Preventative Effect of Angiotensin Receptor Blockers on Moderate-to-Severe Cerebral Vasospasm Among Patients Who Received Interventions for Aneurysmal Subarachnoid Hemorrhage
Abstract
1. Introduction
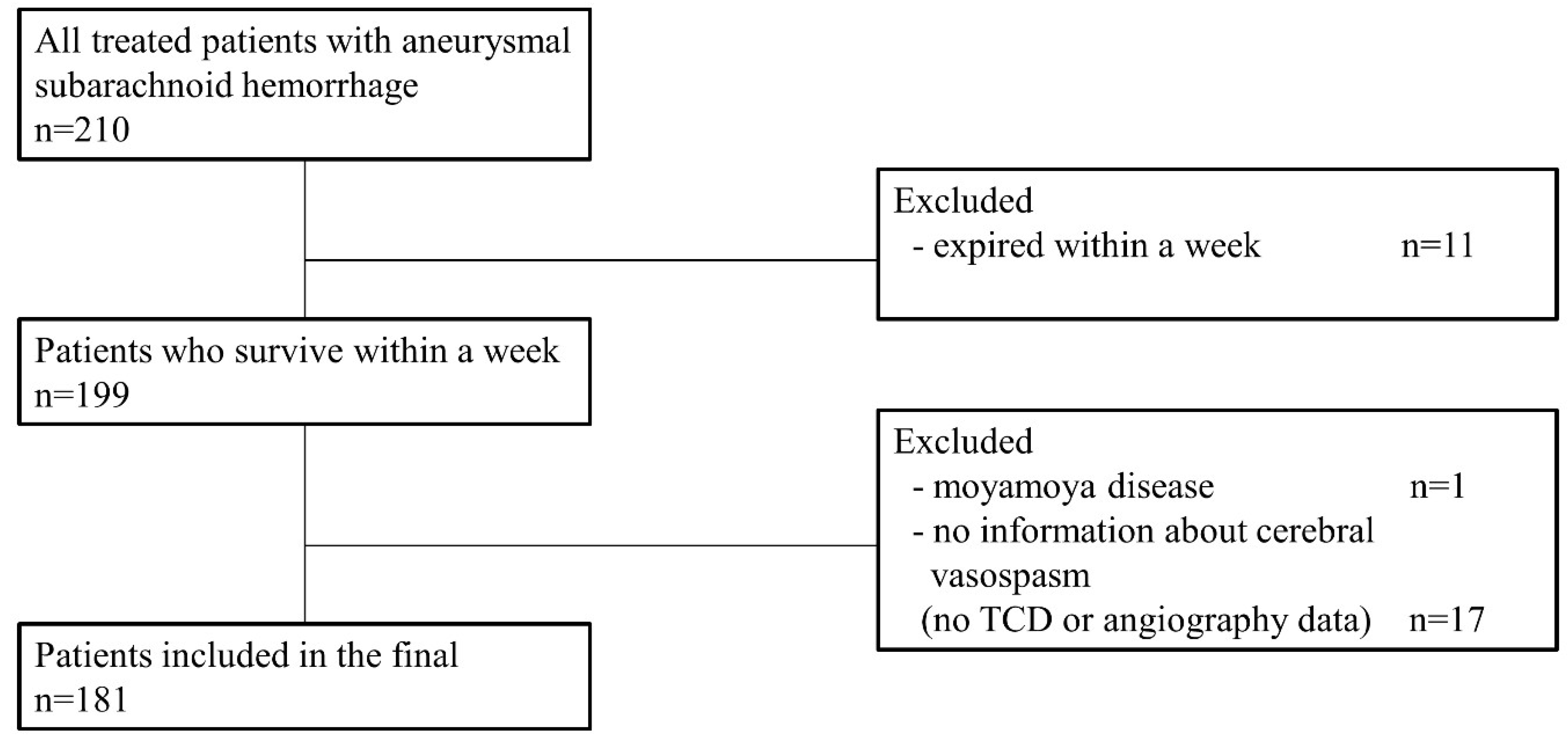
2. Materials and Methods
2.1. Patient Collection and Baseline Characteristics
2.2. Patient Management
2.3. Definition of Moderate-to-Severe Vasospasm and DCI from Cerebral Vasospasm
2.4. Primary and Secondary Endpoint
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Incidence of Moderate-to-Severe Vasospasm
3.3. Risk Factors for Moderate-to-Severe Vasospasm
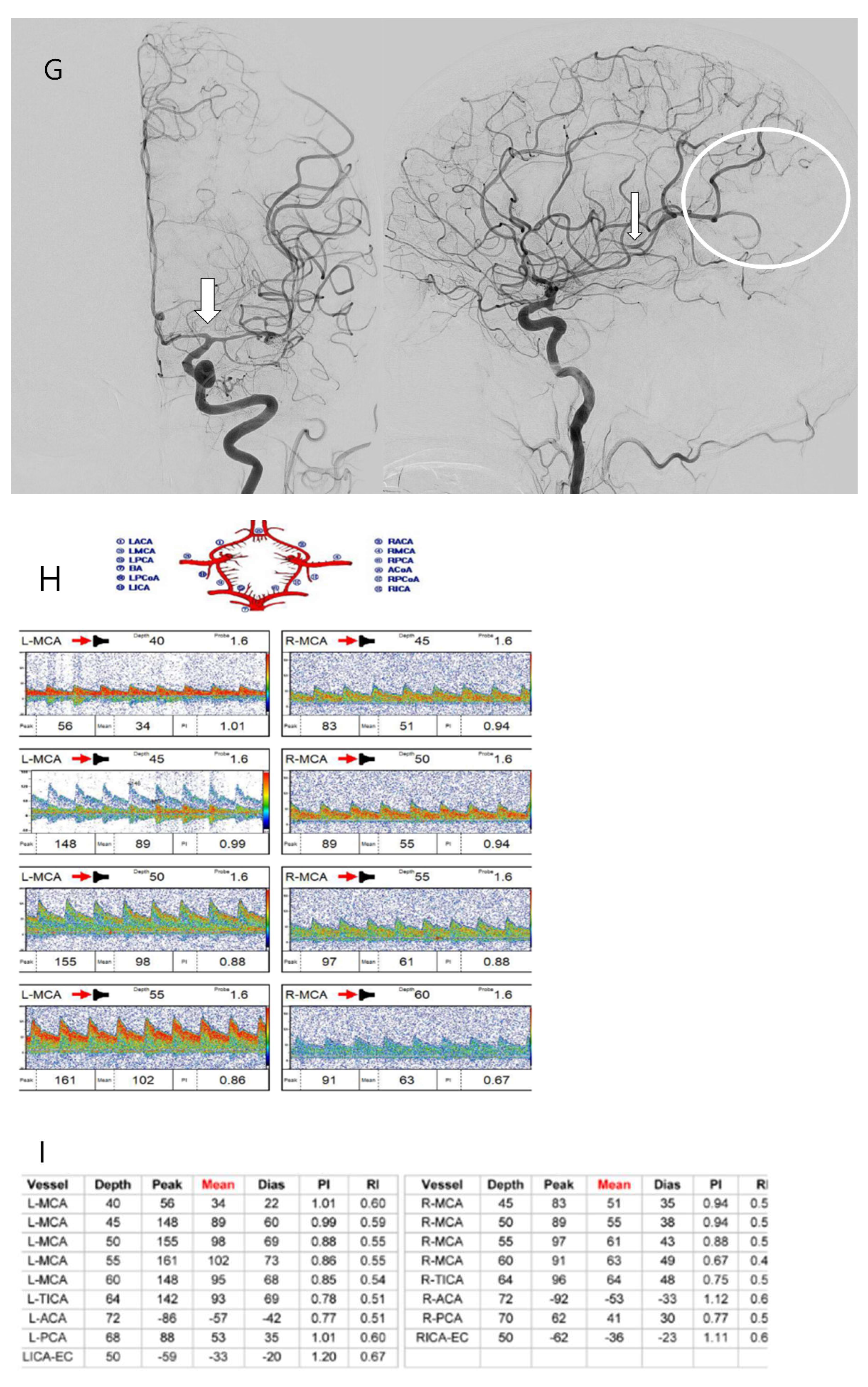
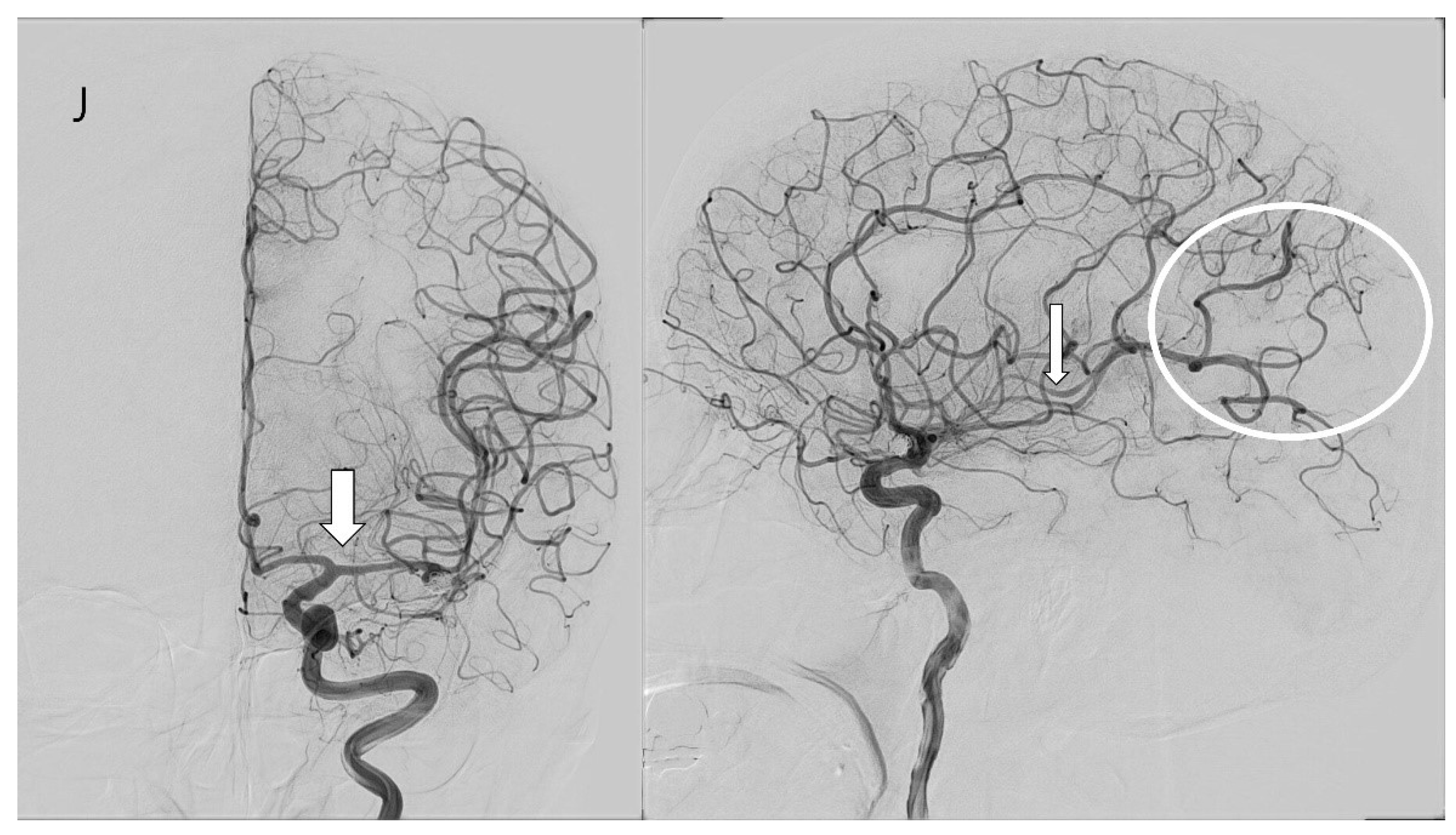
3.4. Case Illustration
4. Discussion
4.1. Diagnosic Modalities and Criteria of Cerebral Vasospasm
4.2. Incidence and Risk Factors of Cerebral Vasospasm
4.3. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARB | angiotensin receptor blocker |
| CCB | calcium channel blocker |
| CT | computed tomography |
| DCI | delayed cerebral ischemia |
| ET | endothelin |
| GCS | Glasgow Coma Scale |
| ICA | internal carotid artery |
| MCA | middle cerebral artery |
| MRA | magnetic resonance angiography |
| mRS | Modified Rankin Scale |
| RAA | renin–angiotensin–aldosterone |
| SAH | subarachnoid hemorrhage |
| TCD | transcranial Doppler |
| TFCA | transfemoral cerebral angiography |
References
- Harrod, C.G.; Bendok, B.R.; Batjer, H.H. Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: A review. Neurosurgery 2005, 56, 633–654; discussion 633–654. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, T. Risk Factors for Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage: A Review of the Literature. World Neurosurg. 2016, 85, 56–76. [Google Scholar] [CrossRef]
- Donaldson, L.; Edington, A.; Vlok, R.; Astono, I.; Iredale, T.; Flower, O.; Ma, A.; Davidson, K.; Delaney, A. The incidence of cerebral arterial vasospasm following aneurysmal subarachnoid haemorrhage: A systematic review and meta-analysis. Neuroradiology 2022, 64, 2381–2389. [Google Scholar] [CrossRef]
