Hemodynamic Factors Driving Peripheral Chemoreceptor Hypersensitivity: Is Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation a Valuable Human Model?
Abstract
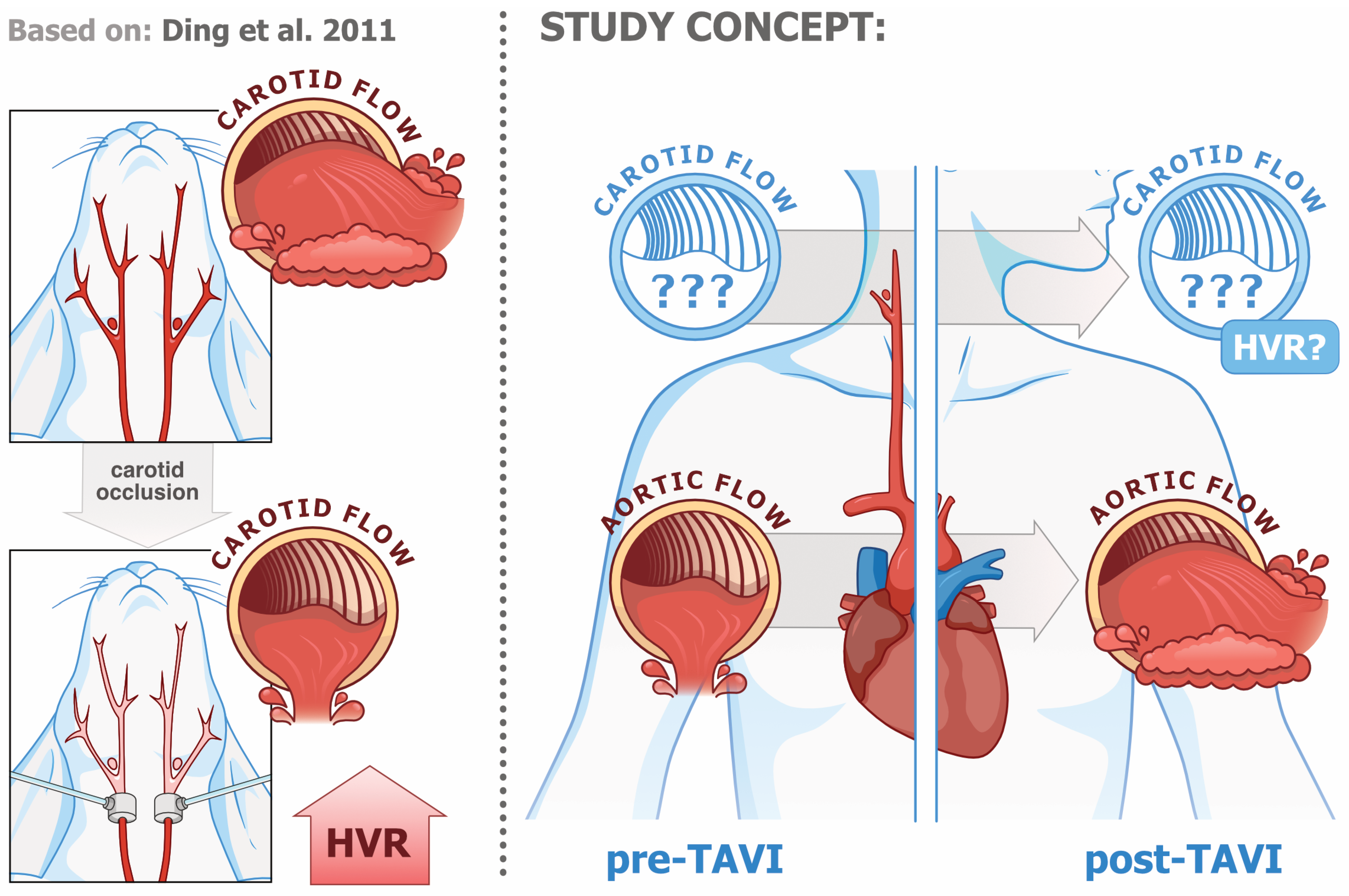
1. Introduction
2. Methods
2.1. Study Population and Ethical Approval
2.2. Study Protocol
2.3. Transcatheter Aortic Valve Implantation
2.4. Assessment of Peripheral Chemoreflex Function with the Transient Hypoxia Test
2.5. Quantification of Peripheral Chemoreflex Sensitivity
2.6. Ultrasound Measurement of Carotid Blood Flow
2.7. Echocardiography and Laboratory Tests
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. The Effect of TAVI on Clinical Parameters
3.3. The Effect of TAVI on Peripheral Chemoreflex Function
3.4. Peripheral Chemoreflex Function Before TAVI as Compared with the Literature Data
4. Discussion
4.1. TAVI Does Not Affect the Ventilatory Component of the Peripheral Chemoreflex
4.2. Heart Rate Response to Hypoxia Is Suppressed in Severe Aortic Stenosis with Partial Restoration Post-TAVI
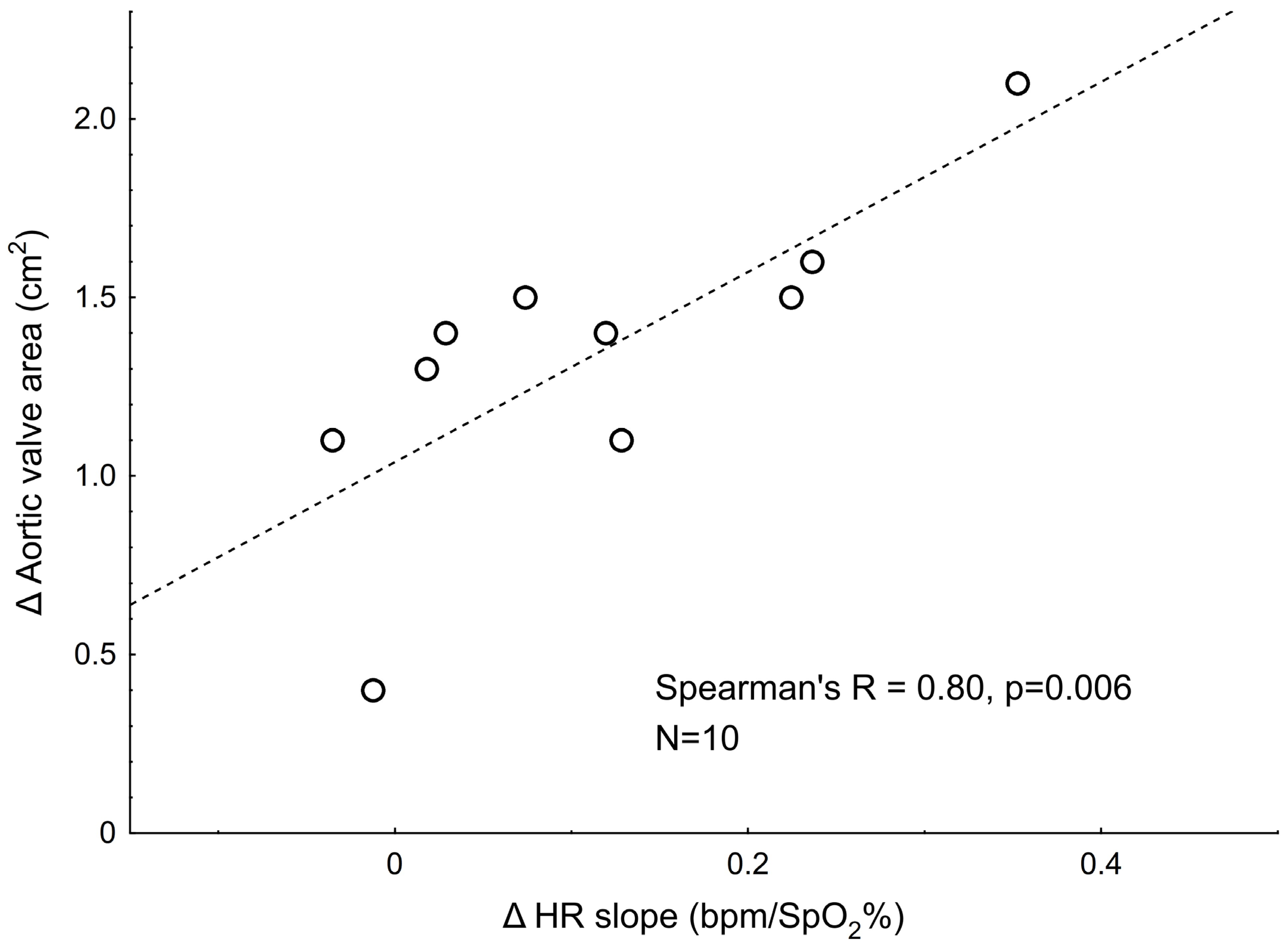
4.3. Relations Between Pre- vs. Post-TAVI Changes in Hypoxic Responses and the Changes in Hemodynamic Variables
4.4. Severe AS Treated with TAVI as a Human Model of PCh Function Under Low- vs. High-Blood-Flow and/or WSS Conditions
5. Limitations
6. Clinical Significance
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teppema, L.J.; Dahan, A. The ventilatory response to hypoxia in mammals: Mechanisms, measurement, and analysis. Physiol. Rev. 2010, 90, 675–754. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Alcayaga, J.; Chapleau, M.W.; Somers, V.K. Carotid body chemoreceptors: Physiology, pathology, and implications for health and disease. Physiol. Rev. 2021, 101, 1177–1235. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Adaptive cardiorespiratory changes to chronic continuous and intermittent hypoxia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 188, pp. 103–123. [Google Scholar]
- Prabhakar, N.R.; Peng, Y.-J. Peripheral chemoreceptors in health and disease. J. Appl. Physiol. 2004, 96, 359–366. [Google Scholar] [CrossRef]
- Niewinski, P.; Janczak, D.; Rucinski, A.; Tubek, S.; Engelman, Z.J.; Jazwiec, P.; Banasiak, W.; Sobotka, P.A.; Hart, E.C.J.; Paton, J.F.R.; et al. Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp. Physiol. 2014, 99, 552–561. [Google Scholar] [CrossRef]
- Comroe, J.H.; Mortimer, L. The respiratory and cardiovascular responses of temporally separated aortic and carotid bodies to cyanide, nicotine, phenyldiguanide and serotonin. J. Pharmacol. Exp. Ther. 1964, 146, 33–41. [Google Scholar] [CrossRef]
- Tubek, S.; Niewinski, P.; Reczuch, K.; Janczak, D.; Rucinski, A.; Paleczny, B.; Engelman, Z.J.; Banasiak, W.; Paton, J.F.R.; Ponikowski, P. Effects of selective carotid body stimulation with adenosine in conscious humans. J. Physiol. 2016, 594, 6225–6240. [Google Scholar] [CrossRef] [PubMed]
