Advancing Bilateral Limbal Deficiency Surgery: A Comprehensive Review of Innovations with Mucosal Cells
Abstract
:1. Introduction
2. Cultivated Oral Mucosal Epithelial Sheets
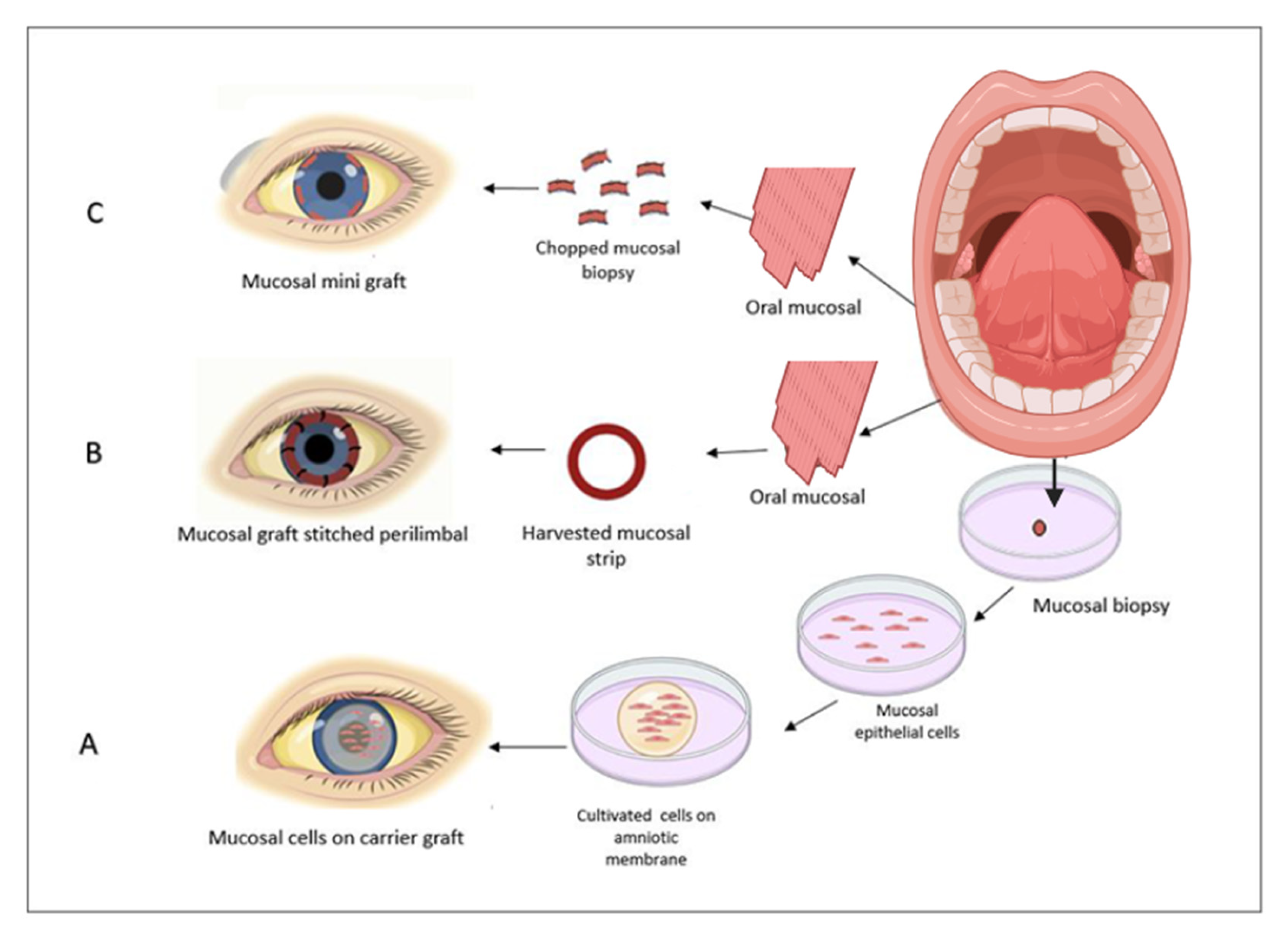
3. A Direct Autologous Oral Mucosal Epithelium Graft Transplantation
4. Simple Limbal Epithelial Cells Transplantation (SLET) and Simple Oral Mucosal Epithelial Transplantation (SOMET)
5. Environmental and Acellularized Scaffold
6. Conclusions
Funding
Conflicts of Interest
References
- Soleimani, M.; Cheraqpour, K.; Koganti, R.; Baharnoori, S.M.; Djalilian, A.R. Concise Review: Bioengineering of Limbal Stem Cell Niche. Bioengineering 2023, 10, 111. [Google Scholar] [CrossRef]
- Moshirfar, M.; Masud, M.; Harvey, D.H.; Payne, C.; Bruce, E.; Ronquillo, Y.C.; Hoopes, P.C. The Multifold Etiologies of Limbal Stem Cell Deficiency: A Comprehensive Review on the Etiologies and Additional Treatment Options for Limbal Stem Cell Deficiency. J. Clin. Med. 2023, 12, 4418. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.; Mehta, J.S.; Walter, P.; Fuest, M. Simple limbal epithelial transplantation (SLET): A simple technique for the treatment of unilateral complete limbal stem cell deficiency. Video article. Ophthalmologe 2021, 118, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Chanda, S.; Donthineni, P.R.; Basu, S. Surgical Management of Unilateral Partial Limbal Stem Cell Deficiency: Conjunctival Autografts versus Simple Limbal Epithelial Transplantation. Clin. Ophthalmol. 2021, 15, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Sugita, S.; Yokokura, S.; Nakazawa, T.; Tomida, D.; Satake, Y.; Shimazaki, J.; Hara, Y.; Shiraishi, A.; Quantock, A.J.; et al. Clinical Trial of Autologous Cultivated Limbal Epithelial Cell Sheet Transplantation for Patients with Limbal Stem Cell Deficiency. Ophthalmology 2023, 130, 608–614. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Holland, E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017, 28, 377–381. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, X.Y.; Huang, D.T.; Yang, X.Y. The capacity of goat epidermal adult stem cells to reconstruct the damaged ocular surface of total LSCD and activate corneal genetic programs. J. Mol. Histol. 2020, 51, 277–286. [Google Scholar] [CrossRef]
- Call, M.; Meyer, E.A.; Kao, W.W.; Kruse, F.E.; Schlötzer-Schrehardt, U. Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier. Bio Protoc. 2018, 8, e2849. [Google Scholar] [CrossRef]
- Booranapong, W.; Kosrirukvongs, P.; Duangsa-Ard, S.; Kasetsinsombat, K.; Sa-Ngiamsuntorn, K.; Wongkajornsilp, A. Transplantation of autologous cultivated oral mucosal epithelial sheets for limbal stem cell deficiency at Siriraj Hospital: A case series. J. Med. Case Rep. 2022, 16, 298. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takeda, K.; Inatomi, T.; Sotozono, C.; Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J. Ophthalmol. 2011, 95, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Dogan, L. Simple Oral Mucosal Epithelial Transplantation in a Patient With Bilateral Limbal Stem Cell Deficiency. Eye Contact Lens 2021, 47, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheha, H.; Fu, Y.; Giegengack, M.; Tseng, S.C. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am. J. Ophthalmol. 2011, 152, 739–747.e731. [Google Scholar] [CrossRef]