- Frontera, J.A.; Fernandez, A.; Schmidt, J.M.; Claassen, J.; Wartenberg, K.E.; Badjatia, N.; Connolly, E.S.; Mayer, S.A. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke 2009, 40, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.W.; Medel, R.; Dumont, A.S.; Ilodigwe, D.; Kassell, N.F.; Mayer, S.A.; Ruefenacht, D.; Schmiedek, P.; Weidauer, S.; Pasqualin, A.; et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 2011, 42, 919–923. [Google Scholar] [CrossRef]
- Bederson, J.B.; Connolly, E.S., Jr.; Batjer, H.H.; Dacey, R.G.; Dion, J.E.; Diringer, M.N.; Duldner, J.E., Jr.; Harbaugh, R.E.; Patel, A.B.; Rosenwasser, R.H.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009, 40, 994–1025. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Bektasoglu, P.K.; Dogruel, Y.; Gungor, A. Beyond nimodipine: Advanced neuroprotection strategies for aneurysmal subarachnoid hemorrhage vasospasm and delayed cerebral ischemia. Neurosurg. Rev. 2024, 47, 305. [Google Scholar] [CrossRef] [PubMed]
- Fassot, C.; Lambert, G.; Gaudet-Lambert, E.; Friberg, P.; Elghozi, J.L. Beneficial effect of renin-angiotensin system for maintaining blood pressure control following subarachnoid haemorrhage. Brain Res. Bull. 1999, 50, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Adzhienko, L.M. Effect of angiotensin II and antagonists of the renin-angiotensin system on the cerebral circulation. Eksperimental’naia I Klin. Farmakol. 2000, 63, 74–79. [Google Scholar]
- Findlay, J.M.; Nisar, J.; Darsaut, T. Cerebral Vasospasm: A Review. Can. J. Neurol. Sci. 2016, 43, 15–32. [Google Scholar] [CrossRef]
- Wanderer, S.; Mrosek, J.; Vatter, H.; Seifert, V.; Konczalla, J. Crosstalk between the angiotensin and endothelin system in the cerebrovasculature after experimental induced subarachnoid hemorrhage. Neurosurg. Rev. 2018, 41, 539–548. [Google Scholar] [CrossRef]