- Niewinski, P.; Janczak, D.; Rucinski, A.; Tubek, S.; Engelman, Z.J.; Piesiak, P.; Jazwiec, P.; Banasiak, W.; Fudim, M.; Sobotka, P.A.; et al. Carotid body resection for sympathetic modulation in systolic heart failure: Results from first-in-man study. Eur. J. Heart Fail. 2017, 19, 391–400. [Google Scholar] [CrossRef]
- Kumar, P.; Bin-Jaliah, I. Adequate stimuli of the carotid body: More than an oxygen sensor? Respir. Physiol. Neurobiol. 2007, 157, 12–21. [Google Scholar] [CrossRef]
- Lahiri, S.; Nishino, T.; Mokashi, A.; Mulligan, E. Relative responses of aortic body and carotid body chemoreceptors to hypotension. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 781–788. [Google Scholar] [CrossRef]
- Landgren, S.; Neil, E. Chemoreceptor impulse activity following haemorrhage. Acta Physiol. Scand. 1951, 23, 158–167. [Google Scholar] [CrossRef]
- Ponikowski, P.; Chua, T.P.; Anker, S.D.; Francis, D.P.; Doehner, W.; Banasiak, W.; Poole-Wilson, P.A.; Piepoli, M.F.; Coats, A.J. Peripheral chemoreceptor hypersensitivity: An ominous sign in patients with chronic heart failure. Circulation 2001, 104, 544–549. [Google Scholar] [CrossRef]
- Niewinski, P.; Engelman, Z.J.; Fudim, M.; Tubek, S.; Paleczny, B.; Jankowska, E.A.; Banasiak, W.; Sobotka, P.A.; Ponikowski, P. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J. Card. Fail. 2013, 19, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Siński, M.; Lewandowski, J.; Przybylski, J.; Bidiuk, J.; Abramczyk, P.; Ciarka, A.; Gaciong, Z. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens. Res. 2012, 35, 487–491. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Ratcliffe, L.E.K.; Hart, E.C.; Briant, L.J.B.; Chrostowska, M.; Wolf, J.; Szyndler, A.; Hering, D.; Abdala, A.P.; Manghat, N.; et al. Unilateral Carotid Body Resection in Resistant Hypertension: A Safety and Feasibility Trial. JACC Basic. Transl. Sci. 2016, 1, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, A.; Gentile, F.; Buoncristiani, F.; Borrelli, C.; Sciarrone, P.; Spiesshoefer, J.; Bramanti, F.; Iudice, G.; Javaheri, S.; Emdin, M.; et al. Chemoreflex and Baroreflex Sensitivity Hold a Strong Prognostic Value in Chronic Heart Failure. JACC Heart Fail. 2022, 10, 662–676. [Google Scholar] [CrossRef]
- Del Rio, R.; Marcus, N.J.; Schultz, H.D. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of cardiorespiratory function. J. Am. Coll. Cardiol. 2013, 62, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.J.; Del Rio, R.; Schultz, E.P.; Xia, X.-H.; Schultz, H.D. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J. Physiol. 2014, 592, 391–408. [Google Scholar] [CrossRef]
- Toledo, C.; Andrade, D.C.; Lucero, C.; Schultz, H.D.; Marcus, N.; Retamal, M.; Madrid, C.; Del Rio, R. Contribution of peripheral and central chemoreceptors to sympatho-excitation in heart failure. J. Physiol. 2017, 595, 43–51. [Google Scholar] [CrossRef]