- Choe, H.R.; Yoon, C.H.; Kim, M.K.; Kang, H.G.; Choi, E.Y.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Kim, M. Ocular surface reconstruction using circumferentially-trephined autologous oral mucosal graft transplantation in limbal stem cell deficiency. Korean J. Ophthalmol. 2019, 33, 16–25. [Google Scholar] [CrossRef]
- Malyugin, B.E.; Gerasimov, M.Y.; Borzenok, S.A. Glueless Simple Limbal Epithelial Transplantation: The Report of the First 2 Cases. Cornea 2020, 39, 1588–1591. [Google Scholar] [CrossRef]
- Doctor, M.B.; Rajagopal, R.N.; Basu, S. Simple oral mucosal epithelial transplantation (SOMET) for ocular surface reconstruction in Stevens-Johnson Syndrome: A case report. Int. J. Surg. Case Rep. 2023, 110, 108643. [Google Scholar] [CrossRef]
- Inamochi, A.; Tomioka, A.; Kitamoto, K.; Miyai, T.; Usui, T.; Aihara, M.; Yamagami, S. Simple oral mucosal epithelial transplantation in a rabbit model. Sci. Rep. 2019, 9, 18088. [Google Scholar] [CrossRef]
- Duan, C.Y.; Xie, H.T.; Zhao, X.Y.; Xu, W.H.; Zhang, M.C. Limbal niche cells can reduce the angiogenic potential of cultivated oral mucosal epithelial cells. Cell Mol. Biol. Lett. 2019, 24, 3. [Google Scholar] [CrossRef]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, K.; Witt, J.; Mertsch, S.; Borrelli, M.; Geerling, G.; Schrader, S. Generation and characterisation of decellularised human corneal limbus. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Iyer, G.K.; Krishnakumar, S. Culture & characterisation of limbal epithelial cells & oral mucosal cells. Indian. J. Med. Res. 2010, 131, 422–428. [Google Scholar]
- Wang, J.; Qi, X.; Dong, Y.; Cheng, J.; Zhai, H.; Zhou, Q.; Xie, L. Comparison of the efficacy of different cell sources for transplantation in total limbal stem cell deficiency. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1253–1263. [Google Scholar] [CrossRef]
- Shimazaki, J.; Higa, K.; Kato, N.; Satake, Y. Barrier function of cultivated limbal and oral mucosal epithelial cell sheets. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5672–5680. [Google Scholar] [CrossRef]
- Han, E.S.; Wee, W.R.; Lee, J.H.; Kim, M.K. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1697–1704. [Google Scholar] [CrossRef]
- Soma, T.; Hayashi, R.; Sugiyama, H.; Tsujikawa, M.; Kanayama, S.; Oie, Y.; Nishida, K. Maintenance and distribution of epithelial stem/progenitor cells after corneal reconstruction using oral mucosal epithelial cell sheets. PLoS ONE 2014, 9, e110987. [Google Scholar] [CrossRef]
- Dobrowolski, D.; Orzechowska-Wylegala, B.; Wowra, B.; Wroblewska-Czajka, E.; Grolik, M.; Szczubialka, K.; Nowakowska, M.; Puzzolo, D.; Wylegala, E.A.; Micali, A.; et al. Cultivated oral mucosa epithelium in ocular surface reconstruction in aniridia patients. BioMed Res. Int. 2015, 2015, 281870. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Oliva, J.; Wood, A.; Hoft, R.; Pan, D.; Thropay, J.; Makalinao, A.; French, S.W.; Niihara, Y. Carrier-free Cultured Autologous Oral Mucosa Epithelial Cell Sheet (CAOMECS) for Corneal Epithelium Reconstruction: A Histological Study. Ocul. Surf. 2015, 13, 150–163. [Google Scholar] [CrossRef]
