Genetic Profiling of MC3T3-E1 Cells in Different Media: Implications for In Vitro Screening Development
Abstract
1. Introduction
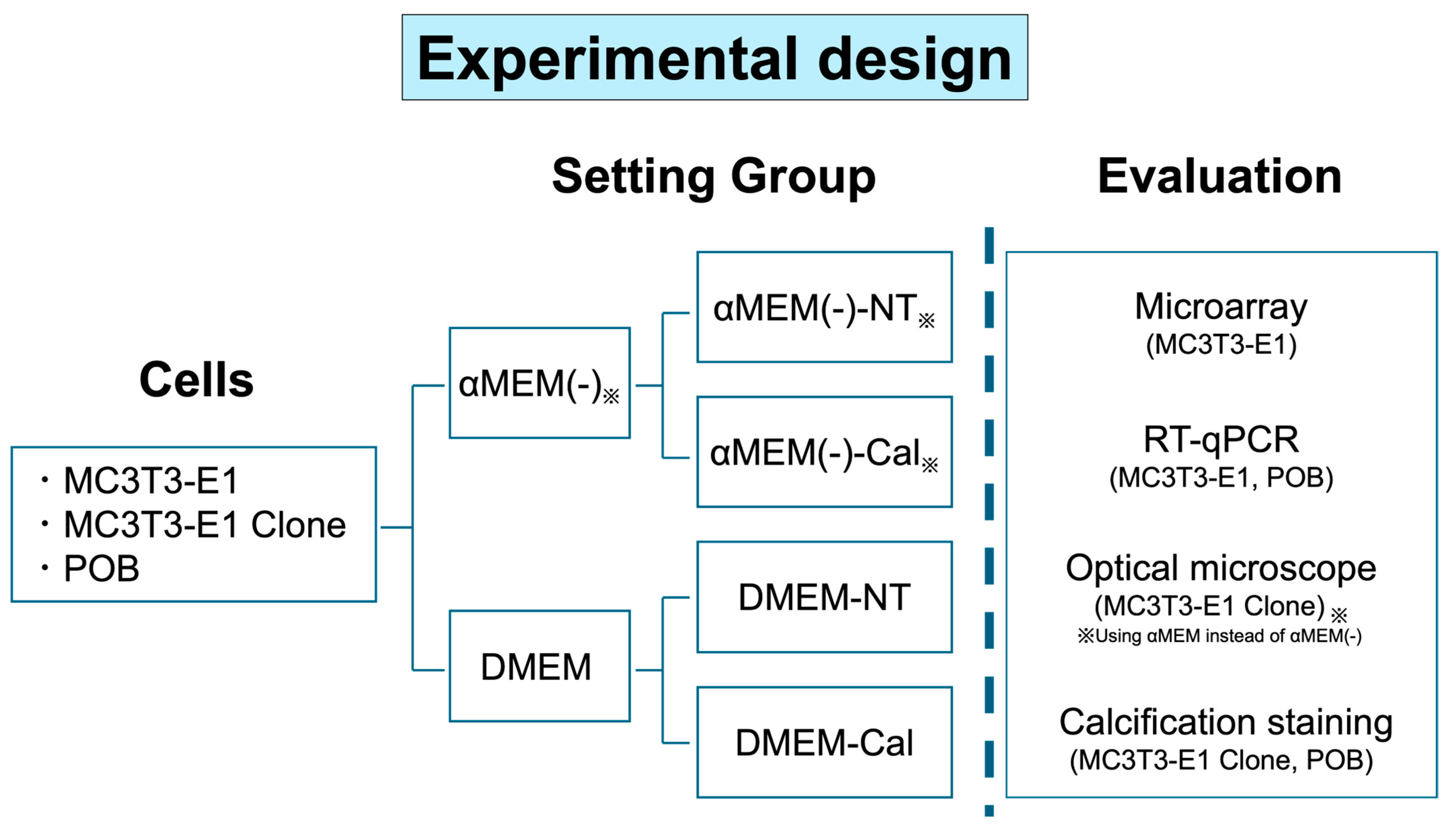
2. Materials and Methods
2.1. Cells
2.1.1. MC3T3-E1
2.1.2. MC3T3-E1 Clone
2.1.3. Primary Osteoblasts
2.2. DNA Microarray
2.3. RT-Quantitative PCR
2.4. Optical Microscopy
2.5. Calcification Evaluation
2.6. Statistical Analysis
3. Results
3.1. Comprehensive Gene Analysis of MC3T3-E1 Cells Cultured in Different Media After Calcification Stimulation
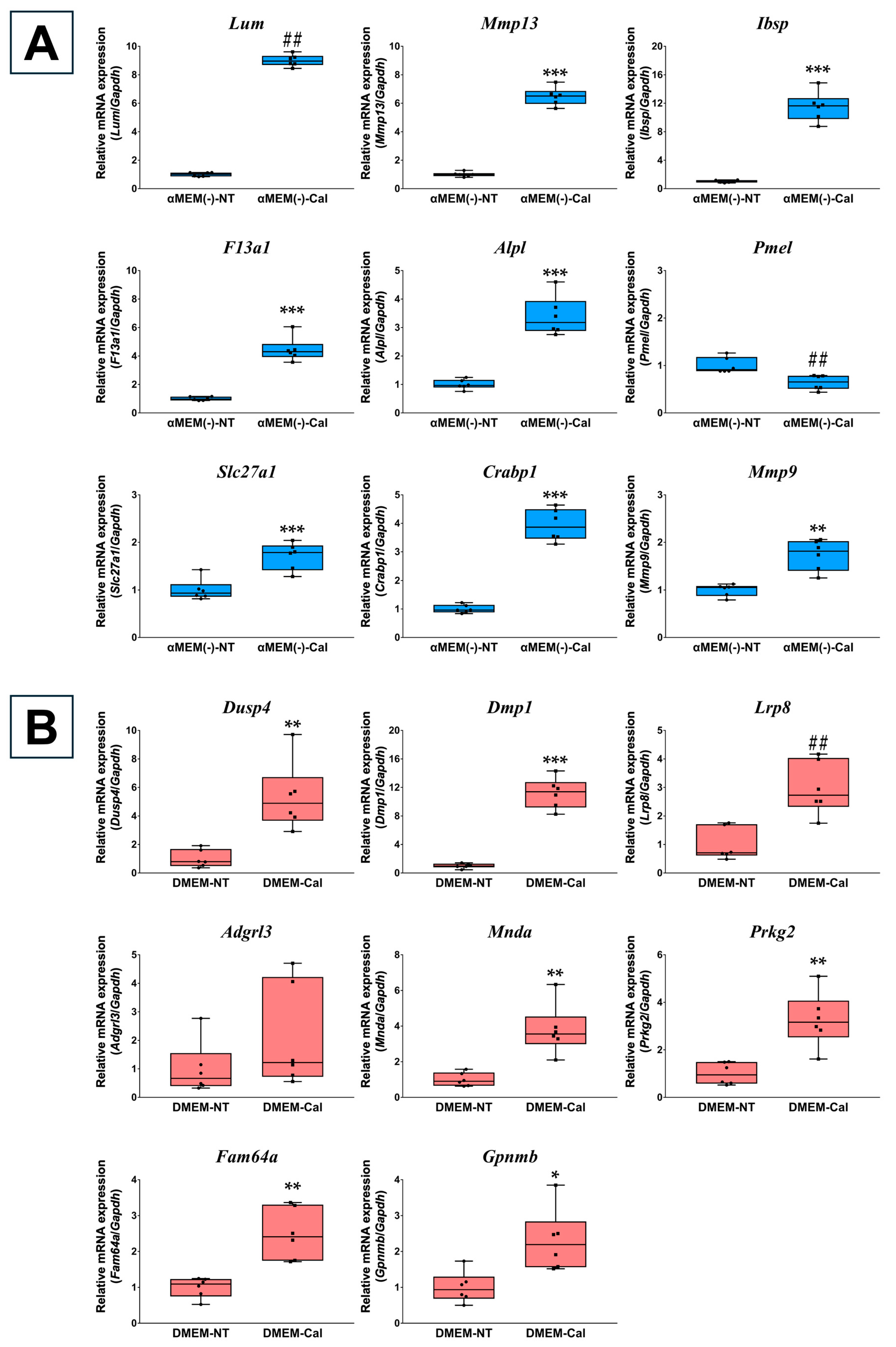
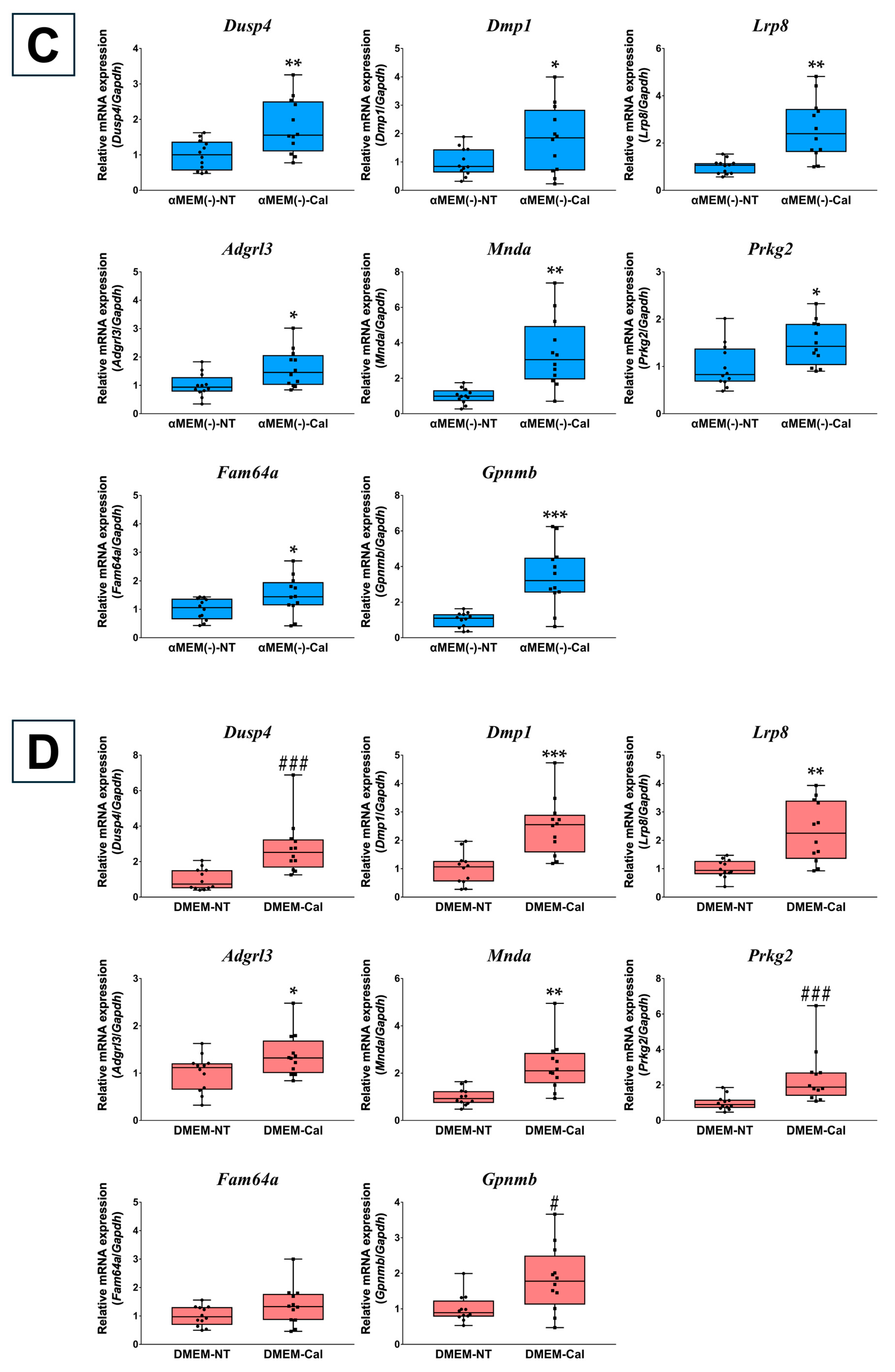
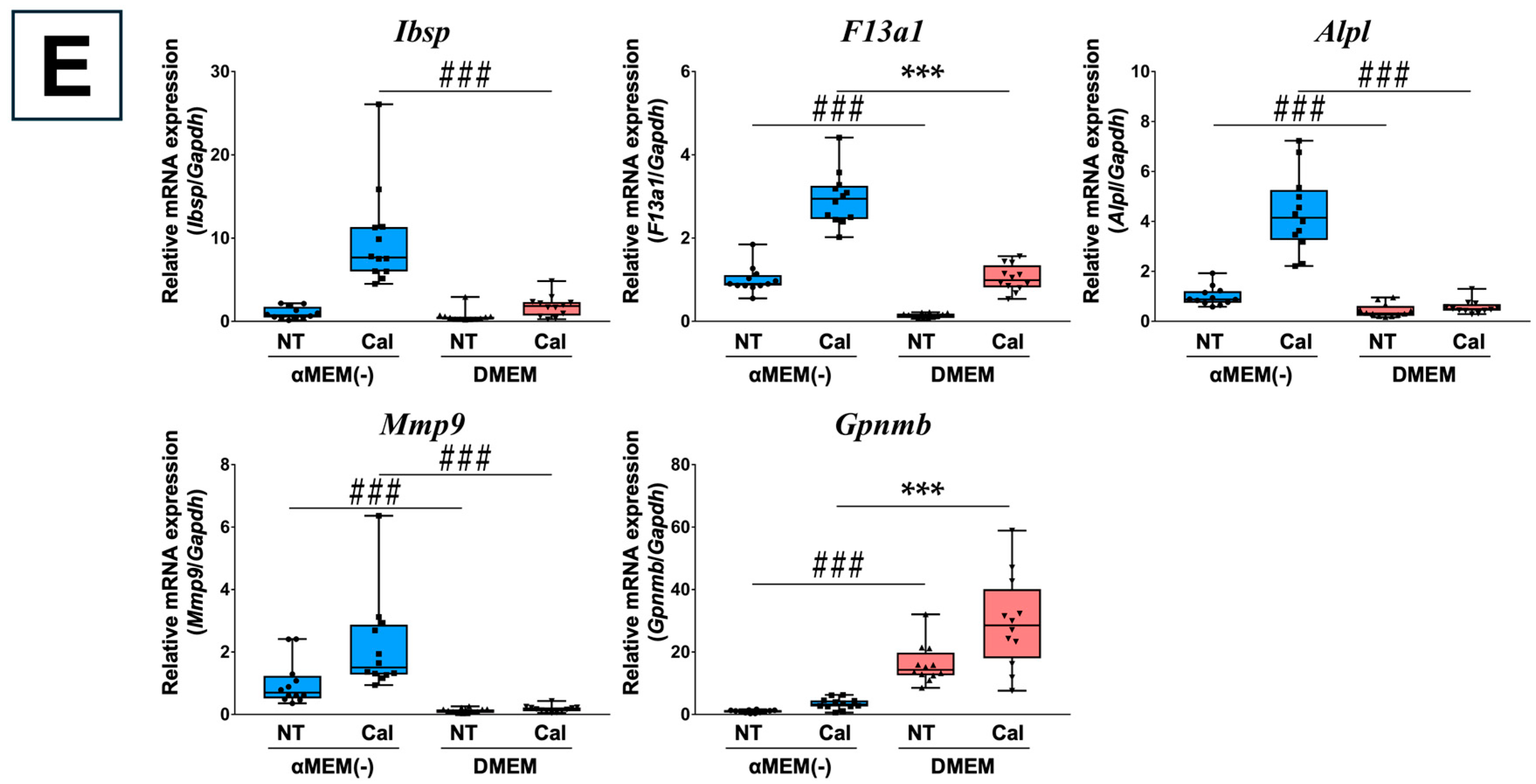
3.2. Validation of Microarray Data Using RT-qPCR
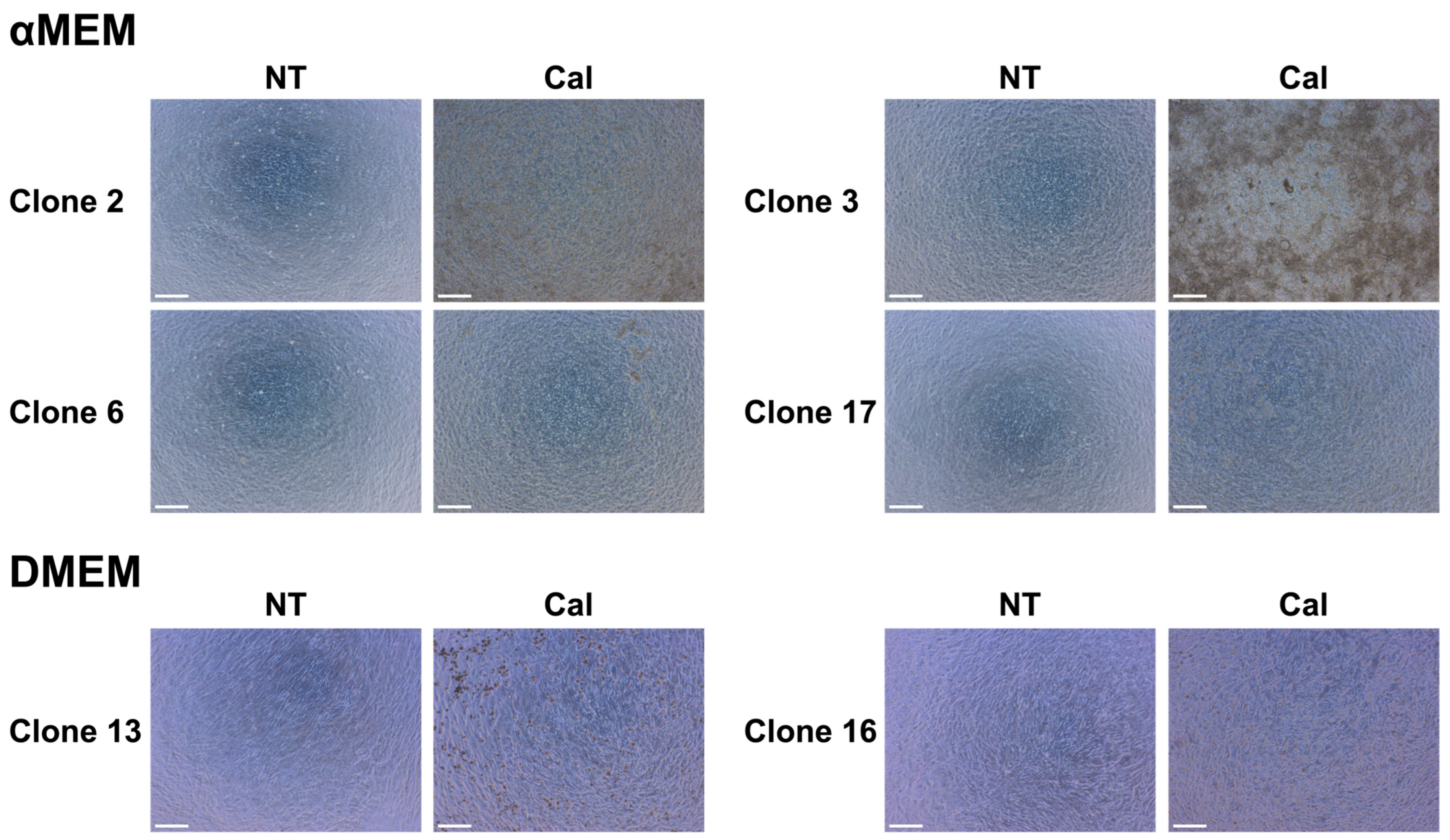
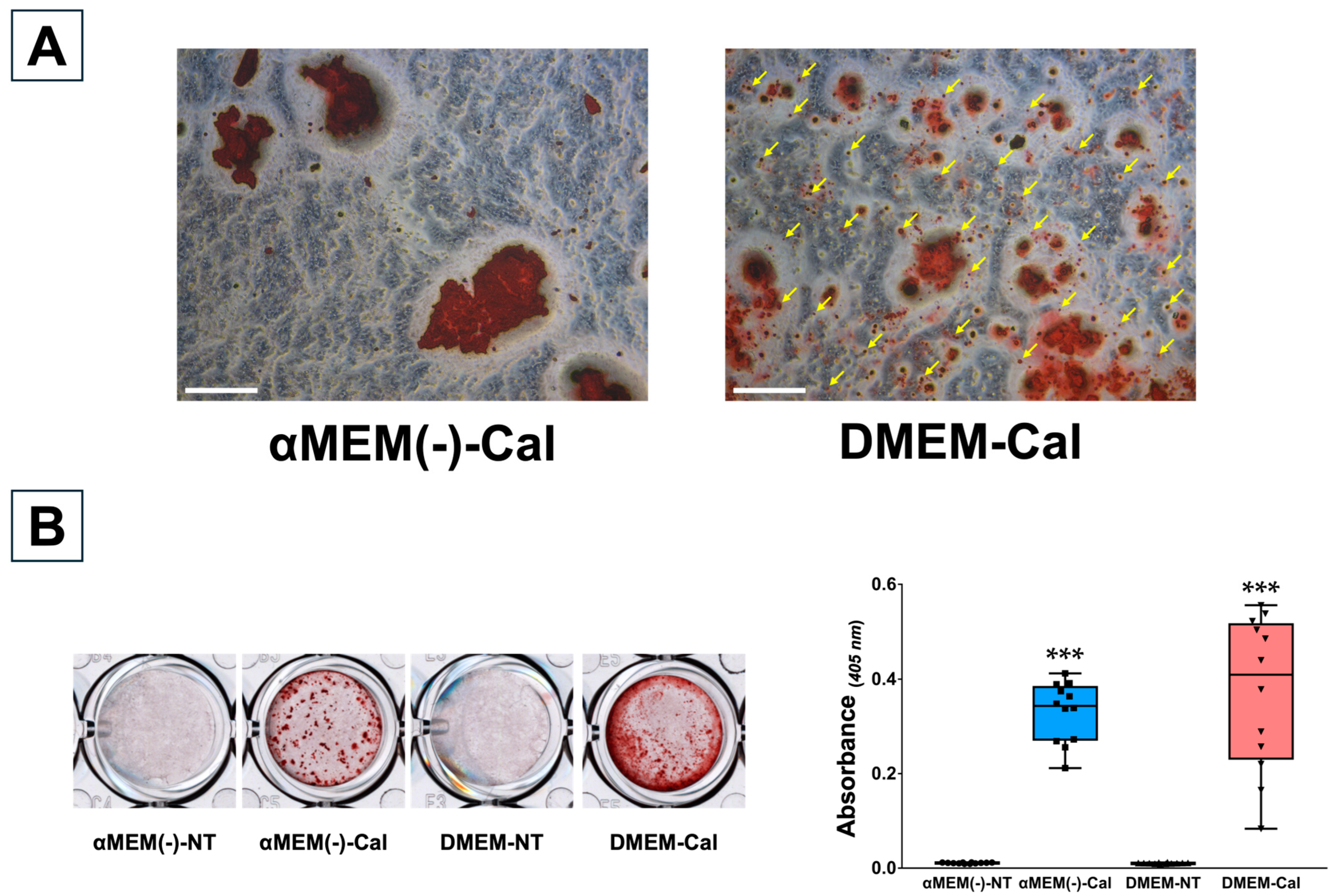
3.3. Optical Microscopy of MC3T3-E1 Clone Cells Cultured in Different Media
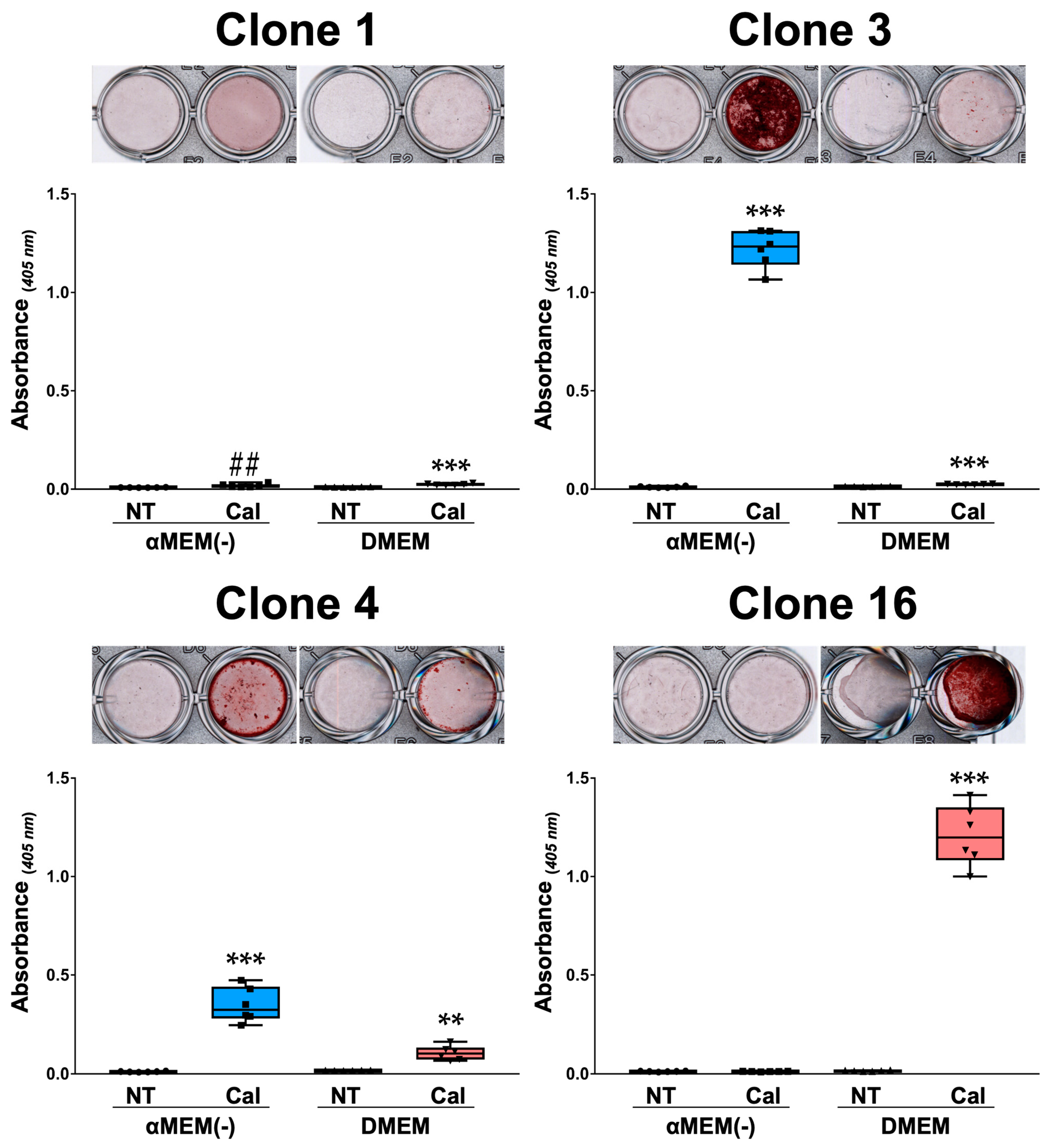
3.4. Calcification of MC3T3-E1 Clone Cells in Different Media
3.5. RT-qPCR Verification of Primary Osteoblasts Derived from Calvaria
3.6. Calcification Response of Primary Osteoblasts Derived from Calvaria in Different Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| αMEM | α Minimum Essential Medium |
| αMEM(−) | α Minimum Essential Medium without L-ascorbic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| FBS | Fetal bovine serum |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| POB | Primary osteoblast |
| POBs | Primary osteoblasts |
References