- Niewiński, P.; Janczak, D.; Rucinski, A.; Jazwiec, P.; Sobotka, P.A.; Engelman, Z.J.; Fudim, M.; Tubek, S.; Jankowska, E.A.; Banasiak, W.; et al. Carotid body removal for treatment of chronic systolic heart failure. Int. J. Cardiol. 2013, 168, 2506–2509. [Google Scholar] [CrossRef]
- Pijacka, W.; Moraes, D.J.A.; Ratcliffe, L.E.K.; Nightingale, A.K.; Hart, E.C.; da Silva, M.P.; Machado, B.H.; McBryde, F.D.; Abdala, A.P.; Ford, A.P.; et al. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med. 2016, 22, 1151–1159. [Google Scholar] [CrossRef]
- Schultz, H.D.; Marcus, N.J.; Del Rio, R. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp. Physiol. 2015, 100, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Y.; Cornish, K.G.; Schultz, H.D. Reduced Blood Flow in Carotid Arteries is a Trigger Contributing to Peripheral Chemoreflex Hypersensitivity in Chronic Heart Failure Rabbits. FASEB J. 2007, 21, A1268–A1269. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.-L.; Schultz, H.D. Role of blood flow in carotid body chemoreflex function in heart failure. J. Physiol. 2011, 589, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Andrade, D.C.; Toledo, C.; Diaz, H.S.; Lucero, C.; Arce-Alvarez, A.; Marcus, N.J.; Schultz, H.D. Carotid Body-Mediated Chemoreflex Drive in The Setting of low and High Output Heart Failure. Sci. Rep. 2017, 7, 8035. [Google Scholar] [CrossRef]
- Marcus, N.J.; Del Rio, R.; Ding, Y.; Schultz, H.D. KLF2 mediates enhanced chemoreflex sensitivity, disordered breathing and autonomic dysregulation in heart failure. J. Physiol. 2018, 596, 3171–3185. [Google Scholar] [CrossRef]
- Carabello, B.A. Introduction to aortic stenosis. Circ. Res. 2013, 113, 179–185. [Google Scholar] [CrossRef]
- Lindman, B.R.; Bonow, R.O.; Otto, C.M. Current management of calcific aortic stenosis. Circ. Res. 2013, 113, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chrissoheris, M.; Ziakas, A.; Chalapas, A.; Chadjimiltiades, S.; Styliades, I.; Karvounis, C.; Nikolaou, I.; Spargias, K. Acute Invasive Hemodynamic Effects of Transcatheter Aortic Valve Replacement. J. Heart Valve Dis. 2016, 25, 162–172. [Google Scholar]
- Sener, Y.Z.; Keresteci, A.H.; Ates, A.H.; Sahiner, M.L.; Kaya, E.B.; Aytemir, K. Outcomes of TAVI in patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2023, 25, 353. [Google Scholar]
- Clavel, M.a.; Webb, J.g.; Rodés-Cabau, J.; Masson, J.b.; Dumont, E.; De Larochellière, R.; Doyle, D.; Bergeron, S.; Baumgartner, H.; Burwash, I.g.; et al. Comparison Between Transcatheter and Surgical Prosthetic Valve Implantation in Patients With Severe Aortic Stenosis and Reduced Left Ventricular Ejection Fraction. Circulation 2010, 122, 1928–1936. [Google Scholar] [CrossRef]