- Sugiyama, H.; Yamato, M.; Nishida, K.; Okano, T. Evidence of the survival of ectopically transplanted oral mucosal epithelial stem cells after repeated wounding of cornea. Mol. Ther. 2014, 22, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Ekpo, P.; Uiprasertkul, M.; Chotikavanich, S.; Tesavibul, N.; Pornpanich, K.; Luemsamran, P. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016, 17, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Satake, Y.; Higa, K.; Tsubota, K.; Shimazaki, J. Long-term Outcome of Cultivated Oral Mucosal Epithelial Sheet Transplantation in Treatment of Total Limbal Stem Cell Deficiency. Ophthalmology 2011, 118, 1524–1530. [Google Scholar] [CrossRef]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Vitrification and storage of oral mucosa epithelial cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, D.H.; Shin, E.J.; Lee, H.J.; Wee, W.R.; Jeon, S.; Kim, M.K. Comparative Analysis of Substrate-Free Cultured Oral Mucosal Epithelial Cell Sheets from Cells of Subjects with and without Stevens—Johnson Syndrome for Use in Ocular Surface Reconstruction. PLoS ONE 2016, 11, e0147548. [Google Scholar] [CrossRef]
- Barbaro, V.; Nasti, A.A.; Raffa, P.; Migliorati, A.; Nespeca, P.; Ferrari, S.; Palumbo, E.; Bertolin, M.; Breda, C.; Miceli, F.; et al. Personalized Stem Cell Therapy to Correct Corneal Defects Due to a Unique Homozygous-Heterozygous Mosaicism of Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome. Stem Cells Transl. Med. 2016, 5, 1098–1105. [Google Scholar] [CrossRef]
- Nakamura, T.; Kinoshita, S. Ocular Surface Reconstruction Using Cultivated Mucosal Epithelial Stem Cells. Cornea 2003, 22, S75–S80. [Google Scholar] [CrossRef]
- Hayashida, Y.; Nishida, K.; Yamato, M.; Watanabe, K.; Maeda, N.; Watanabe, H.; Kikuchi, A.; Okano, T.; Tano, Y. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1632–1639. [Google Scholar] [CrossRef]
- Rovere, M.-R.; Rousselle, P.; Haftek, M.; Charleux, B.; Kocaba, V.; Auxenfans, C.; Nataf, S.; Damour, O. Preserving basement membranes during detachment of cultivated oral mucosal epithelial cell sheets for the treatment of total bilateral limbal stem cell deficiency. Cell Transplant. 2018, 27, 264–274. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, S.; Gupta, A.; Gupta, N.; Singh, H.; Roychoudhury, A.; Mohanty, S.; Sen, S.; Nag, T.C.; Tandon, R. Molecular characterization of explant cultured human oral mucosal epithelial cells. Investig. Opthalmology Vis. Sci. 2011, 52, 9548–9554. [Google Scholar] [CrossRef]
- Zsebik, B.; Ujlaky-Nagy, L.; Losonczy, G.; Vereb, G.; Takács, L. Cultivation of human oral mucosal explants on contact lenses. Curr. Eye Res. 2017, 42, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.; Neale, M.H.; Shortt, A.J.; Massie, I.; Vernon, A.J.; Daniels, J.T. Culture and characterization of oral mucosal epithelial cells on a fibrin gel for ocular surface reconstruction. Curr. Eye Res. 2015, 40, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.; Ahmad, S.; Mudhar, H.S.; Meeny, A.; Lako, M.; Figueiredo, F.C. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 2014, 32, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.R.; Dziasko, M.A.; Sheth-Shah, R.; Lewis, M.P.; Daniels, J.T. Oral Mucosa Tissue Equivalents for the Treatment of Limbal Stem Cell Deficiency. Adv. Biosyst. 2020, 4, e1900265. [Google Scholar] [CrossRef]
- Sharma, S.M.; Fuchsluger, T.; Ahmad, S.; Katikireddy, K.R.; Armant, M.; Dana, R.; Jurkunas, U.V. Comparative analysis of human-derived feeder layers with 3T3 fibroblasts for the ex vivo expansion of human limbal and oral epithelium. Stem Cell Rev. Rep. 2012, 8, 696–705. [Google Scholar] [CrossRef]
- Islam, R.; Eidet, J.R.; Badian, R.A.; Lippestad, M.; Messelt, E.; Griffith, M.; Dartt, D.A.; Utheim, T.P. Tissue harvesting site and culture medium affect attachment, growth, and phenotype of ex vivo expanded oral mucosal epithelial cells. Sci. Rep. 2017, 7, 674. [Google Scholar] [CrossRef]