- Stein, M.; Elefteriou, F.; Busse, B.; Fiedler, I.A.; Kwon, R.Y.; Farrell, E.; Ahmad, M.; Ignatius, A.; Grover, L.; Geris, L.; et al. Why animal experiments are still indispensable in bone research: A statement by the European calcified tissue society. J. Bone Miner. Res. 2023, 38, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Amagai, Y.; Sudo, H.; Kasai, S.; Yamamoto, S. Establishment of a clonal osteogenic cell line from newborn mouse calvaria. Jpn. J. Oral Biol. 1981, 23, 899–901. [Google Scholar] [CrossRef]
- Mendes, S.C.; Tibbe, J.M.; Veenhof, M.; Both, S.; Oner, F.C.; van Blitterswijk, C.A.; de Bruijn, J.D. Relation between in vitro and in vivo osteogenic potential of cultured human bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2004, 15, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Hatt, L.P.; Armiento, A.R.; Mys, K.; Thompson, K.; Hildebrand, M.; Nehrbass, D.; Müller, W.E.G.; Zeiter, S.; Eglin, D.; Stoddart, M.J. Standard in vitro evaluations of engineered bone substitutes are not sufficient to predict in vivo preclinical model outcomes. Acta Biomater. 2023, 156, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An in vitro and in vivo comparison of osteogenic differentiation of human mesenchymal stromal/stem cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone remodelling in vitro: Where are we headed?: -A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.W.; Horton, J.A. Variable osteogenic performance of MC3T3-E1 subclones impacts their utility as models of osteoblast biology. Sci. Rep. 2019, 9, 8299. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.T. Restoration of mineral depositions by dexamethasone in the matrix of nonmineralizing osteoblastic cells subcloned from MC3T3-E1 cells. Calcif. Tissue Int. 2000, 67, 416–421. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Almeida Alves, C.F.; Fialho, L.; Marques, S.M.; Pires, S.; Rico, P.; Palacio, C.; Carvalho, S. MC3T3-E1 cell response to microporous tantalum oxide surfaces enriched with Ca, P and Mg. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112008. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, D.; Ge, B.; Li, M.; Yu, H.; Cao, F.; Wang, W.; Zhang, Q.; Yi, P.; Wang, H.; et al. Selective laser melting of the porous ta scaffold with Mg-doped calcium phosphate coating for orthopedic applications. ACS Biomater. Sci. Eng. 2024, 10, 1435–1447. [Google Scholar] [CrossRef]
- Izumiya, M.; Haniu, M.; Ueda, K.; Ishida, H.; Ma, C.; Ideta, H.; Sobajima, A.; Ueshiba, K.; Uemura, T.; Saito, N.; et al. Evaluation of MC3T3-E1 cell osteogenesis in different cell culture media. Int. J. Mol. Sci. 2021, 22, 7752. [Google Scholar] [CrossRef]
- Coates, B.A.; McKenzie, J.A.; Buettmann, E.G.; Liu, X.; Gontarz, P.M.; Zhang, B.; Silva, M.J. Transcriptional profiling of intramembranous and endochondral ossification after fracture in mice. Bone 2019, 127, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Zein, M.R.; Allen, S.; Francis-West, P. Making and shaping endochondral and intramembranous bones. Dev. Dyn. 2021, 250, 414–449. [Google Scholar] [CrossRef]
- Sarugaser, R.; Hanoun, L.; Keating, A.; Stanford, W.L.; Davies, J.E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 2009, 4, e6498. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Klein-Nulend, J. Osteoblast isolation from murine calvaria and long bones. Methods Mol. Biol. 2012, 816, 19–29. [Google Scholar] [CrossRef]