- Rediker, D.E.; Boucher, C.A.; Block, P.C.; Akins, C.W.; Buckley, M.J.; Fifer, M.A. Degree of reversibility of left ventricular systolic dysfunction after aortic valve replacement for isolated aortic valve stenosis. Am. J. Cardiol. 1987, 60, 112–118. [Google Scholar] [CrossRef] [PubMed]
- van Houte, J.; Eerdekens, R.; Dieters, E.; te Pas, M.; Wijnbergen, I.; Tonino, P.; Bouwman, A. Immediate hemodynamic effects of transcatheter aortic valve replacement on left ventricular stroke volume and carotid artery blood flow. WFUMB Ultrasound Open 2023, 1, 100008. [Google Scholar] [CrossRef]
- Pilkiene, K.; Salkauskaite-Rubliauskiene, A.; Ramantauskaite, G.; Mizariene, V. Echocardiographic results after complete transcatheter versus complete surgical treatment in patients with aortic valve stenosis and concomitant coronary artery disease. Eur. J. Heart Fail. 2023, 25 (Suppl. S2), 352–353. [Google Scholar]
- Kleczyński, P.; Petkow Dimitrow, P.; Dziewierz, A.; Surdacki, A.; Dudek, D. Transcatheter aortic valve implantation improves carotid and vertebral arterial blood flow in patients with severe aortic stenosis: Practical role of orthostatic stress test. Clin. Cardiol. 2017, 40, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Romeo, F.; Marchei, M.; Anceschi, A.; Massaro, G.; Muscoli, S.; De Persis, F.; Macrini, M.; Ussia, G.P. Carotid Doppler sonography: Additional tool to assess hemodynamic improvement after transcatheter aortic valve implantation. J. Cardiovasc. Med. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Edelman, N.H.; Epstein, P.E.; Lahiri, S.; Cherniack, N.S. Ventilatory responses to transient hypoxia and hypercapnia in man. Respir. Physiol. 1973, 17, 302–314. [Google Scholar] [CrossRef]
- Paleczny, B.; Niewiński, P.; Rydlewska, A.; Piepoli, M.F.; Borodulin-Nadzieja, L.; Jankowska, E.A.; Ponikowska, B. Age-related reflex responses from peripheral and central chemoreceptors in healthy men. Clin. Auton. Res. 2014, 24, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Paleczny, B.; Seredyński, R.; Tubek, S.; Adamiec, D.; Ponikowski, P.; Ponikowska, B. Hypoxic tachycardia is not a result of increased respiratory activity in healthy subjects. Exp. Physiol. 2019, 104, 476–489. [Google Scholar] [CrossRef]
- Sato, K.; Ogoh, S.; Hirasawa, A.; Oue, A.; Sadamoto, T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J. Physiol. 2011, 589, 2847–2856. [Google Scholar] [CrossRef]
- Wikstrand, J. Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin. Physiol. Funct. Imaging 2007, 27, 341–345. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.—Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Kappetein, A.P.; Wolner, E.; Nataf, P.; Thomas, M.; Schächinger, V.; De Bruyne, B.; Eltchaninoff, H.; Thielmann, M.; Himbert, D.; et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur. Heart J. 2011, 32, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.P.; Coats, A.J. The reproducibility and comparability of tests of the peripheral chemoreflex: Comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur. J. Clin. Investig. 1995, 25, 887–892. [Google Scholar] [CrossRef]