- Priya, C.; Arpitha, P.; Vaishali, S.; Prajna, N.; Usha, K.; Sheetal, K.; Muthukkaruppan, V. Adult human buccal epithelial stem cells: Identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye 2011, 25, 1641–1649. [Google Scholar] [CrossRef]
- Zhurenkov, K.E.; Alexander-Sinkler, E.I.; Gavrilyik, I.O.; Yartseva, N.M.; Aleksandrova, S.A.; Mashel, T.V.; Khorolskaya, J.I.; Blinova, M.I.; Kulikov, A.N.; Churashov, S.V.; et al. Labial Mucosa Stem Cells: Isolation, Characterization, and Their Potential for Corneal Epithelial Reconstruction. Investig. Opthalmology Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Miyakoda, K.; Kaneda, H.; Fukushima, M.; et al. Visual Improvement after Cultivated Oral Mucosal Epithelial Transplantation. Ophthalmology 2013, 120, 193–200. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Asl, N.S.; Ebrahimi, M.; Jabbehdari, S.; Bamdad, S.; Roshandel, D.; Eslani, M.; Momeni, M. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul. Surf. 2018, 16, 146–153. [Google Scholar] [CrossRef]
- Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I.; Vemuganti, G.K.; Balasubramanian, D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Chirapapaisan, C.; Jiravarnsirikul, A.; Ekpo, P.; Uiprasertkul, M.; Thamphithak, R.; Matamnan, S.; Boonwong, C. Phenotypic characterization of corneal epithelium in long-term follow-up of patients post-autologous cultivated oral mucosal epithelial transplantation. Cornea 2021, 40, 842–850. [Google Scholar] [CrossRef]
- Attico, E.; Galaverni, G.; Bianchi, E.; Losi, L.; Manfredini, R.; Lambiase, A.; Rama, P.; Pellegrini, G. SOX2 Is a Univocal Marker for Human Oral Mucosa Epithelium Useful in Post-COMET Patient Characterization. Int. J. Mol. Sci. 2022, 23, 5785. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Kaneda, H.; Fukushima, M.; Kinoshita, S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014, 92, e447–e453. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Xie, H.T.; Liu, X.; Duan, C.Y.; Qu, J.Y.; Zhang, M.C.; Zhao, X.Y. Subconjunctival Injection of Transdifferentiated Oral Mucosal Epithelial Cells for Limbal Stem Cell Deficiency in Rats. J. Histochem. Cytochem. 2021, 69, 177–190. [Google Scholar] [CrossRef]
- Denig, R. Circumcorneal transplantation of buccal mucous membrane as a curative measure in diseases of the eye. Arch. Ophthalmol. 1929, 1, 351–357. [Google Scholar] [CrossRef]
- Gipson, I.K.; Geggel, H.S.; Spurr-Michaud, S.J. Transplant of oral mucosal epithelium to rabbit ocular surface wounds in vivo. Arch. Ophthalmol. 1986, 104, 1529–1533. [Google Scholar] [CrossRef]
- Sudana, P.; Basu, S.; Shanbhag, S.S. Oral mucous membrane grafts for total symblepharon and lid margin keratinisation post Stevens-Johnson syndrome. BMJ Case Rep. 2020, 13, e239383. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Qiu, W.-Y.; Xu, Y.-S.; Yao, Y.-F. Clinical efficacy of a new surgical technique of oral mucosal epithelial transplantation for severe ocular surface disorders. BMC Ophthalmol. 2023, 23, 145. [Google Scholar] [CrossRef]
- Gerasimov, M.Y.; Ostrovskiy, D.S.; Shatskikh, A.V.; Borzenok, S.A.; Malyugin, B.E. Labial mucosal epithelium grafting in an ex vivo human donor cornea model. Exp. Eye Res. 2022, 216, 108931. [Google Scholar] [CrossRef]