- Wanderer, S.; Gruter, B.E.; Strange, F.; Sivanrupan, S.; Di Santo, S.; Widmer, H.R.; Fandino, J.; Marbacher, S.; Andereggen, L. The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies. Brain Sci. 2020, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Proust, F.; Callonec, F.; Clavier, E.; Lestrat, J.P.; Hannequin, D.; Thiebot, J.; Freger, P. Usefulness of transcranial color-coded sonography in the diagnosis of cerebral vasospasm. Stroke 1999, 30, 1091–1098. [Google Scholar] [CrossRef]
- Kincaid, M.S.; Souter, M.J.; Treggiari, M.M.; Yanez, N.D.; Moore, A.; Lam, A.M. Accuracy of transcranial Doppler ultrasonography and single-photon emission computed tomography in the diagnosis of angiographically demonstrated cerebral vasospasm. J. Neurosurg. 2009, 110, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Dolatowski, K.; Malinova, V.; Frolich, A.M.; Schramm, R.; Haberland, U.; Klotz, E.; Mielke, D.; Knauth, M.; Schramm, P. Volume perfusion CT (VPCT) for the differential diagnosis of patients with suspected cerebral vasospasm: Qualitative and quantitative analysis of 3D parameter maps. Eur. J. Radiol. 2014, 83, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, F.; Roh, D.; Lahiri, S.; Meyers, E.; Witsch, J.; Frey, H.P.; Dangayach, N.; Falo, C.; Mayer, S.A.; Agarwal, S.; et al. Ultra-early angiographic vasospasm associated with delayed cerebral ischemia and infarction following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 126, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Mastantuono, J.M.; Combescure, C.; Elia, N.; Tramer, M.R.; Lysakowski, C. Transcranial Doppler in the Diagnosis of Cerebral Vasospasm: An Updated Meta-Analysis. Crit. Care Med. 2018, 46, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.H.; Young, C.C.; Bass, D.I.; Moore, A.; Levitt, M.R. Transcranial Doppler ultrasonography in neurological surgery and neurocritical care. Neurosurg. Focus 2019, 47, E2. [Google Scholar] [CrossRef]
