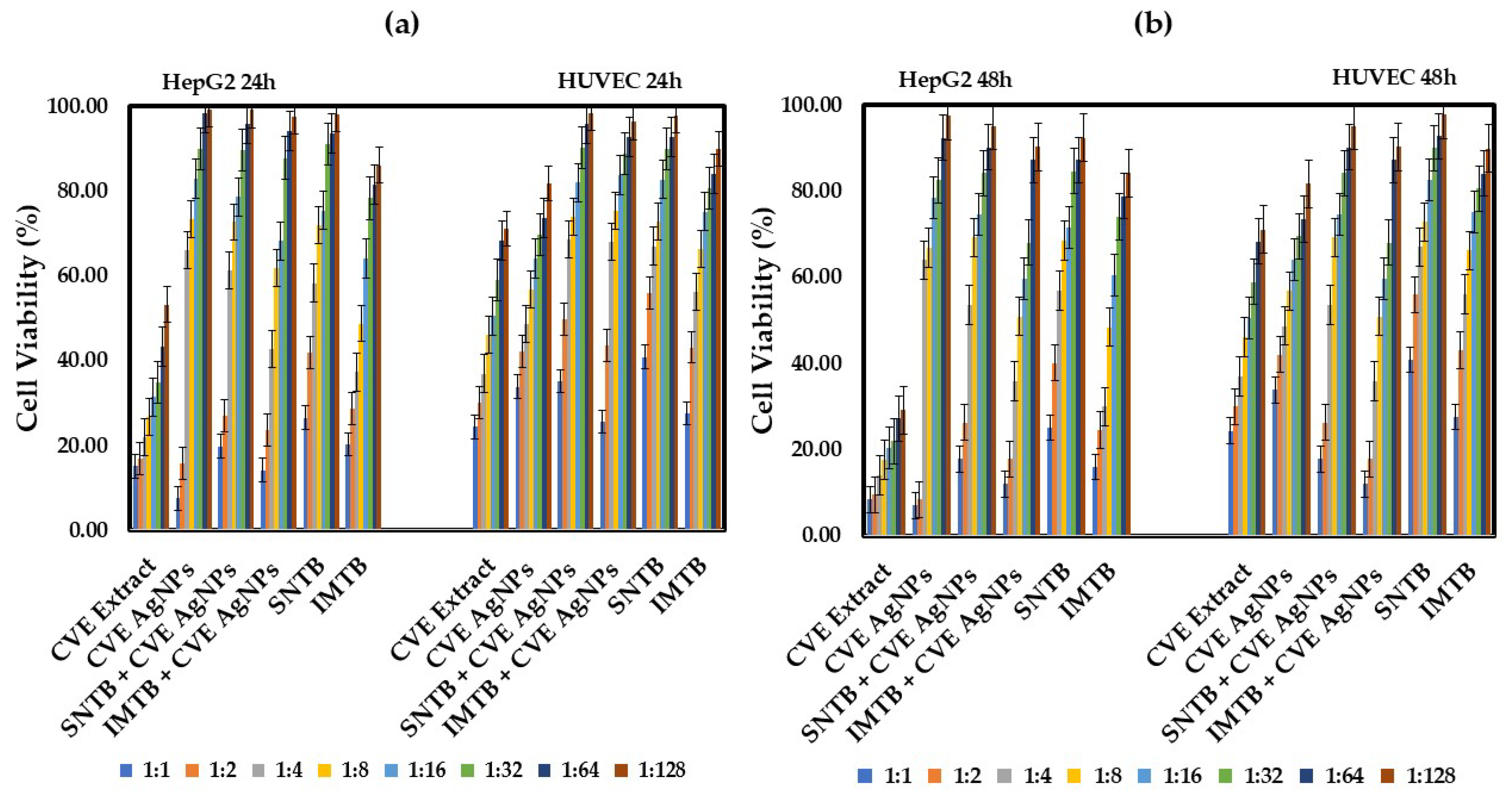
- Clematis vitalbae extractum (CVE)-based samples
3.3. IC50 Determination and SI After 24 h and 48 h
- IC50
- Selectivity Index and Therapeutic Potential
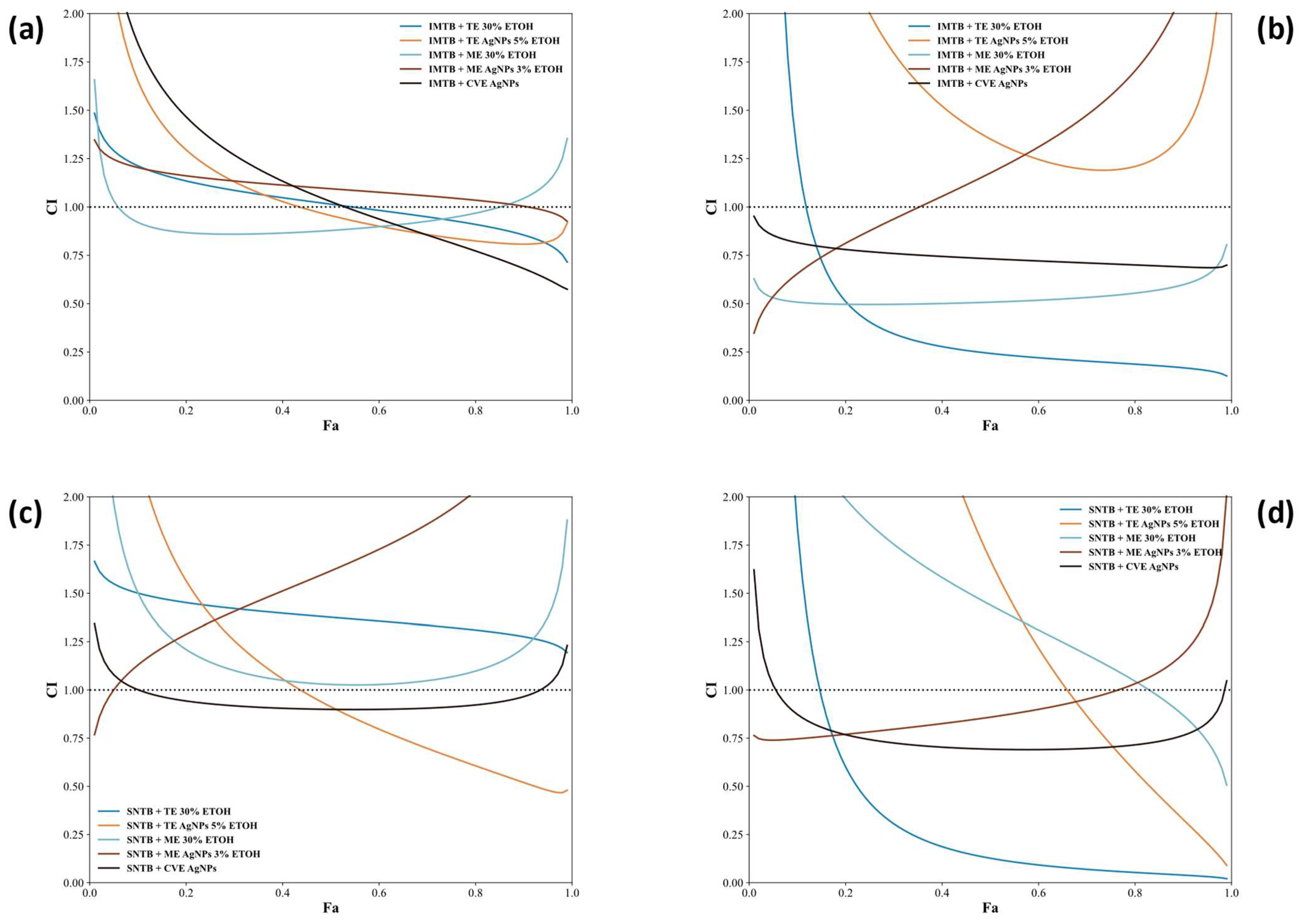
- Chou–Talalay Method
3.4. Antitumor Effects of AgNPs Combined with Sunitinib and Imatinib: Mechanisms and Recent Literature Insights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TE | Taraxaci extractum |
| ME | Melissae extractum |
| CVE | Clematis vitalbae extractum |
| SNTB | Sunitinib |
| IMTB | Imatinib |
| HepG2 | Human hepatocellular carcinoma cell line |
| HUVEC | Human umbilical vein endothelial cells |
| AgNPs | Silver nanoparticles |
| DLS | Dynamic light scattering |
| ELS | Electrophoretic light scattering |
| SEM | Scanning electron microscopy |
| FWHM | Full width at half maximum |
| ROS | Reactive oxygen species |
| DNA | Deoxyribonucleic acid |
| Bax/Bak | Bcl-2-associated X protein/Bcl-2 antagonist killer |
| PI3K/AKT | Phosphoinositide 3-kinase/Protein kinase B |
| PDGFR | Platelet-derived growth factor receptor |
| VEGFR | Vascular endothelial growth factor receptor |
| PKC | Protein Kinase C |
References
- Ritter, J.M.; Robinson, E.; Fullerton, J.; Flower, R.J.; Henderson, G.; Loke, Y.K.; MacEwan, D. Rang & Dale’s Pharmacology, 10th ed.; Elsevier eBooks: London, UK, 2024; pp. 764–782. [Google Scholar]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging cytotoxic alkaloids in the battle against cancer: Overview of molecular mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.T.; Shin, H.; Ramesh, M. Plant derived bioactive compounds, their anti-cancer effects and in silico approaches as an alternative target treatment strategy for breast cancer: An updated overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Costea, L.; Ghica, M.; Costea, T.; Gird, C.E. Spectrophotometric evaluation of flavonoids, phenolcarboxylic acids and total phenolic contents of several indigenous herbal products with potential hepatoprotective effect. Farmacia 2021, 69, 1176–1181. [Google Scholar] [CrossRef]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.-A.; et al. The polyphenolic profile and antioxidant activity of five vegetal extracts with hepatoprotective potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef]
- Ren, F.; Wu, K.; Yang, Y.; Yang, Y.; Wang, Y.; Li, J. Dandelion polysaccharide exerts anti-angiogenesis effect on hepatocellular carcinoma by regulating VEGF/HIF-1α expression. Front. Pharmacol. 2020, 11, 460. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.; Qin, Y.; Shang, J.; Wang, Y.; Wei, P.; Guo, J.; Jia, H.; Zhao, T. Taraxasterol prompted the anti-tumor effect in mice burden hepatocellular carcinoma by regulating T lymphocytes. Cell Death Discov. 2022, 8, 264. [Google Scholar] [CrossRef]
