Abstract
Background/Objectives: Radon is a significant carcinogen, particularly as a leading cause of lung cancer among non-smokers. While its carcinogenic effects are well documented, the relationship between radon exposure and inflammatory reactions remains underexplored. This systematic review investigates inflammatory biomarkers in individuals exposed to chronic radon exposure and conducts a meta-analysis on serum C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) levels. Methods: A systematic search was conducted in PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar using the keywords “radon” AND “inflammation biomarkers” following established guidelines. Studies reporting inflammatory biomarker levels in biological fluids of human participants exposed to residential or occupational radon were included. Statistical analyses, including pooled mean estimates, influence analysis, publication bias, and meta-regression, were performed in RStudio. Results: Ten studies involving 33,099 individuals met the inclusion criteria. Eight studies focused on residential radon exposure, and two examined occupational exposure among uranium miners. Inflammatory biomarkers were analyzed in serum, bronchoalveolar lavage fluid, and saliva. Among individuals exposed to high residential radon levels, serum CRP and TNF-α were the most frequently assessed biomarkers, with pooled mean levels of 2.11 mg/L (95% CI, 1.32–2.89) and 2.20 pg/mL (95% CI, 0.25–4.64), respectively. Conclusions: Serum CRP and TNF-α levels appear lower in adults with chronic radon exposure, suggesting potential anti-inflammatory effects despite radon’s established carcinogenicity. Future longitudinal studies using standardized methods are crucial to elucidate the long-term health impacts of radon exposure.
1. Introduction
Radon (Rn-222) is an odorless, tasteless, and radioactive gas that is a byproduct of the nuclear decay of radium (Ra-226). Radon is the primary source of natural radiation exposure to humans. It is formed within the Earth’s crust as part of the uranium decay series and can seep into the atmosphere, water, and soil [1]. Radon concentrations differ depending on environmental and structural factors. Elevated levels of radon are commonly found in enclosed spaces such as caves, mines, and buildings due to its ability to accumulate in poorly ventilated areas [2]. Radon levels inside buildings are often elevated due to soil infiltration and pressure differences between indoor and outdoor environments [2]. This paper highlights the two main pathways of radon exposure: residential and occupational.
The presence of radon in a residential or an occupational setup is dependent upon the underlying geological environment and building type, as well as the lifestyle being led [3]. High radon levels have been recorded in areas with soil rich in uranium, homes with poor ventilation, and workplaces such as uranium mines [4]. Uranium mining activities disturb the natural equilibrium of radioactive elements in the Earth’s crust, releasing radon gas into the surrounding environment [4]. Workers in these settings face chronic exposure to high radon levels, often exceeding those encountered in residential environments. The restricted spaces and poor ventilation in mines lead to higher radon levels, which significantly heightens the health risks for miners. This occupational exposure has historically been associated with an increased risk of lung cancer [5]. While substantial research has illuminated radon’s carcinogenic properties, particularly its role in lung cancer etiology, systematic investigations into its broader biological effects, especially its relationship with inflammatory biomarkers, remain sparse. This gap in the environmental health literature highlights the need for focused research to assess the non-carcinogenic pathways through which radon affects human health.
It is well documented that radon is a significant carcinogen, especially as a major cause of lung cancer in non-smokers. According to the World Health Organization, radon exposure accounts for approximately 3% to 14% of global lung cancer cases, with the variation largely dependent on regional radon levels and smoking prevalence [6]. Interestingly, despite its known hazards, radon has historically been used in balneotherapy. In this context, low-dose radon inhalation and water therapies have reportedly exhibited pain-relieving and anti-inflammatory properties [7].
Inflammation, a cornerstone of the body’s response to environmental insults, is a key mechanism linking radon exposure to broader health outcomes. Biomarkers such as C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) are central to the inflammatory cascade and serve as indicators of systemic inflammatory status [8,9]. These markers are not only pivotal in understanding inflammation’s role in disease progression but also valuable in assessing the impact of environmental exposures on human health. Multiple studies suggest that radon’s ionizing radiation may influence inflammatory pathways, contributing to pain reduction in clinical experience [10].
Although radon exposure and its health effects are often discussed, there is a notable absence of comprehensive reviews examining its association with inflammatory reactions. This systematic review seeks to bridge this gap by investigating inflammatory biomarkers across various biological fluids in individuals exposed to chronic levels of radon exposure. Furthermore, as a secondary aim, we conduct a meta-analysis of the most frequently analyzed inflammatory biomarkers based on our systematic review findings, specifically mean serum CRP and TNF-α levels, among participants with chronic residential radon (CRR) exposure. This dual approach not only bridges critical gaps in the literature but also offers insights to inform public health strategies and future research directions.
2. Materials and Methods
The study protocol has been registered with PROSPERO, the International Prospective Register of Systematic Reviews maintained by the National Institute for Health Research [11] (ID: CRD42025630827).
2.1. Search Strategy
An initial search of the PROSPERO database to identify registrations of comparable studies revealed no similar systematic review protocols. Subsequently, a systematic search was conducted across five literature databases: PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar. The search began on 21 November 2024 and concluded on 20 December 2024. The search strategy employed the following keywords: “radon” OR “radioactive gas” AND “inflammation biomarkers” OR “inflammatory markers”. No restrictions were placed on publication date; however, the results were limited to English-language publications and studies conducted on humans. Filters were applied, where available, to include only research articles and exclude other types of publications
2.2. Eligibility Criteria
The eligibility criteria for study inclusion were as follows: studies involving human participants, including both adults and children, with documented radon exposure in occupational or residential settings. Eligible studies were required to quantitatively measure radon exposure levels or provide background data on radon levels and assess inflammatory biomarkers in biological fluids such as serum, urine, alveolar fluid, or saliva. Original research articles presenting primary data, including cohort studies with cross-sectional data on inflammatory biomarkers, case–control studies, and cross-sectional studies published in peer-reviewed journals, were included. Studies were excluded if they focused on animal models or in vitro research; examined the efficacy of radon therapy in any form; or were published as reviews, conference abstracts, commentaries, editorials, or short communications. Additional exclusions applied to studies lacking information on inflammatory biomarkers and publications not available in English.
2.3. Selection of Studies and Data Extraction
The literature review and synthesis were carried out in adherence to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12]. Two authors (AL and YK) dependently screened the titles and abstracts of the search results, following the removal of duplicates, to assess their relevance. Full-text articles of studies that met the initial screening criteria were retrieved and evaluated against the pre-specified inclusion and exclusion criteria. Data extracted from the eligible studies included the first author’s last name, year of publication, study design, region and country, population description, sample size, age, type of radon exposure (residential or occupational), radon level assessment method, radon exposure level, biomarkers assessed (pro-inflammatory, anti-inflammatory, and others), and biomarker levels. Two authors independently performed the data extraction process. Any discrepancies were resolved through consultation with a third author (YO) to ensure consensus among all three authors involved in study selection and data extraction.