- Cankaya, C. Conjunctival Limbal Autograft Combined with Amniotic Membrane Transplantation to Treat a Moderate Chemical Eye Injury. Beyoglu Eye J. 2019, 4, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Umfress, A.C.; Mawn, L.A.; Joos, K.M.; Donahue, S.P.; Schmitt, A.D.; Shieh, C. Surgical management of large bilateral epibulbar dermoids with autologous oral mucous membrane transplantation. Am. J. Ophthalmol. Case Rep. 2020, 20, 100982. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Yan, C.; Yu, F.; Yan, D.; Wu, N.; Chen, L.; Zhang, S.; Fu, Y. Direct oral mucosal epithelial transplantation supplies stem cells and promotes corneal wound healing to treat refractory persistent corneal epithelial defects. Exp. Eye Res. 2022, 215, 108934. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiao, Y.; Xu, A.; Duan, C. Effects of Exosomal microRNAs on Oral Mucosal Epithelial Cells Cocultured with Limbal Niche Cells. Contrast Media Mol. Imaging 2022, 2022, 9794950. [Google Scholar] [CrossRef]
- Duan, C.-Y.; Xie, H.-T.; Zhao, X.-Y.; Zhang, M.-C. Limbal niche cells: A novel feeder cell for autologous cultivated oral mucosal epithelial transplantation. Regen. Med. 2019, 14, 49–62. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Xie, H.T.; Duan, C.Y.; Li, J.; Zhang, M.C. Rat limbal niche cells can induce transdifferentiation of oral mucosal epithelial cells into corneal epithelial-like cells in vitro. Stem Cell Res. Ther. 2018, 9, 256. [Google Scholar] [CrossRef]
- Huang, M.; Li, N.; Wu, Z.; Wan, P.; Liang, X.; Zhang, W.; Wang, X.; Li, C.; Xiao, J.; Zhou, Q.; et al. Using acellular porcine limbal stroma for rabbit limbal stem cell microenvironment reconstruction. Biomaterials 2011, 32, 7812–7821. [Google Scholar] [CrossRef]
- Shafiq, M.A.; Milani, B.Y.; Djalilian, A.R. In vivo evaluation of a decellularized limbal graft for limbal reconstruction. Int. J. Tissue Eng. 2014, 2014, 754245. [Google Scholar] [CrossRef]
- Isidan, A.; Liu, S.; Chen, A.M.; Zhang, W.; Li, P.; Smith, L.J.; Hara, H.; Cooper, D.K.; Ekser, B. Comparison of porcine corneal decellularization methods and importance of preserving corneal limbus through decellularization. PLoS ONE 2021, 16, e0243682. [Google Scholar] [CrossRef]
- Sánchez-Porras, D.; Caro-Magdaleno, M.; González-Gallardo, C.; García-García, Ó.D.; Garzón, I.; Carriel, V.; Campos, F.; Alaminos, M. Generation of a biomimetic substitute of the corneal limbus using decellularized scaffolds. Pharmaceutics 2021, 13, 1718. [Google Scholar] [CrossRef]
- Bibak-Bejandi, Z.; Mohammadi, S.F.; Davoudi, M.; Bahmanpour, A.; Asadi-Amoli, F. A New Technique for Harvesting Limbal Stem Cell Tissue for Transplantation Using an Automated Microkeratome and a Novel Globe-Fixation System. Cornea 2024, 43, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Schmid, A.; Schlötzer-Schrehardt, U.; Maier, P.; Lang, S.J.; Steinberg, T.; Schlunck, G.; Reinhard, T. A decellularized human corneal scaffold for anterior corneal surface reconstruction. Sci. Rep. 2021, 11, 2992. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Protocols | Verifications | Outcome |
|---|---|---|---|