- Rehman, G.; Hamayun, M.; Iqbal, A.; Khan, S.A.; Khan, H.; Shehzad, A.; Khan, A.L.; Hussain, A.; Kim, H.-Y.; Ahmad, J.; et al. Effect of methanolic extract of dandelion roots on cancer cell lines and AMP-activated protein kinase pathway. Front. Pharmacol. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.; Wu, K.; Kang, L.; Luo, Y.; Zhang, H.; Yuan, Z. Dandelion polysaccharides exert anticancer effect on Hepatocellular carcinoma by inhibiting PI3K/AKT/mTOR pathway and enhancing immune response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- Moacă, E.A.; Farcaş, C.; Ghiţu, A.; Coricovac, D.; Popovici, R.; Cărăba-Meiţă, N.L.; Ardelean, F.; Antal, D.S.; Dehelean, C.; Avram, Ş. A comparative study of Melissa officinalis leaves and stems ethanolic extracts in terms of antioxidant, cytotoxic, and antiproliferative potential. Evid.-Based Complement. Altern. Med. 2018, 2018, 7860456. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihăiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The influence of phytosociological cultivation and fertilization on polyphenolic content of Menthae and Melissae folium and evaluation of antioxidant properties through in vitro and in silico methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Waleed, A.; Al-Khafaji, K.; Abid, M.M.; Kadhom, M.; Alhaydary, E.; Yousif, E. Comprehensive analysis of anticancer activities in Melissa officinalis extract: Gas chromatography profiling, biological assessment, network pharmacology, and molecular docking. Lett. Appl. NanoBioSci. 2025, 14, 15. [Google Scholar] [CrossRef]
- Dehghan-Nayeri, N.; Darvishi, M.; Mashati, P.; Rezapour-Kalkhoran, M.; Rezaiefard, M.; Younesian, S. Comparison of cytotoxic activity of herbal extracts on the most commonly used breast cancer cell lines (MCF7 and SKBR3): A systematic review. J. Res. Pharm. 2020, 24, 1–22. [Google Scholar] [CrossRef]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; de Ciriano, M.G.Í.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, Í.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef]
- Jun, H.J.; Roh, M.; Kim, H.W.; Houng, S.J.; Cho, B.; Yun, E.J.; Hossain, A.; Lee, H.; Kim, K.H.; Lee, S.-J. Dual inhibitions of lemon balm (Melissa officinalis) ethanolic extract on melanogenesis in B16-F1 murine melanocytes: Inhibition of tyrosinase activity and its gene expression. Food Sci. Biotechnol. 2011, 20, 1051–1059. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef]