- Paleczny, B.; Seredyński, R.; Wyciszkiewicz, M.; Nowicka-Czudak, A.; Łopusiewicz, W.; Adamiec, D.; Wiecha, S.; Mroczek, D.; Chmura, P.; Konefał, M.; et al. Low ventilatory responsiveness to transient hypoxia or breath-holding predicts fast marathon performance in healthy middle-aged and older men. Sci. Rep. 2021, 11, 10255. [Google Scholar] [CrossRef]
- Natsuaki, M.; Itoh, T.; Tomita, S.; Naito, K. Reversibility of cardiac dysfunction after valve replacement in elderly patients with severe aortic stenosis. Ann. Thorac. Surg. 1998, 65, 1634–1638. [Google Scholar] [CrossRef]
- Irace, C.; Gnasso, A.; Cirillo, F.; Leonardo, G.; Ciamei, M.; Crivaro, A.; Renzulli, A.; Cotrufo, M. Arterial remodeling of the common carotid artery after aortic valve replacement in patients with aortic stenosis. Stroke 2002, 33, 2446–2450. [Google Scholar] [CrossRef]
- Shaw, R.A.; Schonfeld, S.A.; Whitcomb, M.E. Progressive and transient hypoxic ventilatory drive tests in healthy subjects. Am. Rev. Respir. Dis. 1982, 126, 37–40. [Google Scholar]
- Pfoh, J.R.; Tymko, M.M.; Abrosimova, M.; Boulet, L.M.; Foster, G.E.; Bain, A.R.; Ainslie, P.N.; Steinback, C.D.; Bruce, C.D.; Day, T.A. Comparing and characterizing transient and steady-state tests of the peripheral chemoreflex in humans. Exp. Physiol. 2016, 101, 432–447. [Google Scholar] [CrossRef]
- van Bilsen, M.; Patel, H.C.; Bauersachs, J.; Böhm, M.; Borggrefe, M.; Brutsaert, D.; Coats, A.J.S.; de Boer, R.A.; de Keulenaer, G.W.; Filippatos, G.S.; et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2017, 19, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, N.; Vaccaro, A.; Despas, F.; Labrunee, M.; Marcheix, B.; Lambert, E.; Esler, M.; Carrie, D.; Senard, J.-M.; Galinier, M.; et al. Transcatheter aortic valve implantation reduces sympathetic activity and normalizes arterial spontaneous baroreflex in patients with aortic stenosis. JACC Cardiovasc. Interv. 2013, 6, 1195–1202. [Google Scholar] [CrossRef]
- Arslan, U.; Özdemir, M.; Kocaman, S.A.; Balcıoğlu, S.; Cemri, M.; Çengel, A. Heart rate variability and heart rate turbulence in mild-to-moderate aortic stenosis. EP Eur. 2008, 10, 1434–1441. [Google Scholar] [CrossRef]
- Zuern, C.S.; Eick, C.; Rizas, K.D.; Stoleriu, C.; Barthel, P.; Scherer, C.; Müller, K.A.L.; Gawaz, M.; Bauer, A. Severe autonomic failure in moderate to severe aortic stenosis: Prevalence and association with hemodynamics and biomarkers. Clin. Res. Cardiol. 2012, 101, 565–572. [Google Scholar] [CrossRef]
- Zuern, C.S.; Rizas, K.D.; Eick, C.; Vogtt, M.-I.; Bigalke, B.; Gawaz, M.; Bauer, A. Severe autonomic failure as a predictor of mortality in aortic valve stenosis. Int. J. Cardiol. 2014, 176, 782–787. [Google Scholar] [CrossRef]