2.4. Risk of Bias (Quality) Assessment
The studies included in this review were assessed for risk of bias (quality) using the Newcastle–Ottawa Scale (NOS) for case–control studies and an adapted version for cross-sectional studies, as recommended by the Cochrane Non-Randomized Studies Methods Working Group [13]. The NOS for case–control studies evaluates each study based on eight criteria, which are divided into three main categories: Selection of study groups (four items), Comparability of these groups (one item), and Exposure (three items). Each criterion can receive up to one point, while the comparability criterion can be awarded a maximum of two points. The total score ranges from 0 to 9, with higher scores indicating better study quality.
For cross-sectional studies, the adapted NOS assesses each study across six criteria, divided into three main categories: Selection (three items), Comparability (one item), and Outcome (two items). Each criterion can receive up to one point, and the comparability criterion can be awarded a maximum of two points, resulting in a total score ranging from 0 to 7, with higher scores reflecting better study quality.
Risk of bias (quality) assessments were independently conducted by two authors (AL and YK) after they had agreed upon the assessment procedures. Inter-rater agreement between the two assessors was calculated by a third author (YO). In this review, studies scoring seven points or higher for case–control studies and five points or higher for cross-sectional studies were classified as having satisfactory quality and included in the systematic review [14,15].
2.5. Qualitative Data Synthesis
The data synthesis involved organizing the included studies to ensure a clear and systematic presentation of the findings. Studies were first sorted chronologically by publication year and then alphabetically by the last name of the first author. To facilitate a structured comparison, the studies were categorized based on the types of biological fluids assessed in participants, with the results presented in a table. For inflammation biomarkers, biomarker levels were reported separately for exposed and non-exposed groups, where such data were available, to highlight differences associated with radon exposure. Biomarkers were divided into pro-inflammatory (e.g., CRP, TNF-α, ICAM-1, IL-1β, IL-2, INF-γ, IL-6, IL-8, MPO, 8-epi-PGF2α, VCAM-1, MCP-1, P-selectin, TNFR-2, MIF, and VEGF), anti-inflammatory (e.g., IL-4 and IL-10), and other biomarkers.
2.6. Meta-Analysis
The pooled mean serum CRP and TNF-α levels for participants with high residential radon exposure were calculated using a random-effects model for meta-analysis in RStudio (version 4.3.2), employing the “meta” and “metafor” packages. The results from the random-effects model were visualized through forest plots. Heterogeneity among the studies was evaluated using the I2 statistic, accompanied by an influence analysis. Publication bias was assessed using Egger’s test, with results illustrated through funnel plots. A meta-regression analysis was conducted to examine the effect of age on the pooled mean serum CRP and TNF-α levels. A subgroup analysis was not performed due to the limited number of studies included in the meta-analysis.
2.7. Evaluation of Certainty of Evidence
We evaluated the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, following the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions [16]. The evaluation encompassed five domains: Risk of Bias, assessed via the NOS checklist described above; Inconsistency, gauged by the I2 statistic, with I2 > 75% labeled as “Serious”, I2 > 50% as “Moderate”, and I2 > 25% as “Not serious”; Indirectness, examined based on PICO criteria; Imprecision, determined by whether the 95% confidence interval (CI) of the pooled estimate crossed the threshold of interest; and Publication Bias, evaluated using funnel plots and Egger’s test. This assessment adhered to the procedures detailed in the research notes on GRADE evaluation in systematic reviews [17]. Certainty of evidence was calculated in RStudio, adhering to those guidelines and using the “grade” package.
3. Results
3.1. Included Study Characteristics
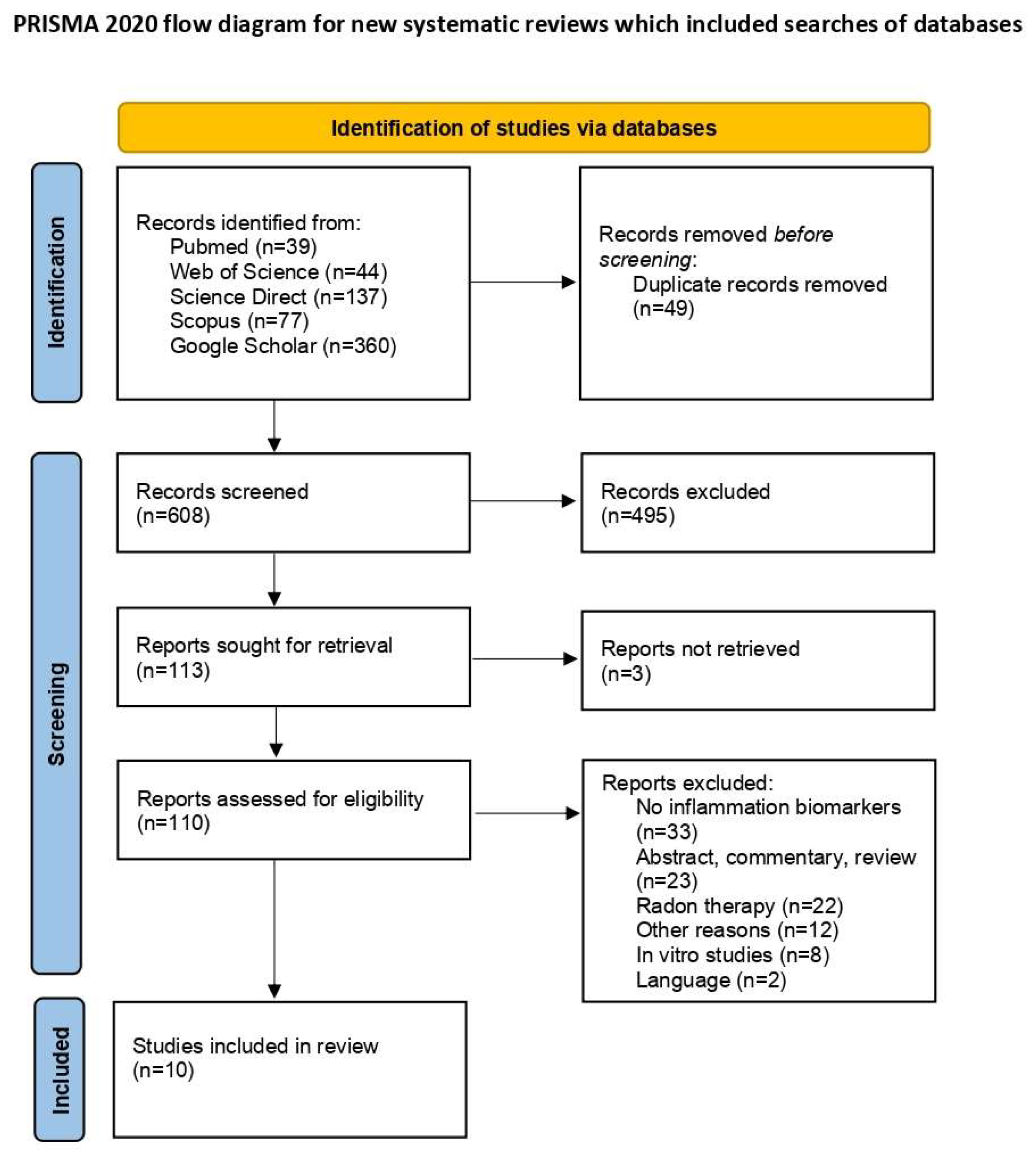
The initial database search identified 657 articles. After removing 49 duplicates, 608 unique articles remained for screening. Of these, 110 articles were selected for full-text review following the exclusion of 495 irrelevant titles and three articles for which full texts were unavailable. Upon further assessment, 10 articles met the inclusion criteria. The exclusions included 33 studies that lacked information on inflammatory biomarkers; 23 studies that were abstracts, commentaries, or reviews; 22 studies that focused on radon therapy rather than residential or occupational radon exposure; 12 studies excluded for other reasons; 8 in vitro studies; and 2 studies not published in English [18,19]. A PRISMA flow diagram detailing the study selection process is presented in Figure 1 [12].