- Jonason, J.H.; O’Keefe, R.J. Isolation and culture of neonatal mouse calvarial osteoblasts. Methods Mol. Biol. 2014, 1130, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.R.; Hajjawi, M.O.; Huesa, C.; MacRae, V.E.; Arnett, T.R. Optimisation of the differing conditions required for bone formation in vitro by primary osteoblasts from mice and rats. Int. J. Mol. Med. 2014, 34, 1201–1208. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112463. [Google Scholar] [CrossRef] [PubMed]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.Y.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-dependent osteoblasts response to TiO2 nanotubes of different crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Tornero, C.; de Miguel, E.; Navarro-Compán, V.; Balsa, A.; Aguado, P. Prevalence of chondrocalcinosis and calcium pyrophosphate deposition disease in a cohort of adult patients with low alkaline phosphatase levels and a positive versus negative genetic ALPL study. JBMR Plus 2024, 8, ziae124. [Google Scholar] [CrossRef]
- Fedde, K.N.; Blair, L.; Silverstein, J.; Coburn, S.P.; Ryan, L.M.; Weinstein, R.S.; Waymire, K.; Narisawa, S.; Millán, J.L.; MacGregor, G.R.; et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999, 14, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nam, H.K.; Campbell, C.; Gasque, K.C.S.; Millán, J.L.; Hatch, N.E. Tissue-nonspecific alkaline phosphatase deficiency causes abnormal craniofacial bone