- Vukasovic, J.L.; Florenzano, F.; Adriazola, P.; Escobar, E. Heart rate variability in severe aortic stenosis. J. Heart Valve Dis. 1999, 8, 143–148. [Google Scholar] [PubMed]
- Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Torres-Arellano, J.M.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío, J.; Fajardo, E.; Lerma, C. Inflammation and Reduced Parasympathetic Cardiac Modulation in Aortic-Valve Sclerosis. Appl. Sci. 2019, 9, 4020. [Google Scholar] [CrossRef]
- Duckheim, M.; Bensch, C.; Kittlitz, L.; Götz, N.; Klee, K.; Groga-Bada, P.; Mizera, L.; Gawaz, M.; Zuern, C.; Eick, C. Deceleration capacity of heart rate predicts 1-year mortality of patients undergoing transcatheter aortic valve implantation. Clin. Cardiol. 2017, 40, 919–924. [Google Scholar] [CrossRef]
- Ardakani, J.S.; Jafarnejad, M.; Firoozabadi, B.; Saidi, M.S. Investigation of Wall Shear Stress Related Factors in Realistic Carotid Bifurcation Geometries and Di erent Flow Conditions. Sci. Iran. 2010, 17, 358–366. [Google Scholar]
- Khan, Q.; Heath, D.; Smith, P. Anatomical variations in human carotid bodies. J. Clin. Pathol. 1988, 41, 1196–1199. [Google Scholar] [CrossRef]
- Dawes, G.S.; Comroe, J.H. Chemoreflexes from the heart and lungs. Physiol. Rev. 1954, 34, 167–201. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.; Hainsworth, R.; Sofola, O.A.; Wood, L.M. Responses of the heart to stimulation of aortic body chemoreceptors in dogs. Circ. Res. 1980, 46, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Siebenmann, C.; Ryrsø, C.K.; Oberholzer, L.; Fisher, J.P.; Hilsted, L.M.; Rasmussen, P.; Secher, N.H.; Lundby, C. Hypoxia-induced vagal withdrawal is independent of the hypoxic ventilatory response in men. J. Appl. Physiol. 2019, 126, 124–131. [Google Scholar] [CrossRef]
- Lhuissier, F.J.; Canouï-Poitrine, F.; Richalet, J.-P. Ageing and cardiorespiratory response to hypoxia. J. Physiol. 2012, 590, 5461–5474. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.P.P.; Xu, X.Y.; Torii, R.; Wood, N.B.; Delahunty, N.; Mullen, M.; Moat, N.; Mohiaddin, R. Comparison of Aortic Flow Patterns Before and After Transcatheter Aortic Valve Implantation. Cardiovasc. Eng. Tech. 2012, 3, 123–135. [Google Scholar] [CrossRef]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall Shear Stress: Theoretical Considerations and Methods of Measurement. Progress. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Assessment of aortic stenosis severity: When the gradient does not fit with the valve area. Heart 2010, 96, 1431–1433. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Low-Flow, Low-Gradient Aortic Stenosis With Normal and Depressed Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2012, 60, 1845–1853. [Google Scholar] [CrossRef]
- Sharma, U.C.; Barenbrug, P.; Pokharel, S.; Dassen, W.R.M.; Pinto, Y.M.; Maessen, J.G. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann. Thorac. Surg. 2004, 78, 90–95. [Google Scholar] [CrossRef]