- Shamseini, M.; Mohammadi, M.; Shirazi, F.H.; Andalib, S.; Gholami, S.; Hosseini, S.H.; Noubarani, M.; Kamalinejad, M.; Eskandari, M.R. Prevention of liver cancer by standardized extract of Melissa officinalis L. in a rat model of hepatocellular carcinoma: Its potential role as a chemopreventive agent. Int. Pharm. Acta 2019, 2, 2e8-1–2e8-7. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, M.; Randy, A.; Son, Y.J.; Hong, C.R.; Nho, C.W. Lemon balm extract and its major chemical compound, rosmarinic acid, alleviate damages of liver in an animal model of nonalcoholic steatohepatitis (NASH)(P06-093-19). Curr. Dev. Nutr. 2019, 3, nzz031-P06. [Google Scholar] [CrossRef]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef]
- Yesilada, E.; Küpeli, E. Clematis vitalba L. aerial part exhibits potent anti-inflammatory, antinociceptive and antipyretic effects. J. Ethnopharmacol. 2007, 110, 504–515. [Google Scholar] [CrossRef]
- Șuțan, N.A.; Popescu, D.I.; Drăghiceanu, O.A.; Topală, C.; Şuţan, C.; Negrea, A.D.; Vîlcoci, D.Ş.; Cîrstea, G.; Moga, S.G.; Soare, L.C. In vitro cytotoxic activity of phytosynthesized silver nanoparticles using Clematis vitalba L. (Ranunculaceae) aqueous decoction. Caryologia 2023, 76, 67–81. [Google Scholar]
- Şuțan, N.A.; Dobrescu, C.M.; Drăghiceanu, O.A.; Fierăscu, I.; Fierăscu, R.C.; Şuțan, C.; Soare, L.C. Phytotoxicity of Clematis vitalba L. (Ranunculaceae) aqueous extract and nanostructured mixture. Chem. Proc. 2022, 7, 21. [Google Scholar] [CrossRef]
- Buzzini, P.; Pieroni, A. Antimicrobial activity of extracts of Clematis vitalba towards pathogenic yeast and yeast-like microorganisms. Fitoterapia 2003, 74, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R. Phytochemicals and Pharmacological Effects of Clematis Species—A Report. J. Emerg. Technol. Innov. Res. 2023, 10, k200–k207. [Google Scholar] [CrossRef]
- Petran, M.; Dragos, D.; Gilca, M. Historical Ethnobotanical Review of Medicinal Plants Used to Treat Children’s Diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomed. 2020, 16, 1–33. [Google Scholar] [CrossRef]
- Sandulovici, R.C.; Mihăilescu, C.M.; Grigoroiu, A.; Moldovan, C.A.; Savin, M.; Ordeanu, V.; Voicu, S.N.; Cord, D.; Costache, G.M.; Galatanu, M.L.; et al. The physicochemical and antimicrobial properties of silver/gold nanoparticles obtained by “green synthesis” from willow bark and their formulations as potential innovative pharmaceutical substances. Pharmaceuticals 2022, 16, 48. [Google Scholar] [CrossRef]
- Ungureanu, A.R.; Ozon, E.A.; Musuc, A.M.; Anastasescu, M.; Atkinson, I.; Mitran, R.A.; Rusu, A.; Popescu, L.; Gîrd, C.E. Preparation and preliminary analysis of several nanoformulations based on plant extracts and biodegradable polymers as a possible application for chronic venous disease therapy. Polymers 2024, 16, 1362. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Triantafyllidi, E.; Sideris, M.; Pittaras, T.; Papalois, A.E. Herbals and plants in the treatment of pancreatic cancer: A systematic review of experimental and clinical studies. Nutrients 2022, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.S.; Rasul, K.H.; Mustafa, I.A.; Abdulqadir, S.Z. In vitro anti-cancer potentiality of quince and dandelion leaves extract against leukemia cell lines and database retrieval expression of BCR ABL and TCR genes in leukemia. Immunopathol. Persa 2025, x, e41730. [Google Scholar] [CrossRef]
- Rîmbu, M.C.; Cord, D.; Savin, M.; Grigoroiu, A.; Mihăilă, M.A.; Gălățanu, M.L.; Ordeanu, V.; Panțuroiu, M.; Țucureanu, V.; Mihalache, I.; et al. Eco-friendly dandelion and sweet wormwood-synthesized nanoparticles: Polyphenolic drug delivery systems with antitumoral and antibacterial properties and low toxicity. Manuscript no. HELIYON-D-24-63740R1. in revision.
- Faivre, S.; Raymond, E.; Boucher, E.; Douillard, J.; Lim, H.Y.; Kim, J.S.; Zappa, M.; Lanzalone, S.; Lin, X.; DePrimo, S.; et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: An open-label, multicentre, phase II study. Lancet Oncol. 2009, 10, 794–800. [Google Scholar] [CrossRef]
- Rini, B.I.; Michaelson, M.D.; Rosenberg, J.E.; Bukowski, R.M.; Sosman, J.A.; Stadler, W.M.; Hutson, T.E.; Margolin, K.; Harmon, C.S.; DePrimo, S.E.; et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Duda, D.G.; Sahani, D.V.; Jain, R.K. Development of sunitinib in hepatocellular carcinoma: Rationale, early clinical experience, and correlative studies. Cancer J. 2009, 15, 263–268. [Google Scholar] [CrossRef]
- Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Pipitone, R.M.; Dusonchet, L.; Meli, M.; Crosta, L.; Gebbia, N.; Invidiata, F.P.; Titone, L.; et al. Galangin increases the cytotoxic activity of imatinib mesylate in imatinib-sensitive and imatinib-resistant Bcr-Abl expressing leukemia cells. Cancer Lett. 2008, 265, 289–297. [Google Scholar] [CrossRef]
- Keshavarz-Pakseresht, B.; Shandiz, S.A.S.; Baghbani-Arani, F. Imatinib induces up-regulation of NM23, a metastasis suppressor gene, in human Hepatocarcinoma (HepG2) Cell Line. Gastroenterol. Hepatol. Bed Bench 2017, 10, 29–33. [Google Scholar]
- Sandulovici, R.C.; Gălăţanu, M.L.; Cima, L.M.; Panus, E.; Truţă, E.; Mihăilescu, C.M.; Sârbu, I.; Cord, D.; Rîmbu, M.C.; Anghelache, Ş.A.; et al. Phytochemical characterization, antioxidant, and antimicrobial activity of the vegetative buds from Romanian Spruce, Picea abies (L.) H. Karst. Molecules 2024, 29, 2128. [Google Scholar] [CrossRef]
- Ghica, A.; Tănase, M.L.; Niculițe, C.M.; Tocilă, A.; Popescu, L.; Luță, E.A.; Olaru, O.T.; Popovici, V.; Balaci, T.D.; Duțu, L.E.; et al. In vitro toxicity evaluation of some plant extracts and their potential application in Xerosis cutis. Cosmetics 2024, 11, 124. [Google Scholar] [CrossRef]