- Esmael, A.; Flifel, M.E.; Elmarakby, F.; Belal, T. Predictive value of the transcranial Doppler and mean arterial flow velocity for early detection of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Ultrasound 2021, 29, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Chang, J.Y.; Zhou, C.X.; Cui, J.B. Clinical diagnosis of cerebral vasospasm after subarachnoid hemorrhage by using transcranial Doppler sonography. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2029–2035. [Google Scholar] [CrossRef]
- Mortimer, A.M.; Steinfort, B.; Faulder, K.; Bradford, C.; Finfer, S.; Assaad, N.; Harrington, T. The detrimental clinical impact of severe angiographic vasospasm may be diminished by maximal medical therapy and intensive endovascular treatment. J. NeuroInterventional Surg. 2015, 7, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Cattin, F.; Bonneville, J.F. Transcranial Doppler and cerebral vasospasm. J. Neuroradiol. 1999, 26, S22–S27. [Google Scholar]
- Danura, H.; Schatlo, B.; Marbacher, S.; Kerkeni, H.; Diepers, M.; Remonda, L.; Fathi, A.R.; Fandino, J. Acute angiographic vasospasm and the incidence of delayed cerebral vasospasm: Preliminary results. Acta Neurochir. Suppl. 2015, 120, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Mijiti, M.; Mijiti, P.; Axier, A.; Amuti, M.; Guohua, Z.; Xiaojiang, C.; Kadeer, K.; Xixian, W.; Geng, D.; Maimaitili, A. Incidence and Predictors of Angiographic Vasospasm, Symptomatic Vasospasm and Cerebral Infarction in Chinese Patients with Aneurysmal Subarachnoid Hemorrhage. PLoS ONE 2016, 11, e0168657. [Google Scholar] [CrossRef] [PubMed]
- Rumalla, K.; Lin, M.; Ding, L.; Gaddis, M.; Giannotta, S.L.; Attenello, F.J.; Mack, W.J. Risk Factors for Cerebral Vasospasm in Aneurysmal Subarachnoid Hemorrhage: A Population-Based Study of 8346 Patients. World Neurosurg. 2021, 145, e233–e241. [Google Scholar] [CrossRef]