| Minghai Huang et al. (2011) [67] | De-epithelized rabbit limbal autograft stroma (auto-DERLS) and de-epithelized porcine limbal stroma (DEPLS): 4 °C for 20 min, frozen at −70 °C for 1 h, warmed at 37 °C, and then washed by phosphate-buffered saline (PBS) with continuous shaking in a thermostat-controlled water bath for 2 h freeze–thaw (3 times). Acellular porcine limbal stroma (APLS) and acellular porcine corneal stroma (APCS): saline solution of bicarbonate mixture I (phospholipase A2 with sodium deoxycholate) for 6 h at 37 °C and followed by sodium bicarbonate mixture II (sodium deoxycholate free phospholipase A2) for 2 h at 37 °C. | 1. Subcutaneous implantation 2. Transplantation on limbal defect model 3. Follow-up of transplantation models via slit lamp examination (integrity of epithelium, transparency, and neovascularization of cornea, ingrowth of conjunctival epithelial, and rejection over 6 months) 4. Histological examination (postoperative months 1, 3, and 6) 5. Immunofluorescent staining (K13, CD4 and 8, Muc5AC, goat rabbit IgG for Ki67, p63, and K3) | 1. Thin fibrous around auto-DERLS, APLS, and APCS but thick fibrous around DEPLS 2. Re-epithelialization occurred in Auto-DERLS, DEPLS, and APLS in 3 to 4 days post-transplantation 3. Acute graft rejection has occurred in 8–10 days post-transplantation in DEPLS 4. The graft integration with the cornea was observed 5. APCS, APLS, and auto-DERLS groups: few T-cells (CD4+ and CD8+) |
| Maryam A. Shafiq et al. (2014) [68] | 1. In the first step, 1.5 M NaCl solution for 48 h with a change in NaCl each 24 h. 2. In the second step, corneas were treated with both DNase and RNase (5 U/mL, Sigma-Aldrich, St. Louis, MO, USA) for 48 h. 3. Then, the corneas were washed with PBS for 72 h and PBS was changed every 24 h. | 1. Slit-lamp imaging after limbal transplantation on the limbal deficient model 2. Immunohistologic evaluation (anti-p63, anti-keratin 12, and anti-Ki67) | 1. A minimal amount of fluorescein staining and also stromal haziness was shown in eyes that received the graft; however, non-grafted eyes indicated peripheral neovascularization. Epithelial cells growing on the graft showed keratin 12. 2. Ki67 as a proliferation maker and DeltaNp63 as a stem cell marker were expressed more in the limbal corneal epithelial cells rather than the center of the cornea. |
| Kristina Spaniol (2017) [22] | 1. For tissue incubation at 4 °C under continuous agitation at 200 rpm, PBS containing 5% penicillin/streptomycin was used and washed three times. 2. Decellularization solution containing 4% sodium deoxycholate monohydrate solution in a continuous agitation of 200 rpm for 48 h with changes of decellularization solution two times. 3. After washing with PBS transfer to DNase solution under continuous situation. 4. Wash with PBS under continuous agitation. 5. 25 kGy γ-irradiation used for sterilizing. | 1. Using scanning electron microscopy for surface structure analysis 2. Histological characterization (H&E staining) 3. DNA content 4. Determination of the components of BM and leukocytes (fibronectin, laminin, collagen IV, CD45) 5. Cell viability assay | 1. H&E (regular lamellar collagen in connective tissue). 2. 1.5 ± 0.3 μg/mg of DNA content before and 0.15 ± 0.01 μg/mg after the procedure. |
| Abdulkadir Isidan et al. (2021) [69] | Corneoscleral with or without limbus (n = 6), underwent 3 decellularized protocols: 1. 0.1% SDS for 48 h with continuous shaking. 2. HS (Hypertonic Saline): ultrapure water for 12 h + 30 min 2 M HS followed by 30 min ultrapure water. 3. NG (Nitrogen Gas). | 1. Macroscopic assessments (transparency + severity of edema + tissue thickness) 2. H&E staining for histopathological analysis 3. Hoechst staining for DNA analysis 4. Transmission electron microscopy (TEM) for ultrastructural analysis | 1. Compared to the HS and NG groups, the SDS group experienced more edema. 2. Thickness reduced in NG and SDS with limbus groups after glycerol treatment for 2 h. 3. Transparency increased in all groups after glycerol treatment for 2 h except the SDS without limbus group. 4. 94.3% and 100% of nucleic acid were successfully removed with SDS in corneas with and without limbus groups, respectively. 5. SDS disorganized lamellar collagen fiber and increased inter-fiber spacing in both with and without limbus groups. |