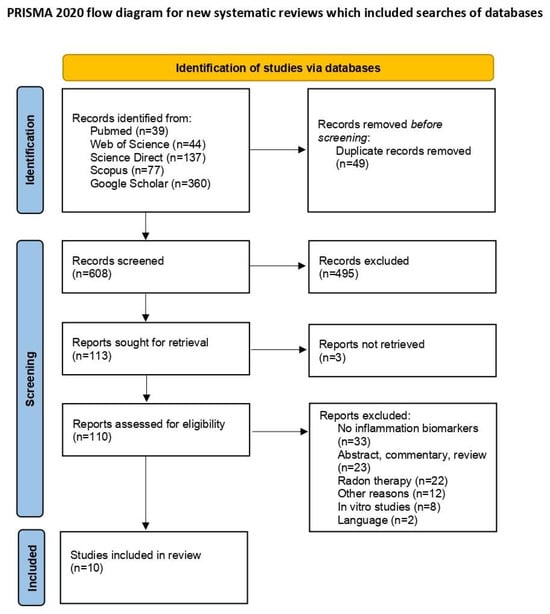
Figure 1.
PRISMA flow diagram of study selection process [12].
The included studies were published between 2000 and 2023. Five studies were conducted in the United States; two in Iran; and one study each in Germany, Thailand, and Indonesia. Eight studies focused on residential radon exposure, including one study that exclusively assessed children, while the others focused on adults. Two studies examined occupational radon exposure among uranium miners, one conducted in Germany and the other in the United States. Three studies included participants diagnosed with cancer. In total, 33,099 participants were assessed. Radon level assessments and the reported radon levels varied across the included studies. Eight studies assessed inflammation biomarker levels in serum, with one study assessing both serum and human bronchial epithelial cells. One study each assessed the bronchoalveolar lavage fluid and saliva. Detailed information on the included articles is provided in Table 1.

Table 1.
Description of the included studies.
3.2. Inflammation Biomarker Levels in Various Biological Fluids in Studies on Radon Exposure
Table 2 provides a summary of data on inflammation biomarker levels across various biological fluids, including serum, bronchoalveolar lavage fluid, and saliva. Six out of ten studies presented data on inflammation biomarker levels among radon-exposed and non-exposed participants. Of these, one study assessed biomarkers in bronchoalveolar lavage fluid, while the remaining studies evaluated them in serum. Among participants with CRR exposure, the most frequently assessed pro-inflammatory biomarkers in serum were CRP, reported in three studies and eight participant groups, and TNF-α, reported in three studies and four groups. These were followed by ICAM-1, assessed in two studies with three participant groups, IL-2 in two studies, INF-γ in two studies, and IL-6 in two studies. Other pro-inflammatory biomarkers assessed in serum included MPO in one study, 8-epi-PGF2α in one study, VCAM-1 in one study, MCP-1 in one study, P-selectin in one study, TNFR-2 in one study, MIF in one study, and VEGF in one study. Among the anti-inflammatory biomarkers in serum, IL-4 was assessed in three studies, while IL-10 was assessed in two studies. In bronchoalveolar lavage fluid, TNF-α was the only pro-inflammatory biomarker evaluated. In saliva, the assessed pro-inflammatory biomarkers included CRP, IL-1β, IL-6, IL-8, and TNF-α. Notably, no anti-inflammatory biomarkers were assessed in either bronchoalveolar lavage fluid or saliva.

Table 2.
Summary of inflammation biomarker levels in various biological fluids.
3.3. Risk of Bias (Quality) Assessment
All the case–control studies included had a NOS score of 7 or higher out of 8, while all cross-sectional studies had a NOS score of 6 out of 7. These scores suggest that the studies were of high quality and had a low risk of bias, as shown in Table 3.

Table 3.
Newcastle–Ottawa risk of bias (quality) assessment results.