development in the Alpl(−/−) mouse model of infantile hypophosphatasia. Bone 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Teixeira, J.F.L.; de Souza, J.A.C.; Magalhães, F.A.C.; de Oliveira, G.J.P.L.; de Santis, J.B.; de Souza Costa, C.A.; de Souza, P.P.C. Laser-modified ti surface improves paracrine osteogenesis by modulating the expression of DKK1 in osteoblasts. J. Funct. Biomater. 2023, 14, 224. [Google Scholar] [CrossRef]
- Tikhomirov, E.; Franconetti, A.; Johansson, M.; Sandström, C.; Carlsson, E.; Andersson, B.; Hailer, N.P.; Ferraz, N.; Palo-Nieto, C. A simple and cost-effective FeCl3-catalyzed functionalization of cellulose nanofibrils: Toward adhesive nanocomposite materials for medical implants. ACS Appl. Mater. Interfaces 2024, 16, 30385–30395. [Google Scholar] [CrossRef] [PubMed]
- Bronk, J.K.; Russell, B.H.; Rivera, J.J.; Pasqualini, R.; Arap, W.; Höök, M.; Barbu, E.M. A multifunctional streptococcal collagen-mimetic protein coating prevents bacterial adhesion and promotes osteoid formation on titanium. Acta Biomater. 2014, 10, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. In vitro osteogenic performance of two novel strontium and zinc-containing glass polyalkenoate cements. J. Biomed. Mater. Res. A 2021, 109, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, W.B.; Kim, H.J.; Mi Woo, K.; Baek, J.H.; Choi, J.Y.; Hur, C.G.; Ryoo, H.M. Bone-related gene profiles in developing calvaria. Gene 2006, 372, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; Sundivakkam, P.; Song, Y.; Ravindran, S.; Ramachandran, A.; Tiruppathi, C.; George, A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J. Biol. Chem. 2010, 285, 36339–36351. [Google Scholar] [CrossRef]