- Manolache, M.; Cadar, E.; Antonescu, D.; Mircioiu, C.; Prasacu, I.; Sandulovici, R. Bioethics approach of biostatistics in clinical trials: Avoiding the use of excessive or inadequate numbers of research subjects. J. Sci. Arts 2018, 18, 239–246. [Google Scholar]
- Gherghiceanu, F.; Sandulovici, R.; Prasacu, I.; Anuta, V.; Mircioiu, C. Bioequivalence implies therapeutic equivalence. I. Biostatistical approach. Farmacia 2016, 64, 823–827. [Google Scholar]
- Truţă, E.; Vartic, M.; Cristea, A.N. Clinical study regarding preemptive analgesic effect of ketamine and remifentanil in laparoscopic cholecystectomy. Farmacia 2011, 59, 200–205. [Google Scholar]
- Truță, E.; Davitoiu, A.M.; Jinescu, G.; Mitu, A.M.; Caragea, G.; Ionica, M.; Stanciulescu, E.L. Study on the correlation of urinary leaden levels and attention deficit hyperactivity disorder. Rev. Chim. Buchar. 2016, 67, 609–612. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Guerrini, R.; Ingallina, C.; Spano, M.; Mannina, L. NMR Metabolomics of Arctium lappa L.; Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes. Foods 2024, 13, 1642. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ahmeda, A.; Zangeneh, A.; Zangeneh, M.M. Preparation, formulation, and chemical characterization of silver nanoparticles using Melissa officinalis leaf aqueous extract for the treatment of acute myeloid leukemia in vitro and in vivo conditions. Appl. Organomet. Chem. 2020, 34, e5378. [Google Scholar] [CrossRef]
- Da-Cheng, H.A.O.; Pei-Gen, X.I.A.O.; Hong-Ying, M.A.; Yong, P.E.N.G.; Chun-Nian, H.E. Mining chemodiversity from biodiversity: Pharmacophylogeny of medicinal plants of Ranunculaceae. Chin. J. Nat. Med. 2015, 13, 507–520. [Google Scholar] [CrossRef]
- Wen-Tsai, W. A revision of Clematis sect. Clematis (Ranunculaceae). J. Syst. Evol. 2003, 41, 1–62. [Google Scholar]
- Hao, D.C.; He, C.N.; Shen, J.; Xiao, P.G. Anticancer chemodiversity of Ranunculaceae medicinal plants: Molecular mechanisms and functions. Curr. Genom. 2017, 18, 39–59. [Google Scholar] [CrossRef]
- Bianco, S.; Gasparini, F.; Schettini, R. Color coding for data visualization. In Encyclopedia of Information Science and Technology, 3rd ed.; IGI Global: New York, NY, USA, 2015; pp. 1682–1691. [Google Scholar] [CrossRef]
- Bosdriesz, E.; Neto, J.M.F.; Sieber, A.; Bernards, R.; Blüthgen, N.; Wessels, L.F.A. Identifying mutant-specific multi-drug combinations using comparative network reconstruction. iScience 2022, 25, 104760. [Google Scholar] [CrossRef]