3.4. Meta-Analysis of Mean Serum CRP and TNF-α Levels Among Participants with Chronic Residential Radon Exposure
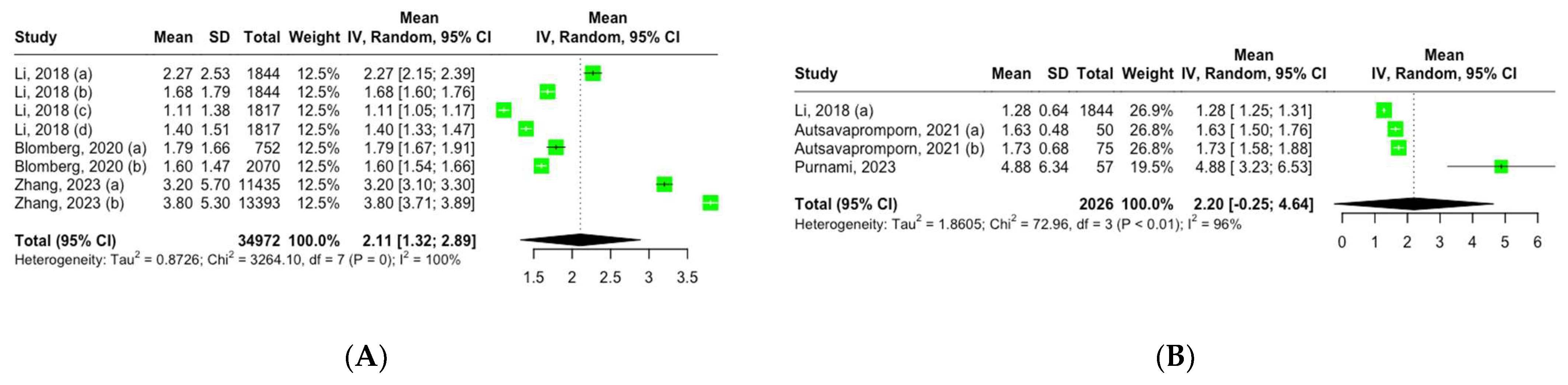
Three studies, encompassing eight groups, reported the mean serum CRP levels among adult participants with CRR exposure. Using a random-effects model, the pooled mean CRP level across these groups was 2.11 mg/L (95% CI, 1.32–2.89), showing high heterogeneity: I2 = 100%, Q (df = 7) = 3264, p = 0 (Figure 2A).
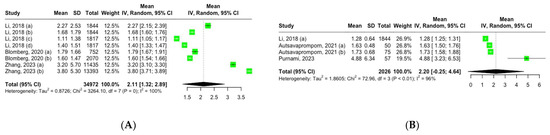
Figure 2.
Forest plot of mean inflammation biomarker levels among participants with chronic residential radon exposure: (A) CRP levels; (B) TNF-α levels. Abbreviations: SD—standard deviation. Group definitions: Li, 2018 (a) [26] —offspring cohort cycle 7; Li, 2018 (b) [26] —offspring cohort cycle 8; Li, 2018 (c) [26]—third-generation cohort cycle 1; Li, 2018 (d) [26]—third-generation cohort cycle 2; Blomberg, 2020 (a) [27] —first visit; Blomberg, 2020 (b) [27] —all visits; Zhang, 2023 (a) [30] —never smokers; Zhang, 2023 (b) [30] —ever smokers; Autsavapromporn, 2021 (a) [28] —high-residential-radon-exposure participants; Autsavapromporn, 2021 (b) [28] —high-residential-radon-exposure participants with lung cancer.
Three studies comprising four groups reported the mean serum TNF-α levels among adult participants with CRR exposure. Using a random-effects model, the pooled mean TNF-α level across these groups was 2.20 pg/mL (95% CI, 0.25–4.64), showing high heterogeneity: I2 = 96%, Q (df = 3) = 72.96, p < 0.01 (Figure 2B).
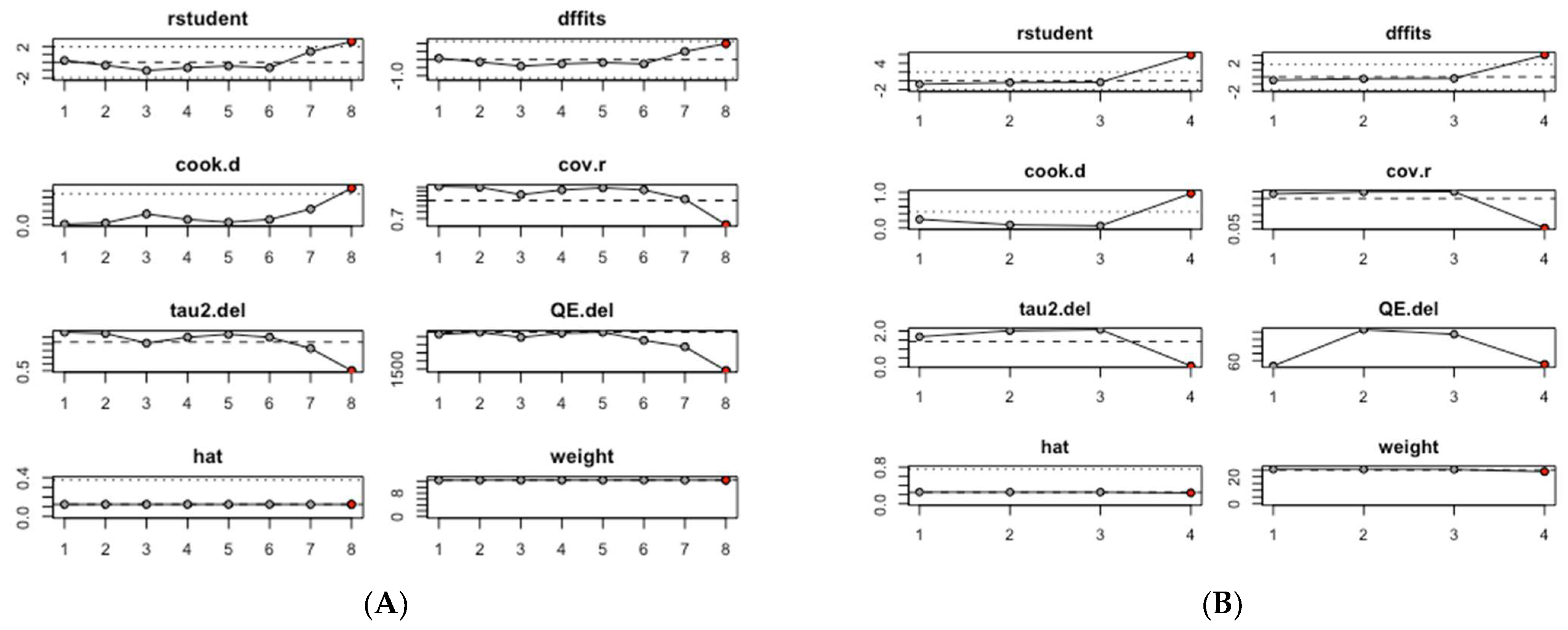
An influence analysis was conducted to identify which studies had the greatest impact on the pooled estimates of mean serum CRP and TNF-α levels. Among the studies assessing serum CRP levels, the pooled mean CRP value was predominantly influenced by study #8, Zhang, 2023 (b) [30], which focused on ever smokers (Figure 3A). Similarly, in the group of studies evaluating serum TNF-α levels, the pooled mean TNF-α value was primarily influenced by study #4, Purnami, 2023 [29] (Figure 3B).
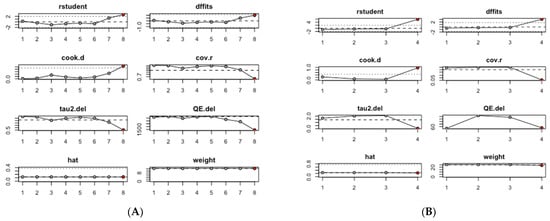
Figure 3.