| David Sánchez-Porras et al. (2021) [70] | 1. P1 Protocol: 24 h of ddH2O; 0.1% SDS (each 24 h: 3 incubations). 2. P2 Protocol: 24 h of ddH2O; 24 h of 0.1% SDS; washing via PBS; 1.5 M NaCl (each 24 h: 2 incubations). 3. P3 Protocol: 24 h of ddH2O; 24 h of 0.1% SDS; washing with PBS; 24 h of 1% SDC; washing with PBS; 24 h of 0.6% triton X-100; washing with PBS; 45 min of 100 mg/L DNase and 20 mg/L RNase. 4. P4 Protocol: 24 h of ddH2O; 24 h of 0.1% SDS; washing with PBS; 24 h of 1% SDC; washing with PBS; 24 h of 0.6% triton X-100; washing with PBS; 45 min of 100 mg/L DNase and 20 mg/L RNase; washing with PBS; 1 h of 0.05% Trypsin. | 1. H&E staining 2. 4′,6-diamidino 2-phenylindole (DAPI) staining 3. Alcian blue (AB) staining (proteoglycans) 4. PSR staining 5. Historical analysis of re-cellularized limbus 6. Evaluation of p63 and pan-cytokeratin as markers of limbal cells 7. Evaluation of collagen IV and laminin (immunohistochemically) as BM components 8. PSR and AB staining for analysis of ECM components | 1. <50 mg of DNA per 1 mg in all the protocols. 2. Except in protocol 1 with HL, the PSR intensity decreased; however, the area fraction increased. 3. Corneal epithelial cell numbers were high on day 7 with minimal change to days 14 and 21. Intercellular space becomes less over the 21 days. HADSC attached to DL, but on day 7, the number of cells was inadequate and increased by day 21. 4. Re-cellularized limbus with SIRC revealed p63 from day 7 to 21 and also pan cytokeratin and CRY-Z were positive at 7, 14, and 21 days. Positive p63 was shown at day 21 in hADSCs. From day 14 to 21, pan cytokeratin became positive. CRY-Z become positive from day 14 to 21. 5. At days 14 and 21, laminin was negative and collagen IV was positive in RLs with SIRC cells. RLs with hADSC were positive for laminin and collagen IV from day 14. 6. PSR area fractions were lower in two conditions of RL. In all RL than native limbus, AB-positive staining was lower. |
| Naresh Polisetti et al. (2021) [72] | 1% SD in ultrapure water (30 min), then DPBS (3 × 30 min), then DNase I, 1 mg/mL (overnight), and then washed for 4 × 30 min in DPBS. All of the steps were carried out under 4% dextran. | 1. H&E staining 2. Human leukocyte antigen (HLA-ABC) 3. DAPI 4. Light and electron microscopy for evaluating limbal architecture 5. Periodic acid Schiff (PAS) and AB staining to evaluate ECM Components (collagen III, IV, and XVIII, again, junctional adhesion molecule C (JAM-C), tenascin C (TN-C), fibronectin (FN), laminin (LN) chains (α3, α5, β2, β3, and γ2), and vitronectin (VN)) 6. In vitro recellularization (limbal epithelial progenitor cells (LEPC) and limbal melanocytes (LM)): intercellular E (epithelial)-cadherin, pan-cytokeratin, vimentin, p63, CK15, Ki-67, CK3, and Melan-A staining 7. Ex vivo transplantation (pan-cytokeratin, CK3, p63, Ki-67, Melan-A, and vimentin staining) | 1. Multilayer epithelium, dark stained cells in the basal layer, arranged, and regular collagen fiber. 