- Patel, T.A.; Kevadiya, B.D.; Bajwa, N.; Singh, P.A.; Zheng, H.; Kirabo, A.; Patel, K.P. Role of nanoparticle-conjugates and nanotheranostics in abrogating oxidative stress and ameliorating neuroinflammation. Antioxidants 2023, 12, 1877. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, H.; Solanki, R.; Rawat, L.; Tabasum, S.; Tanwar, P.; Sabarwal, A. Recent developments in receptor tyrosine kinase inhibitors: A promising mainstay in targeted cancer therapy. Med. Drug Discov. 2024, 2024, 100195. [Google Scholar] [CrossRef] [PubMed]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The role of silver nanoparticles in the diagnosis and treatment of cancer: Are there any perspectives for the future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Bonavida, B.; Chen, Z.S.J.; Yang, D.H. Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Chen, K.; Li, Y.; Zhang, X.; Ullah, R.; Tong, J.; Shen, Y. The role of the PI3K/AKT signalling pathway in the corneal epithelium: Recent updates. Cell Death Dis. 2022, 13, 513. [Google Scholar] [CrossRef]
- Yang, S.; Fei, W.; Zhao, Y.; Wang, F.; Ye, Y.; Wang, F. Combat against gynecological cancers with blood vessels as entry point: Anti-angiogenic drugs, clinical trials and pre-clinical nano-delivery platforms. Int. J. Nanomed. 2023, 18, 3035–3046. [Google Scholar] [CrossRef]
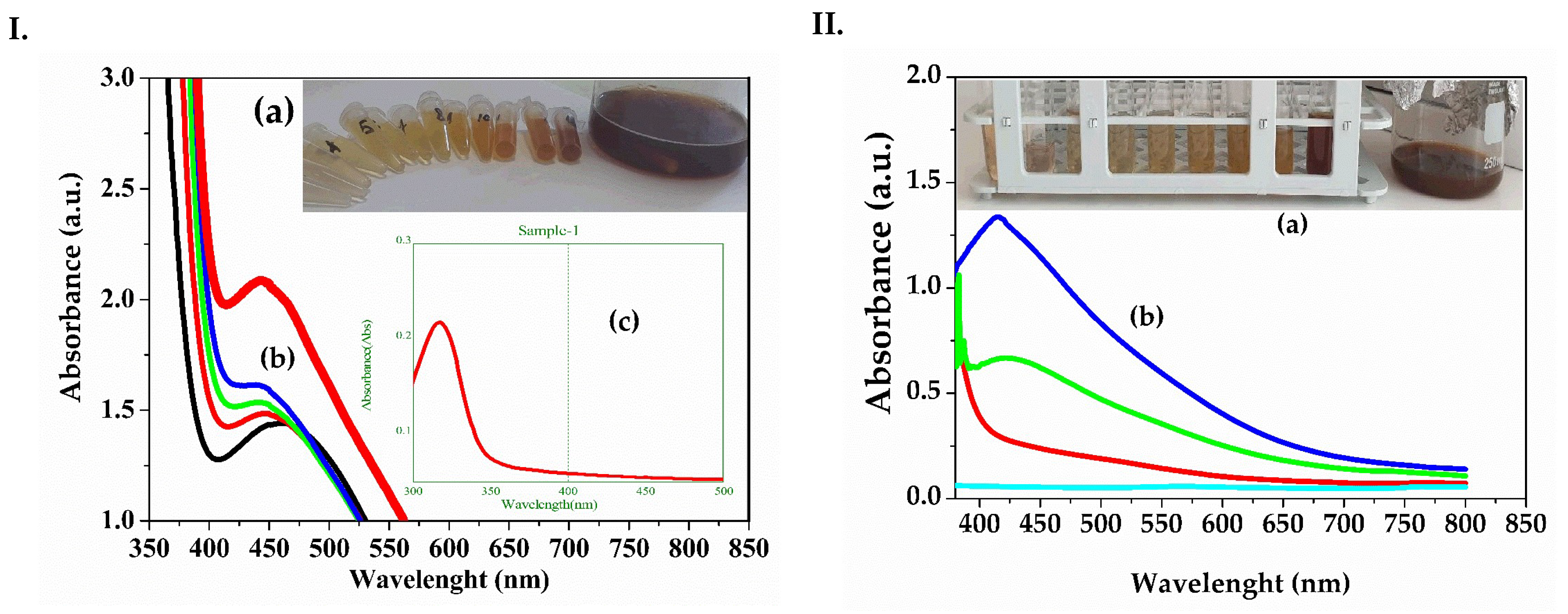
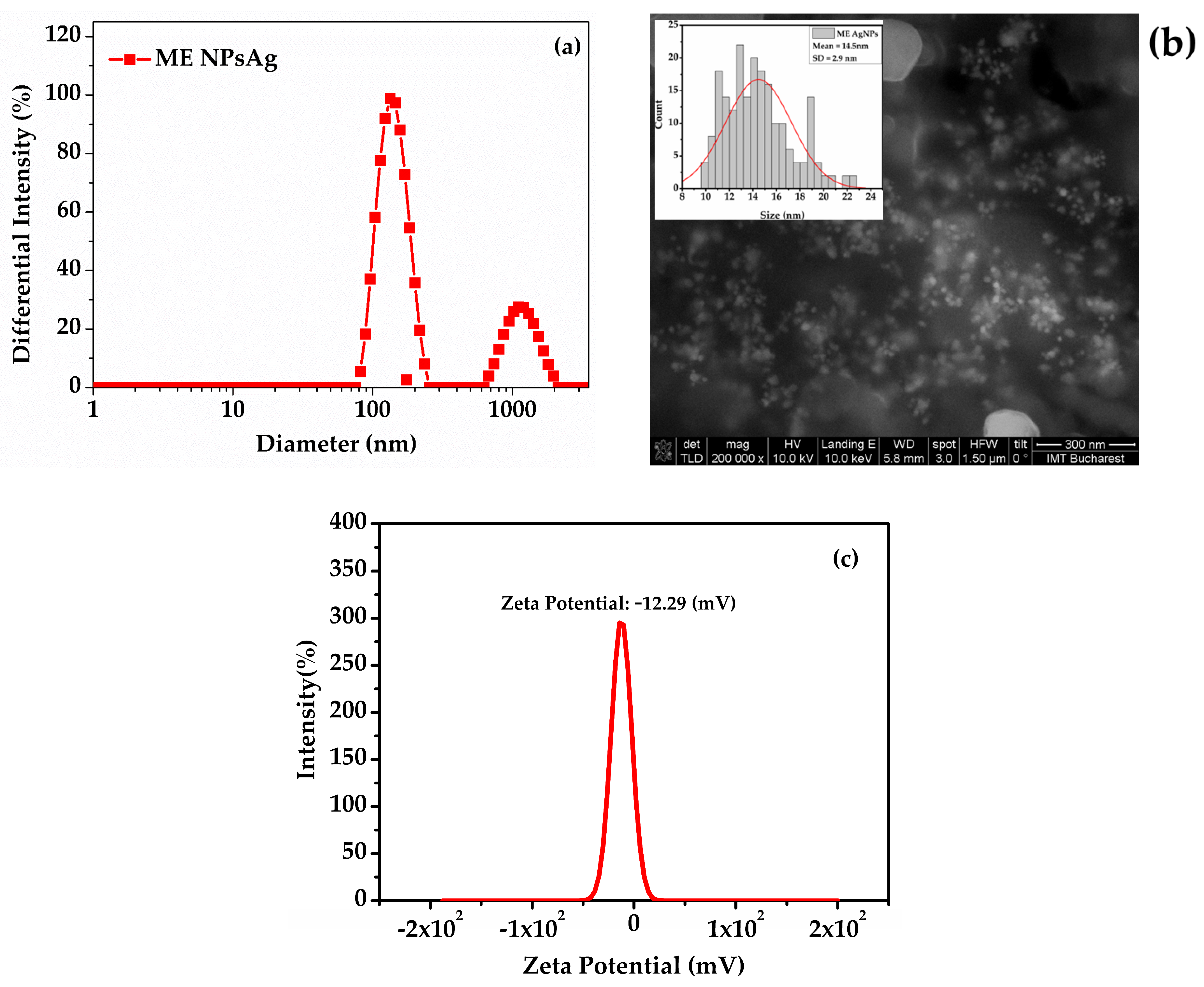



| TE 30% ETOHExtract—dandelion 30% ehtanolic extract (Taraxaci extractum); |
| TE AgNPs 5% ETOH—dandelion silver nanoparticles 5% ethanolic extract; |
| SNTB + TE 30% ETOH—sunitinib + dandelion 30% ehtanolic extract; |
| IMTB + TE 30% ETOH—imatinib + dandelion 30% ehtanolic extract; |
| SNTB + TE AgNPs 5% ETOH—sunitinib + dandelion silver nanoparticles 5% ehtanolic extract; |
| IMTB + TE AgNPs 5% ETOH—imatinib + dandelion silver nanoparticles 5% ehtanolic extract. |
| ME 30% ETOHExtract—lemon balm 30% ehtanolic extract (Melissae extractum); |
| ME AgNPs 3% ETOH—lemon balm silver nanoparticles 3% ethanolic extract; |
| SNTB + ME 30% ETOH—sunitinib + lemon balm 30% ehtanolic extract; |
| IMTB + ME 30% ETOH—imatinib + lemon balm 30% ehtanolic extract; |
| SNTB + ME AgNPs 3% ETOH—sunitinib + lemon balm silver nanoparticles 3% ehtanolic extract; |
| IMTB + ME AgNPs 3% ETOH—imatinib + lemon balm silver nanoparticles 3% ehtanolic extract. |
| CVE Extract—traveller’s joy aqueous extract (Clematis vitalbae extractum); |
| CVE AgNPs—Clematis vitalbae extractum silver nanoparticles; |
| SNTB + CVE AgNPs—sunitinib + Clematis vitalbae extractum silver nanoparticles; |
| IMTB + CVE AgNPs—imatinib + Clematis vitalbae extractum silver nanoparticles. |
| SNTB—sunitinib (synthetic anticancer drug); |
| IMTB—imatinib (synthetic anticancer drug). |
| Sample | Nanoparticle Diameter (nm) | ||
|---|---|---|---|
| Min–Max | FWHM | Mean ± SD | |
| CVE AgNPs | 6–18 | 9–13 | 11.1 ± 1.9 |