- Frara, N.; Abdelmagid, S.M.; Sondag, G.R.; Moussa, F.M.; Yingling, V.R.; Owen, T.A.; Popoff, S.N.; Barbe, M.F.; Safadi, F.F. Transgenic expression of Osteoactivin/gpnmb enhances bone formation in vivo and osteoprogenitor differentiation ex vivo. J. Cell Physiol. 2016, 231, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann. N. Y. Acad. Sci. 2010, 1192, 190–200. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Gene Name | Takara Bio Primer_Set ID |
|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | MA050371 |
| Lum | Lumican | MA206078 |
| Mmp13 | Matrix metallopeptidase 13 | MA198906 |
| Ibsp | Integrin binding sialoprotein | MA220893 |
| F13a1 | Coagulation factor XIII, A1 subunit | MA217968 |
| Alpl | Alkaline phosphatase, liver/bone/kidney | MA127137 |
| Pmel | Premelanosome protein | MA232167 |
| Slc27a1 | Solute carrier family 27 (fatty acid transporter), member 1 | MA201988 |
| Crabp1 | Cellular retinoic acid binding protein I | MA211028 |
| Mmp9 | Matrix metallopeptidase 9 | MA031311 |
| Dusp4 | Dual specificity phosphatase 4 | MA089467 |
| Dmp1 | Dentin matrix protein 1 | MA155586 |
| Lrp8 | Low-density lipoprotein receptor-related protein 8, apolipoprotein E receptor | MA203890 |
| Adgrl3 | Adhesion G protein-coupled receptor L3 | MA237640 |
| Mnda | Interferon-activated gene 211 | MA197419 |
| Olfr775 | Olfactory receptor family 6 subfamily C member 205 | MA231969 |
| Prkg2 | Protein kinase, cGMP-dependent, type II | MA220730 |