Influence analysis of mean inflammation biomarker levels among participants with chronic residential radon exposure: (A) CRP levels; (B) TNF-α levels.
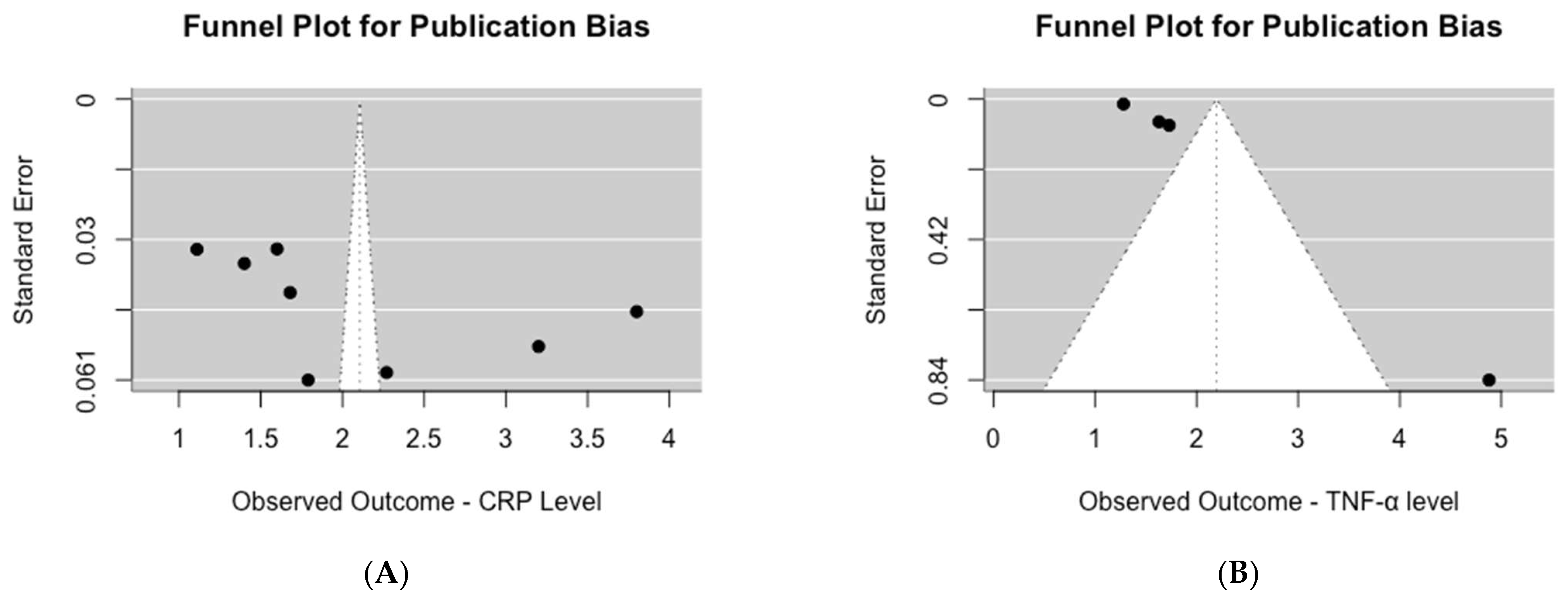
Upon visual inspection of the funnel plot for studies assessing serum CRP levels, asymmetry was evident (Figure 4A). This observation was further supported by the significant results of Egger’s test for publication bias (p = 0.04). Similarly, for studies evaluating serum TNF-α levels, visual inspection of the funnel plot also revealed asymmetry (Figure 4B), which was confirmed by the highly significant results of Egger’s test for publication bias (p < 0.0001).
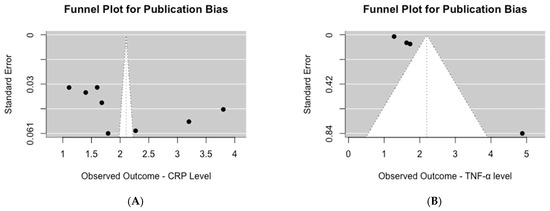
Figure 4.
Publication bias assessment of mean inflammation biomarker levels among participants with chronic residential radon exposure: (A) CRP levels; (B) TNF-α levels.
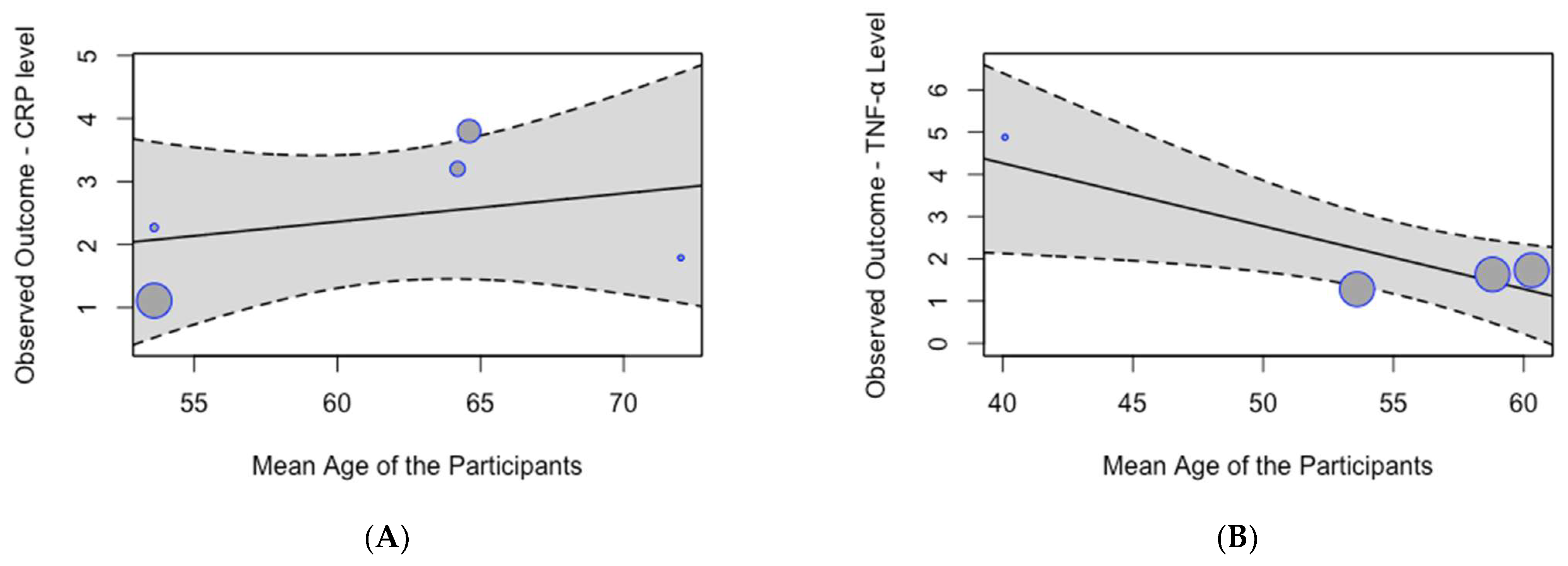
A meta-regression analysis did not identify a significant effect of age on the pooled mean serum CRP levels (p = 0.63; Figure 5A). However, the analysis demonstrated a significant effect of age on the pooled mean serum TNF-α levels (p = 0.01; Figure 5B), indicating that mean serum TNF-α levels decrease with age.