| ME AgNPs | 9–22 | 11–17 | 14.5 ± 2.9 |
| Sample | PDI * | NPs Hydrodynamic Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 24 h | |||
| ME AgNPs 3% ETOH | 0.200 | 940 | −31.04 |
| TE AgNPs 5% ETOH | 0.248 | 218.7 | −39.54 |
| CVE AgNPs aqueous | 1.588 | 5000 | −34.05 |
| 48 h | |||
| ME AgNPs 3% ETOH | 0.171 | 362.2 | −35.61 |
| TE AgNPs 5% ETOH | 0.249 | 209.3 | −38.15 |
| CVE AgNPs aqueous | 0.814 | 2000 | −34.60 |
| 72 h | |||
| ME AgNPs 3% ETOH | 0.247 | 581.4 | −29.76 |
| TE AgNPs 5% ETOH | 0.291 | 243,9 | −31.25 |
| CVE AgNPs aqueous | 0.564 | 4000 | −27.54 |
| One month | |||
| ME AgNPs 3% ETOH | 0.240 | 143 | −12.29 |
| TE AgNPs 5% ETOH [32] | 0.179 | 200 | −35.20 |
| CVE AgNPs aqueous | 0.179 | 507 | −0.60 |
| (I) HepG2_Treatment_ME_vs_SNTB | (J) Control_Group | Mean Difference (J–I) | Std. Error | Sig. (p Value) |
|---|---|---|---|---|
| ME AgNPs 1:1 | Control_SNTB | 0.61259 * | 0.08632 | 0.003 |
| ME AgNPs 1:2 | Control_SNTB | 0.39760 * | 0.07941 | 0.022 |
| SNTB + ME AgNPs 1:1 | Control_SNTB | 0.49988 * | 0.07853 | 0.002 |
| (I) HepG2_Treatment_ME_vs_IMTB | (J) Control_Group | Mean Difference (J–I) | Std. Error | Sig. (p Value) |
|---|---|---|---|---|
| ME AgNPs 1:1 | Control_IMTB | 0.45994 * | 0.10684 | 0.028 |
| IMTB + ME AgNPs 1:1 | Control_IMTB | 0.37692 * | 0.07202 | 0.036 |
| (I) HepG2_Treatment_CVE_vs_SNTB | (J) Control_Group | Mean Difference (J–I) | Std. Error | Sig. (p Value) |
|---|---|---|---|---|
| CVE 1:1 | Control_SNTB | 0.51588 * | 0.07305 | 0.004 |
| CVE 1:2 | Control_SNTB | 0.46707 * | 0.06980 | 0.010 |
| CVE 1:4 | Control_SNTB | 0.40876 * | 0.09289 | 0.035 |
| CVE AgNPs 1:1 | Control_SNTB | 0.68835 * | 0.06493 | 0.005 |
| CVE AgNPs 1:2 | Control_SNTB | 0.50431 * | 0.08197 | 0.005 |
| SNTB + CVE AgNPs 1:1 | Control_SNTB | 0.37086 * | 0.04998 | 0.000 |
| SNTB + CVE AgNPs 1:2 | Control_SNTB | 0.26627 * | 0.05312 | 0.018 |
| (I) HepG2_Treatment_CVE_vs_IMTB | (J) Control_Group | Mean Difference (J–I) | Std. Error | Sig. (p Value) |
|---|---|---|---|---|
| CVE 1:1 | Control_IMTB | 0.43148 * | 0.08789 | 0.023 |
| CVE 1:2 | Control_IMTB | 0.37713 † | 0.08879 | 0.066 |
| CVE AgNPs 1:1 | Control_IMTB | 0.62611 * | 0.06099 | 0.011 |
| CVE AgNPs 1:2 | Control_IMTB | 0.42165 * | 0.09653 | 0.028 |
| IMTB + CVE AgNPs 1:1 | Control_IMTB | 0.40090 * | 0.05021 | 0.001 |
| COMPOUND | IC50 (µg/mL) | |||
|---|---|---|---|---|
| HUVEC | HepG2 | |||
| 24 h | 48 h | 24 h | 48 h | |
| TE 30% ETOHExtract | 12,757.22 ± 11,832.83 | 16,454.15 ± 9213.11 | 5874.16 ± 593.80 | 30,530.13 ± 36,511.17 |
| TE AgNPs 5% ETOH | 4.87 ± 1.77 | 5.91 ± 4.20 | 1.91 ± 0.30 | 1.11 ± 2.42 |
| ME 30% ETOHExtract | 20,693.57 ± 10,867.49 | 23,879.18 ± 11,180.72 | 29,077.68 ± 36,206.09 | 25,208.79 ± 11,539.90 |