| Fam64a | PICALM interacting mitotic regulator | MA235428 |
| Gpnmb | Glycoprotein (transmembrane) nmb | MA212827 |
| αMEM(−) Top 10 Genes | |||||
| Gene Symbol | Gene ID | mRNA Accession | Name | Fold Change | |
| αMEM(−) | DMEM | ||||
| Lum | 17022 | NM_008524 | Lumican | 8.62 | −1.32 |
| Mmp13 | 17386 | NM_008607 | Matrix metallopeptidase 13 | 7.34 | 1.43 |
| Ibsp | 15891 | NM_008318 | Integrin binding sialoprotein | 6.17 | −1.25 |
| F13a1 | 74145 | NM_001166391 | Coagulation factor XIII, A1 subunit | 5.38 | 1.38 |
| Alpl | 11647 | NM_001287172 | Alkaline phosphatase, liver/bone/kidney | 4.93 | −1.17 |
| Pmel | 20431 | NM_021882 | Premelanosome protein | 4.61 | 1.49 |
| Slc27a1 | 26457 | NM_011977 | Solute carrier family 27 (fatty acid transporter), member 1 | 4.06 | 1.54 |
| Crabp1 | 12903 | NM_001284507 | Cellular retinoic acid binding protein I | 3.92 | 1.95 |
| Mmp9 | 17395 | NM_013599 | Matrix metallopeptidase 9 | 3.74 | −1.41 |
| Gm11099 | - | ENSMUST 00000112589 | Predicted gene 11099 | 3.57 | −1.47 |
| DMEM Top 10 Genes | |||||
| Gene Symbol | Gene ID | mRNA Accession | Name | Fold Change | |
| αMEM(−) | DMEM | ||||
| Dusp4 | 319520 | NM_176933 | Dual specificity phosphatase 4 | −1.49 | 10.67 |
| Dmp1 | 13406 | NM_016779 | Dentin matrix protein 1 | 2.10 | 9.35 |
| Lrp8 | 16975 | NM_001080926 | Low-density lipoprotein receptor-related protein 8, apolipoprotein E receptor | −1.07 | 6.13 |
| Adgrl3 | 319387 | NM_198702 | Adhesion G protein-coupled receptor L3 | −7.91 | 5.78 |
| Mnda | 381308 | NM_001033450 | Interferon-activated gene 211 | 1.07 | 5.59 |