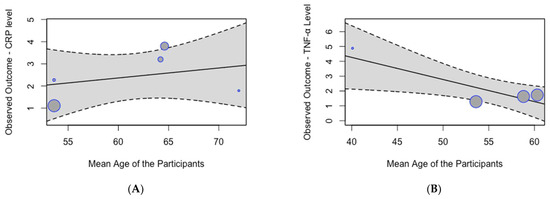
Figure 5.
Meta-regression analysis of mean inflammation biomarker levels among participants with chronic residential radon exposure by age: (A) CRP levels; (B) TNF-α levels.
3.5. Evaluation of Certainty of Evidence
The findings from the GRADE certainty assessment, as detailed in Table 4, indicate that the pooled mean levels of CRP and TNF-α exhibit a low degree of certainty. Consequently, these results should be interpreted with caution.

Table 4.
Evaluation of evidence certainty using the GRADE framework.
4. Discussion
Our systematic review aimed to investigate the inflammatory biomarkers present in various biological fluids among individuals exposed to chronic levels of radon and to conduct a meta-analysis of the mean serum CRP and TNF-α levels in participants with CRR exposure. Inflammatory biomarkers were assessed in serum, bronchoalveolar lavage fluid, and saliva. Among participants with CRR exposure, the most frequently evaluated pro-inflammatory biomarkers in serum were CRP and TNF-α, followed by ICAM-1, IL-2, INF-γ, IL-6, MPO, 8-epi-PGF2α, VCAM-1, MCP-1, P-selectin, TNFR-2, MIF, and VEGF. For anti-inflammatory biomarkers in serum, IL-4 and IL-10 were analyzed. In bronchoalveolar lavage fluid, TNF-α was assessed, while in saliva, the evaluated pro-inflammatory biomarkers included CRP, IL-1β, IL-6, IL-8, and TNF-α. No anti-inflammatory biomarkers were assessed in either bronchoalveolar lavage fluid or saliva.
This systematic review and meta-analysis also revealed an association between CRR exposure and low levels of inflammatory biomarkers, particularly CRP and TNF-α. The pooled mean serum CRP level among participants with CRR exposure was 2.11 mg/L (95% CI, 1.32–2.89), while the pooled mean serum TNF-α level was 2.20 pg/mL (95% CI, 0.25–4.64). Based on the low certainty of evidence using the GRADE framework, the findings of this study should be interpreted with caution. Radon is a well-established carcinogen known to induce oxidative stress and DNA damage. However, our findings suggest that radon exposure may be associated with reduced systemic inflammation, although the mechanisms underlying this observation remain unclear. Importantly, these results should not be interpreted as evidence of a direct anti-inflammatory effect of radon but, rather, as an indication of a complex biological response that warrants further investigation.
To better understand the potential impact of radon on lung-specific inflammation, we propose a hypothetical scheme linking radon exposure to changes in CRP and TNF-α levels in the lung. Radon, as an alpha particle emitter, induces oxidative stress and DNA damage in lung tissue, which may trigger a cascade of cellular responses [34]. Initially, radon-induced DNA damage activates repair mechanisms and inflammatory pathways, leading to the release of pro-inflammatory cytokines such as TNF-α [35]. However, with chronic exposure, the sustained oxidative stress and DNA damage may lead to immune modulation, characterized by a downregulation of inflammatory responses as a protective mechanism [36]. This could explain the observed reductions in serum CRP and TNF-α levels in individuals with CRR exposure. Additionally, radon exposure may alter the lung microenvironment, affecting the production and clearance of these biomarkers. For instance, radon-induced epithelial damage could impair the local production of TNF-α, while systemic immune suppression might reduce CRP synthesis in the liver [37]. This proposed scheme highlights the need for future studies to investigate the temporal dynamics of biomarker changes and to explore the role of lung-specific mechanisms in mediating the observed effects.
Our findings suggest a complex link between radon exposure and its impact on human health. While chronic radon exposure appears to reduce levels of certain inflammatory biomarkers, potentially indicating anti-inflammatory effects, it remains strongly linked to an elevated risk of lung cancer. The health risks of radon are mainly due to its ionizing radiation, which causes DNA damage and triggers carcinogenic processes [6]. The International Commission on Radiological Protection (ICRP) highlights that even minimal exposure to radon carries a lung cancer risk, underscoring its critical public health importance and recommending an integrated strategy for controlling radon exposure [38]. A recent meta-analysis of fifty-five studies confirms that CRR exposure is associated with elevated incidence of lung cancer and pediatric leukemia [39]. The paradoxical association can be partially explained by the dual biological effects of radon’s ionizing radiation, which may suppress systemic inflammation while concurrently inducing DNA damage, chromosomal abnormalities, and alterations in the cell cycle [34]. Furthermore, low levels of inflammatory biomarkers such as CRP and TNF-α observed in radon-exposed individuals may reflect an adaptive response or immune suppression, which could inadvertently compromise the body’s ability to detect and eliminate cancerous cells [10]. The observed reduction in inflammatory biomarkers could also explain the therapeutic application of radon in the form of hot spas for patients with degenerative and inflammatory conditions accompanied by pain [10]. However, in scenarios involving non-chronic exposure relevant to radon therapy for chronic inflammatory diseases, there is a complete lack of epidemiological data to estimate the associated cancer risk [10].
The findings of this study have important practical implications. Future research should prioritize longitudinal studies designed to evaluate the potential benefits and risks of radon therapy for chronic inflammatory diseases. These studies should systematically measure the effects of controlled radon exposure on inflammatory biomarkers while simultaneously monitoring long-term cancer incidence rates. Additionally, experimental studies could elucidate the underlying biological mechanisms by which low-dose, short-term radon exposure exerts anti-inflammatory effects, potentially through pathways involving oxidative stress modulation or immune response alteration. To ensure robust and generalizable findings, future investigations should focus on diverse populations, including individuals with pre-existing inflammatory conditions, varying genetic predispositions, and different environmental radon exposure baselines.