- Ozono, I.; Ikawa, F.; Hidaka, T.; Matsuda, S.; Oku, S.; Horie, N.; Date, I.; Suzuki, M.; Kobata, H.; Murayama, Y.; et al. Different Risk Factors Between Cerebral Infarction and Symptomatic Cerebral Vasospasm in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2023, 173, e487–e497. [Google Scholar] [CrossRef]
- Gonzalez, N.R.; Boscardin, W.J.; Glenn, T.; Vinuela, F.; Martin, N.A. Vasospasm probability index: A combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2007, 107, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Hasegawa, Y.; Miura, M. Current Therapeutic Drugs Against Cerebral Vasospasm after Subarachnoid Hemorrhage: A Comprehensive Review of Basic and Clinical Studies. Curr. Drug Deliv. 2017, 14, 843–852. [Google Scholar] [CrossRef]
- Wanderer, S.; Mrosek, J.; Gessler, F.; Seifert, V.; Konczalla, J. Vasomodulatory effects of the angiotensin II type 1 receptor antagonist losartan on experimentally induced cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir. 2018, 160, 277–284. [Google Scholar] [CrossRef]
- Chehrazi, B.B.; Giri, S.; Joy, R.M. Prostaglandins and vasoactive amines in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 1989, 20, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Provencio, J.J. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: A review. Acta Neurochir. Suppl. 2013, 115, 233–238. [Google Scholar] [CrossRef]
- Erdi, F.; Keskin, F.; Esen, H.; Kaya, B.; Feyzioglu, B.; Kilinc, I.; Karatas, Y.; Cuce, G.; Kalkan, E. Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol. Res. 2016, 38, 224–231. [Google Scholar] [CrossRef] [PubMed]



| Variables | No ARB (n = 152) | ARB (n = 29) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (mean ± SD), years | 58.19 ± 13.136 | 63.31 ± 11.430 | 1.032 (0.999–1.066) | 0.054 |
| Sex (female), n (%) | 96 (63.2) | 19 (65.5) | 0.902 (0.392–2.077) | 0.809 |
| Smoking, n (%) | 48 (31.6) | 8 (27.6) | 1.212 (0.501–2.930) | 0.670 |