| Olfr775 | 258538 | NM_146545 | Olfactory receptor family 6 subfamily C member 205 | 1.10 | 5.00 |
| Prkg2 | 19092 | NM_008926 | Protein kinase, cGMP-dependent, type II | 1.43 | 5.00 |
| Fam64a | 109212 | NM_144526 | PICALM interacting mitotic regulator | −1.33 | 4.97 |
| Gm13212 | 433801 | NM_001205101 | Predicted gene 13212 | 1.50 | 4.81 |
| Gpnmb | 93695 | NM_053110 | Glycoprotein (transmembrane) nmb | 2.09 | 4.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumiya, M.; Nobuoka, H.; Endo, H.; Ueno, R.; Mimura, M.; Saito, N.; Haniu, H. Genetic Profiling of MC3T3-E1 Cells in Different Media: Implications for In Vitro Screening Development. Biomedicines 2025, 13, 489. https://doi.org/10.3390/biomedicines13020489
Izumiya M, Nobuoka H, Endo H, Ueno R, Mimura M, Saito N, Haniu H. Genetic Profiling of MC3T3-E1 Cells in Different Media: Implications for In Vitro Screening Development. Biomedicines. 2025; 13(2):489. https://doi.org/10.3390/biomedicines13020489
Chicago/Turabian StyleIzumiya, Makoto, Hidehiko Nobuoka, Hono Endo, Rintaro Ueno, Masaki Mimura, Naoto Saito, and Hisao Haniu. 2025. "Genetic Profiling of MC3T3-E1 Cells in Different Media: Implications for In Vitro Screening Development" Biomedicines 13, no. 2: 489. https://doi.org/10.3390/biomedicines13020489
APA StyleIzumiya, M., Nobuoka, H., Endo, H., Ueno, R., Mimura, M., Saito, N., & Haniu, H. (2025). Genetic Profiling of MC3T3-E1 Cells in Different Media: Implications for In Vitro Screening Development. Biomedicines, 13(2), 489. https://doi.org/10.3390/biomedicines13020489