2. No cellular and material debris was found in DL. 3. Removed about 98.5 ± 0.3% with dextran and 99.2 ± 0.4% w/o it. 4. Stromal projections demonstrated in DL in both w or w/o. Epithelial basement membrane significantly detected in dextran-treated DHL. 5. Re-cellularized scaffolds phenotype: E-cadherin and pan-cytokeratin in all of the epithelial layers, p63 and CK15 in the basal layer, Ki-67 in the basal limbal layer, vimentin in the basal and stromal layer, and Melan-A in the epithelial layer. 7. Pan-cytokeratin positive in host and graft cells and CK3 positive cells in superficial and all of the epithelial layers in the graft and host, respectively. CK15 presents on the basal epithelial cells of the graft, however, was not positive in the host corneal cells. The basal layer of the graft and also a few cells on the host were positive for P63. Ki-67 was positive in the basal layer and negative in the host tissue. Melan-A positive melanocytes were presented at the corneal region. |
| Simple Oral Mucosal Transplantation (SOMT) | Oral Mucosal Graft Transplantation (OMGT) | Cultured Oral Mucosal Cell Transplantation (COMCT) | |||
|---|---|---|---|---|---|
| Pros | Cons and Future Considerations | Pros | Cons and Future Considerations | Pros | Cons and Future Considerations |
| Needs fewer facilities | Requires more and long-term clinical trials | Needs fewer facilities | Needs more tissue grafts to create the annular or crescent graft | Many in vitro, in vivo, and clinical trials have been conducted | Requires expertise and facilities |
| All the procedures are performed beside the patients in OR | More innovation in Auto-SLET should be assessed, considering the suitable base membrane, adding AM as scaffolds, or enhancing healing | All procedures are performed beside the patients in the operation room | Requires more and long-term clinical trials | Take 2 to 4 weeks for culturing process | |
| Time saving | The consideration of other cell source, such as Auto-SLET should be compared | Time saving | Expensive and not cost-effective | ||
| Cost benefits | Cost benefits | ||||
| Reduced the need for tissue grafts for transplantation | |||||
| Reduced the induction of limbal deficiency | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibak-Bejandi, Z.; Soleimani, M.; Arabpour, Z.; Karaca, E.E.; Jalilian, E.; Asadigandomani, H.; Bibak-Bejandi, R.; D’jalilian, A.R. Advancing Bilateral Limbal Deficiency Surgery: A Comprehensive Review of Innovations with Mucosal Cells. Biomedicines 2025, 13, 630. https://doi.org/10.3390/biomedicines13030630
Bibak-Bejandi Z, Soleimani M, Arabpour Z, Karaca EE, Jalilian E, Asadigandomani H, Bibak-Bejandi R, D’jalilian AR. Advancing Bilateral Limbal Deficiency Surgery: A Comprehensive Review of Innovations with Mucosal Cells. Biomedicines. 2025; 13(3):630. https://doi.org/10.3390/biomedicines13030630
Chicago/Turabian StyleBibak-Bejandi, Zahra, Mohammad Soleimani, Zohreh Arabpour, Emine Esra Karaca, Elmira Jalilian, Hassan Asadigandomani, Reyhaneh Bibak-Bejandi, and Ali R. D’jalilian. 2025. "Advancing Bilateral Limbal Deficiency Surgery: A Comprehensive Review of Innovations with Mucosal Cells" Biomedicines 13, no. 3: 630. https://doi.org/10.3390/biomedicines13030630
APA StyleBibak-Bejandi, Z., Soleimani, M., Arabpour, Z., Karaca, E. E., Jalilian, E., Asadigandomani, H., Bibak-Bejandi, R., & D’jalilian, A. R. (2025). Advancing Bilateral Limbal Deficiency Surgery: A Comprehensive Review of Innovations with Mucosal Cells. Biomedicines, 13(3), 630. https://doi.org/10.3390/biomedicines13030630