This study has several limitations. First, the included studies exhibited significant heterogeneity in radon exposure assessment methods, biomarker measurement techniques, and participant demographics. Consequently, we were unable to compare high- and low-radon-exposure groups effectively. Second, the small number of studies included in the meta-analysis limits the generalizability of the findings. Third, publication bias, as indicated by Egger’s test, suggests that studies reporting null or negative findings may be underrepresented. Fourth, the cross-sectional nature of most included studies precludes causal inferences. Lastly, this study does not account for confounding factors that could influence inflammatory biomarker levels independently of radon exposure, such as smoking status, occupational exposure of residential participants, pre-existing medical conditions, and dietary habits, among others. These confounders may introduce variability into the observed relationships between radon exposure and inflammatory biomarkers, potentially limiting the interpretability of the findings. The included articles lacked uniform and comprehensive information on the aforementioned confounders, which precluded our ability to systematically collect and analyze these variables in the context of the present analysis.
5. Conclusions
In summary, this systematic review emphasizes that CRP and TNF-α are the most commonly studied pro-inflammatory biomarkers in serum among individuals exposed to high levels of residential or occupational radon. The meta-analysis findings reveal that these biomarkers are lower in adults with CRR exposure, pointing to potential anti-inflammatory effects, even as radon remains a well-recognized carcinogen. These insights highlight the complex health implications of radon exposure, extending beyond its established link to cancer. Moving forward, it is essential for future research to focus on longitudinal studies using standardized methods to better understand the long-term health impacts of short-term radon exposure used for health benefits. By addressing these research gaps, we can deepen our understanding of radon’s diverse health effects and create a stronger foundation for informed public health strategies.
Author Contributions
Conceptualization, A.L. and Y.K.; data curation, A.L. and Y.O.; formal analysis, A.L. and Y.O.; funding acquisition, Y.K. and M.B.; methodology, A.L., Y.O., M.B., P.K., S.T., N.A., D.I. and Y.K.; writing—original draft preparation, A.L., Y.O., M.B., P.K., S.T., N.A., D.I. and Y.K.; writing—review and editing, A.L., Y.O., M.B., P.K., S.T., N.A., D.I. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number IRN AP22786933, “Impact of Technogenic Radiation Factors on the Development of Tumor and Non-Tumor Bronchopulmonary Diseases in the Population of Northern Kazakhstan Based on Molecular-Genetic Analysis” (2024–2026). This research was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number 23KK0095).
Institutional Review Board Statement
Ethical review and approval were waived for this study as this is a systematic review of the published literature.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 8-epi-PGF2α | 8-epi-prostaglandin F2 alpha |
| CANC | Patients with lung cancer |
| CON | Healthy controls |
| CRP | C-reactive protein |
| CRR | Chronic residential radon |
| EPA | Environmental Protection Agency |
| FIBR | Patients with lung asbestosis or fibrosis of the lung |
| HRR | High residential radon |
| ICAM-1 | Intercellular adhesion molecule 1 |
| ICRP | International Commission on Radiological Protection |
| IL-1β | Interleukin 1 beta |
| IL-2 | Interleukin 2 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| INF-γ | Interferon gamma |
| LRR | Low residential radon |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MIF | Macrophage migration inhibitory factor |
| MPO | Myeloperoxidase |
| NAS | Normative Aging Study |
| P-selectin | Platelet selectin |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SD | Standard deviation |
| TNF-α | Tumor necrosis factor alpha |
| TNFR-2 | Tumor necrosis factor receptor 2 |
| URAN | Uranium miners |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| WLM | Working level month |
References
- Cinelli, G.; De Cort, M.; Tollefsen, T.; Achatz, M.; Ajtic, J.; Ballabio, C.; Barnet, I.; Bochicchio, F.; Borelli, P.; Bossew, P.; et al. European Atlas of Natural Radiation; Cinelli, G., De Cort, M., Tollefsen, T., Eds.; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Grzywa-Celińska, A.; Krusiński, A.; Mazur, J.; Szewczyk, K.; Kozak, K. Radon—The Element of Risk. The Impact of Radon Exposure on Human Health. Toxics 2020, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Vogiannis, E.G.; Nikolopoulos, D. Radon Sources and Associated Risk in Terms of Exposure and Dose. Front. Public Health 2015, 2, 93477–93487. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Panigrahi, D.C.; Mishra, D.P. A Comprehensive Review on Sources of Radon and Factors Affecting Radon Concentration in Underground Uranium Mines. Environ. Earth Sci. 2016, 75, 617. [Google Scholar] [CrossRef]
- Tirmarche, M.; Harrison, J.; Laurier, D.; Blanchardon, E.; Paquet, F.; Marsh, J. Risk of Lung Cancer from Radon Exposure: Contribution of Recently Published Studies of Uranium Miners. Ann. ICRP 2012, 41, 368–377. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Fact Sheets: Radon. Available online: https://www.who.int/news-room/fact-sheets/detail/radon-and-health (accessed on 8 January 2025).
- Pawlik-Sobecka, L.; Górka-Dynysiewicz, J.; Kuciel-Lewandowska, J. Balneotherapy with the Use of Radon–Sulphide Water: The Mechanisms of Therapeutic Effect. Appl. Sci. 2021, 11, 2849. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 342848–342857. [Google Scholar] [CrossRef]
- Maier, A.; Wiedemann, J.; Rapp, F.; Papenfuß, F.; Rödel, F.; Hehlgans, S.; Gaipl, U.S.; Kraft, G.; Fournier, C.; Frey, B. Radon Exposure—Therapeutic Effect and Cancer Risk. Int. J. Mol. Sci. 2020, 22, 316. [Google Scholar] [CrossRef]
- National Institute for Health and Care Research PROSPERO. International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 27 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 November 2024).