- Gotzmann, M.; Lindstaedt, M.; Bojara, W.; Mügge, A.; Germing, A. Hemodynamic results and changes in myocardial function after transcatheter aortic valve implantation. Am. Heart J. 2010, 159, 926–932. [Google Scholar] [CrossRef]
- Keir, D.A.; Duffin, J.; Millar, P.J.; Floras, J.S. Simultaneous assessment of central and peripheral chemoreflex regulation of muscle sympathetic nerve activity and ventilation in healthy young men. J. Physiol. 2019, 597, 3281–3296. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Morgan, B.J.; Gupta, A.; Pegelow, D.F.; Teodorescu, M.; Dopp, J.M.; Dempsey, J.A. The need for specificity in quantifying neurocirculatory vs. respiratory effects of eucapnic hypoxia and transient hyperoxia. J. Physiol. 2020, 598, 4803–4819. [Google Scholar] [CrossRef] [PubMed]
- Brognara, F.; Felippe, I.S.A.; Salgado, H.C.; Paton, J.F.R. Autonomic innervation of the carotid body as a determinant of its sensitivity: Implications for cardiovascular physiology and pathology. Cardiovasc. Res. 2021, 117, 1015–1032. [Google Scholar] [CrossRef]
- Farag, E.S.; Vendrik, J.; van Ooij, P.; Poortvliet, Q.L.; van Kesteren, F.; Wollersheim, L.W.; Kaya, A.; Driessen, A.H.G.; Piek, J.J.; Koch, K.T.; et al. Transcatheter aortic valve replacement alters ascending aortic blood flow and wall shear stress patterns: A 4D flow MRI comparison with age-matched, elderly controls. Eur. Radiol. 2019, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.R.; Ratcliffe, L.; Hering, D.; Wolf, J.; Sobotka, P.A.; Narkiewicz, K. Revelations About Carotid Body Function Through its Pathological Role in Resistant Hypertension. Curr. Hypertens. Rep. 2013, 15, 273–280. [Google Scholar] [CrossRef]
- Lataro, R.M.; Moraes, D.J.A.; Gava, F.N.; Omoto, A.C.M.; Silva, C.A.A.; Brognara, F.; Alflen, L.; Brazão, V.; Colato, R.P.; do Prado, J.C.; et al. P2X3 receptor antagonism attenuates the progression of heart failure. Nat. Commun. 2023, 14, 1725. [Google Scholar] [CrossRef]



| All Patients (N = 26) | |
|---|---|
| Male, n (%) | 13 (50) |
| Age, years | 77 ± 6 |
| Body mass index, kg/m2 | 29.1 ± 5.1 |
| HF, n (%) | 13 (50) |
| Atrial fibrillation, n (%) | 9 (35) |
| Coronary artery disease, n (%) | 11 (42) |
| Previous myocardial infarction, n (%) | 2 (8) |
| Hypertension, n (%) | 22 (85) |
| Diabetes mellitus, n (%) | 13 (50) |
| Chronic obstructive pulmonary disease, n (%) | 4 (15) |
| Chronic kidney disease, n (%) | 7 (27) |
| Therapies, n (%) | |
| Beta-blocker | 24 (92) |
| ACE-I/ARB/ARNI | 18 (69)/2 (8)/2 (8) |
| MRA | 15 (58) |
| SGLT2i | 4 (15) |
| Loop diuretics | 16 (62) |
| Thiazides | 4 (15) |
| CCB | 10 (38) |
| Statin | 24 (92) |
| N | Before TAVI | After TAVI | p-Value | |
|---|---|---|---|---|
| Ultrasound measurements | ||||
| Aortic valve area, cm2 | 26 | 0.7 [0.5–0.9] | 1.9 [1.7–2.0] | <0.001 |
| Mean aortic valve gradient, mmHg | 26 | 50 ± 15 | 11 ± 4 | <0.001 |