| ME AgNPs 3% ETOH | 0.76 ± 0.72 | 1.04 ± 0.30 | 7.44 ± 4.70 | 7.21 ± 4.32 |
| CVE Extract | 9345.40 ± 4155.15 | 9345.41 ± 4155.15 | 2089.83 ± 2287.86 | 7932.39 ± 5859.23 |
| CVE AgNPs | 5.58 ± 6.97 | 5.58 ± 6.97 | 5.96 ± 3.11 | 5.54 ± 3.54 |
| SNTB | 252.48 ± 357.14 | 247.09 ± 339.23 | 134.48 ± 97.48 | 142.50 ± 112.80 |
| IMTB | 165.37 ± 90.76 | 171.07 ± 108.15 | 44.20 ± 5.00 | 44.99 ± 5.49 |
| SNTB + TE 30% ETOH | 11,290.90 ± 9226.68 | 12,440.12 ± 11,447.12 | 6586.45 ± 3154.23 | 6373.19 ± 2859.54 |
| SNTB + TE AgNPs 5% ETOH | 32.26 ± 10.23 | 31.11 ± 9.27 | 34.34 ± 18.95 | 40.58 ± 20.52 |
| IMTB + TE 30% ETOH | 2847.42 ± 1264.79 | 3754.29 ± 993.94 | 4201.78 ± 242.58 | 3101.41 ± 646.43 |
| IMTB + TE AgNPs 5% ETOH | 20.92 ± 0.66 | 19.61 ± 1.67 | 22.46 ± 1.73 | 22.68 ± 4.13 |
| SNTB + ME 30% ETOH | 38,239.95 ± 68,477.77 | 6091.39 ± 1211.48 | 21,323.24 ± 19,207.04 | 19,279.42 ± 13,526.20 |
| SNTB + ME AgNPs 3% ETOH | 108.86 ± 61.74 | 114.07 ± 58.69 | 71.61 ± 46.60 | 55.92 ± 42.69 |
| IMTB + ME 30% ETOH | 8830.77 ± 1193.40 | 9625.93 ± 1299.08 | 8179.60 ± 2347.55 | 4030.21 ± 1925.17 |
| IMTB + ME AgNPs 3% ETOH | 67.71 ± 26.90 | 60.88 ± 20.97 | 39.72 ± 5.47 | 35.47 ± 13.86 |
| SNTB + CVE AgNPs | 106.09 ± 114.21 | 57.60 ± 24.67 | 62.22 ± 34.39 | 57.60 ± 24.67 |
| IMTB + CVE AgNPs | 96.68 ± 51.42 | 22.58 ± 8.71 | 37.19 ± 10.84 | 22.58 ± 8.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rîmbu, M.C.; Popescu, L.; Mihăilă, M.; Sandulovici, R.C.; Cord, D.; Mihăilescu, C.-M.; Gălățanu, M.L.; Panțuroiu, M.; Manea, C.-E.; Boldeiu, A.; et al. Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer. Biomedicines 2025, 13, 641. https://doi.org/10.3390/biomedicines13030641
Rîmbu MC, Popescu L, Mihăilă M, Sandulovici RC, Cord D, Mihăilescu C-M, Gălățanu ML, Panțuroiu M, Manea C-E, Boldeiu A, et al. Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer. Biomedicines. 2025; 13(3):641. https://doi.org/10.3390/biomedicines13030641
Chicago/Turabian StyleRîmbu, Mirela Claudia, Liliana Popescu, Mirela Mihăilă, Roxana Colette Sandulovici, Daniel Cord, Carmen-Marinela Mihăilescu, Mona Luciana Gălățanu, Mariana Panțuroiu, Carmen-Elisabeta Manea, Adina Boldeiu, and et al. 2025. "Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer" Biomedicines 13, no. 3: 641. https://doi.org/10.3390/biomedicines13030641
APA StyleRîmbu, M. C., Popescu, L., Mihăilă, M., Sandulovici, R. C., Cord, D., Mihăilescu, C.-M., Gălățanu, M. L., Panțuroiu, M., Manea, C.-E., Boldeiu, A., Brîncoveanu, O., Savin, M., Grigoroiu, A., Ungureanu, F. D., Amzoiu, E., Popescu, M., & Truță, E. (2025). Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer. Biomedicines, 13(3), 641. https://doi.org/10.3390/biomedicines13030641