- Karibayeva, I.; Bilibayeva, G.; Yerzhanova, A.; Alekesheva, R.; Iglikova, A.; Maxudova, M.; Ussebayeva, N. Prevalence of Vitamin D Deficiency Among Adults in Kazakhstan: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 2043. [Google Scholar] [CrossRef]
- Breau, G.; Ellis, U. Risk Factors Associated With Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control 2020, 27, 1073274820976670. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; Cochrane GRADEing Methods Group. Assessing Certainty in the Evidence in the Context of a Systematic Review. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2022; Version 6.3. [Google Scholar]
- Brennan, S.E.; Johnston, R.V. Research Note: Interpreting Findings of a Systematic Review Using GRADE Methods. J. Physiother. 2023, 69, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Nikonorov, M.V.; Boriak, V.P. Updated Assessment of Efficacy of Dry Air Radon Baths of Different Concentrations at the Stage of Medical Rehabilitation of Patients with Bronchial Asthma. Vopr. Kurortol. Fizioter. I Lech. Fiz. Kult. 2006, 4, 15–18. [Google Scholar]
- Haustein, U.F. Silica-induced scleroderma in miners in former uranium ore mining (Wismut AG). Der Hautarzt 2021, 72, 644–646. [Google Scholar] [CrossRef]
- Attar, M.; Kondolousy, Y.M.; Khansari, N. Effect of High Dose Natural Ionizing Radiation on the Immune System of the Exposed Residents of Ramsar Town, Iran. Iran. J. Allergy Asthma Immunol. 2007, 6, 73–78. [Google Scholar]
- Ghiassi-Nejad, M.; Mortazavi, S.M.J.; Cameron, J.R.; Niroomand-Rad, A.; Karam, P.A. Very High Background Radiation Areas of Ramsar, Iran: Preliminary Biological Studies. Health Phys. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Yousefian, F.; Nasiri, Z.; Kordi, M.; Marzi, Y.G.; Dehghani, R.; Mirzaei, N.; Janjani, H.; Aghaei, M.; Aboosaedi, Z. Indoor Radon and Its Health Risk Assessment in Iran: A Comprehensive Review Study. Indoor Air 2024, 2024, 2300116. [Google Scholar] [CrossRef]
- Sohrabi, M.; Babapouran, M. New Public Dose Assessment from Internal and External Exposures in Low- and Elevated-Level Natural Radiation Areas of Ramsar, Iran. Int. Congr. Ser. 2005, 1276, 169–174. [Google Scholar] [CrossRef]
- Molaie, Y.; Latifynia, A.; Kalamzadeh, A.; Abofazeli, T.; Nuraie, M.; Khansarii, N. Phagocyte Functions of Human Subjects Living in High Level of Natural Radiation Areas in Iran—PubMed. J. Ayub Med. Coll. Abbottabad 2012, 24, 177–179. [Google Scholar]
- Leng, S.; Thomas, C.L.; Snider, A.M.; Picchi, M.A.; Chen, W.; Willis, D.G.; Carr, T.G.; Krzeminski, J.; Desai, D.; Shantu, A.; et al. Radon Exposure, IL-6 Promoter Variants, and Lung Squamous Cell Carcinoma in Former Uranium Miners. Environ. Health Perspect. 2015, 124, 445. [Google Scholar] [CrossRef]
- Li, W.; Nyhan, M.M.; Wilker, E.H.; Vieira, C.L.Z.; Lin, H.; Schwartz, J.D.; Gold, D.R.; Coull, B.A.; Aba, A.M.; Benjamin, E.J.; et al. Recent Exposure to Particle Radioactivity and Biomarkers of Oxidative Stress and Inflammation: The Framingham Heart Study. Environ. Int. 2018, 121, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A.J.; Nyhan, M.M.; Bind, M.A.; Vokonas, P.; Coull, B.A.; Schwartz, J.; Koutrakis, P. The Role of Ambient Particle Radioactivity in Inflammation and Endothelial Function in an Elderly Cohort. Epidemiology 2020, 31, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Autsavapromporn, N.; Klunklin, P.; Chitapanarux, I.; Jaikang, C.; Chewaskulyong, B.; Sripan, P.; Hosoda, M.; Tokonami, S. A Potential Serum Biomarker for Screening Lung Cancer Risk in High Level Environmental Radon Areas: A Pilot Study. Life 2021, 11, 1273. [Google Scholar] [CrossRef] [PubMed]
- Purnami, S.; Ramadhani, D.; Oktariyani, T.A.; Suvifan, V.A.; Tetriana, D.; Sugoro, I.; Rahajeng, N.; Wanandi, S.I.; Wibowo, H.; Yamaguchi, M.; et al. Immune Status of People Living in the Tande-Tande Sub-Village (Indonesia), an Area with High Indoor Radon Concentration. Radiat. Environ. Biophys. 2023, 62, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Q.; Angley, M.; Lu, L.; Miller, E.C.; Judd, S.E.; Field, R.W.; Kahe, K. Smoking Modifies the Association Between Radon Exposure and Incident Ischemic Stroke: The REGARDS Study. Stroke 2023, 54, 2737–2744. [Google Scholar] [CrossRef]
- Popp, W.; Plappert, U.; Müller, W.U.; Rehn, B.; Schneider, J.; Braun, A.; Bauer, P.C.; Vahrenholz, C.; Presek, P.; Brauksiepe, A.; et al. Biomarkers of Genetic Damage and Inflammation in Blood and Bronchoalveolar Lavage Fluid among Former German Uranium Miners: A Pilot Study. Radiat. Environ. Biophys. 2000, 39, 275–282. [Google Scholar] [CrossRef]
- Walsh, L.; Grosche, B.; Schnelzer, M.; Tschense, A.; Sogl, M.; Kreuzer, M. A Review of the Results from the German Wismut Uranium Miners Cohort. Radiat. Prot. Dosim. 2015, 164, 147–153. [Google Scholar] [CrossRef]
- Taylor, B.K.; Smith, O.T.V.; Miller, G.E. Chronic Home Radon Exposure Is Associated with Higher Inflammatory Biomarker Concentrations in Children and Adolescents. Int. J. Environ. Res. Public Health 2022, 20, 246. [Google Scholar] [CrossRef]
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The Cellular and Molecular Carcinogenic Effects of Radon Exposure: A Review. Int. J. Mol. Sci. 2013, 14, 14024–14034. [Google Scholar] [CrossRef]
- Xin, L.; Sun, J.; Zhai, X.; Chen, X.; Wan, J.; Tian, H. Repeated Radon Exposure Induced Lung Damage via Oxidative Stress-Mediated Mitophagy in Human Bronchial Epithelial Cells and Mice. Environ. Toxicol. Pharmacol. 2022, 90, 103812. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Bailey, T.; Hinrichs, A.; Lerchl, S.; Newman, R.T.; Fournier, C.; Vandevoorde, C. Experimental Setups for In Vitro Studies on Radon Exposure in Mammalian Cells—A Critical Overview. Int. J. Environ. Res. Public Health 2023, 20, 5670. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP) Radiological Protection against Radon Exposure. Ann. ICRP 2014, 126, 1–6.
- Ngoc, L.T.N.; Park, D.; Lee, Y.C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case–Control Studies. Int. J. Environ. Res. Public Health 2023, 20, 97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).