| Peak aortic jet velocity, m/s | 26 | 4.4 ± 0.7 | 2.2 ± 0.4 | <0.001 |
| LVEF, % | 26 | 50 ± 15 | 55 ± 11 | 0.020 |
| CABF †, mL/min | 18 | 576 ± 166 | 578 ± 158 | 0.933 |
| Respiratory and hemodynamic parameters at rest | ||||
| , L/min | 26 | 11 ± 3 | 11 ± 2 | 0.388 |
| HR, bpm | 10 | 62 ± 12 | 63 ± 11 | 0.825 |
| SBP, mmHg | 10 | 113 ± 23 | 128 ± 29 | 0.210 |
| MAP, mmHg | 10 | 74 ± 12 | 83 ± 17 | 0.112 |
| SVR, dyn × s/cm5 | 10 | 1411 ± 251 | 1596 ± 341 | 0.217 |
| Cardiac output, L/min | 10 | 4.4 ± 0.7 | 4.4 ± 0.9 | 0.907 |
| Blood parameters | ||||
| Hemoglobin, g/dL | 26 | 12.9 ± 1.2 | 13.3 ± 1.2 | 0.047 |
| Hematocrit, % | 26 | 38.4 ± 3.9 | 40.1 ± 3.6 | 0.017 |
| Creatinine, mg/dL | 26 | 1.00 [0.89–1.25] | 0.97 [0.83–1.19] | 0.043 |
| Sodium, mmol/L | 26 | 140.4 ± 2.5 | 140.7 ± 2.4 | 0.571 |
| Potassium, mmol/L | 26 | 4.36 ± 0.49 | 4.53 ± 0.62 | 0.079 |
| NT-proBNP, pg/mL | 26 | 1925 [1278–3575] | 698 [422–1005] | 0.016 |
| Δ HVR, L/min/SpO2% | Δ HR Slope, bpm/SpO2% | |
|---|---|---|
| Δ Aortic valve area, cm2 | −0.14 (p = 0.499), N = 26 | −0.80 (p = 0.006), N = 10 |
| Δ Mean aortic valve gradient, mmHg | −0.15 (p = 0.477), N = 26 | −0.43 (p = 0.214), N = 10 |
| Δ Peak aortic jet velocity, m/s | −0.30 (p = 0.137), N = 26 | −0.26 (p = 0.467), N = 10 |
| Δ LVEF, % | −0.09 (p = 0.667), N = 26 | −0.03 (p = 0.933), N = 10 |
| Δ CABF, mL/min | −0.01 (p = 0.958), N = 18 | −0.14 (p = 0.736), N = 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jura, M.; Tubek, S.; Reczuch, J.; Seredyński, R.; Niewiński, P.; Protasiewicz, M.; Ponikowska, B.; Paleczny, B. Hemodynamic Factors Driving Peripheral Chemoreceptor Hypersensitivity: Is Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation a Valuable Human Model? Biomedicines 2025, 13, 611. https://doi.org/10.3390/biomedicines13030611
Jura M, Tubek S, Reczuch J, Seredyński R, Niewiński P, Protasiewicz M, Ponikowska B, Paleczny B. Hemodynamic Factors Driving Peripheral Chemoreceptor Hypersensitivity: Is Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation a Valuable Human Model? Biomedicines. 2025; 13(3):611. https://doi.org/10.3390/biomedicines13030611
Chicago/Turabian StyleJura, Maksym, Stanisław Tubek, Jędrzej Reczuch, Rafał Seredyński, Piotr Niewiński, Marcin Protasiewicz, Beata Ponikowska, and Bartłomiej Paleczny. 2025. "Hemodynamic Factors Driving Peripheral Chemoreceptor Hypersensitivity: Is Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation a Valuable Human Model?" Biomedicines 13, no. 3: 611. https://doi.org/10.3390/biomedicines13030611
APA StyleJura, M., Tubek, S., Reczuch, J., Seredyński, R., Niewiński, P., Protasiewicz, M., Ponikowska, B., & Paleczny, B. (2025). Hemodynamic Factors Driving Peripheral Chemoreceptor Hypersensitivity: Is Severe Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation a Valuable Human Model? Biomedicines, 13(3), 611. https://doi.org/10.3390/biomedicines13030611