| HTN, n (%) | 25 (16.4) | 29 (100) | N/A * | <0.001 |
| Initial GCS < 8 | 17 (11.2) | 7 (24.1) | 2.527 (0.940–6.792) | 0.066 |
| Treated with magnesium, n (%) | 147 (96.7) | 29 (100.0) | N/A | 1.000 |
| Statin use, n (%) | 23 (15.1) | 9 (31.0) | 0.396 (0.161–0.978) | 0.045 |
| HH grade (≥3), n (%) | 67 (44.1) | 13 (44.8) | 0.970 (0.436–2.157) | 0.941 |
| Fisher grade (≥3), n (%) | 85 (55.9) | 22 (75.9) | 0.404 (0.163–1.002) | 0.050 |
| Location of aneurysm, n (%) | 0.302 (0.082–1.107) | 0.071 | ||
| Ant. Circulation | 145 (95.4) | 25 (86.2) | ||
| Post. Circulation | 7 (4.6) | 4 (13.8) | ||
| M1/ICA ratio | ||||
| Initial | 0.547 ± 0.110 | 0.524 ± 0.115 | 0.161 (0.006–4.530) | 0.283 |
| At POD #7 | 0.492 ± 0.129 | 0.485 ± 0.120 | 0.166 (0.073–2.978) | 0.419 |
| TCD Mean Velocity | ||||
| Initial | 41.578 ± 27.611 | 39.43 ± 26.536 | 0.997 (0.983–1.011) | 0.683 |
| At POD #7 | 71.148 ± 37.915 | 63.198 ± 23.829 | 0.997 (0.988–1.006) | 0.544 |
| At POD #10 | 75.854 ± 38.015 | 61.769 ± 27.525 | 0.993 (0.984–1.002) | 0.145 |
| TCD Peak velocity | ||||
| Initial | 62.155 ± 44.418 | 60.596 ± 44.866 | 0.999 (0.991–1.008) | 0.855 |
| At POD #7 | 105.089 ± 55.234 | 90.986 ± 37.729 | 0.998 (0.991–1.004) | 0.457 |
| At POD #10 | 106.526 ± 53.002 | 88.299 ± 41.728 | 0.996 (0.989–1.002) | 0.183 |
| Vasospasm, n (%) | 57 (37.5) | 4 (13.8) | 3.750 (1.242–11.326) | 0.019 |
| Angioplasty, n (%) | 25 (16.4) | 3(10.3) | 1.706 (0.479–6.073) | 0.410 |
| DCI, n (%) | 19 (12.5) | 0 (0) | N/A * | 0.047 |
| Pre-mRS (≤2), n (%) | 83 (54.6) | 14 (48.3) | 1.289 (0.582–2.855) | 0.532 |
| mRS at 3 months (≤2), n (%) | 117 (77.0) | 16 (55.2) | 2.716 (1.192–6.189) | 0.017 |
| Surgical clipping, n (%) | 15 (9.9) | 1 (3.4) | 3.066 (0.389–24.166) | 0.288 |
| Coil embolization, n (%) | 137 (90.1) | 28 (96.6) | 3.066 (0.389–24.166) | 0.288 |
| Variables | Non-Vasospasm (n = 120) | Vasospasm (n = 61) | p-Value |
|---|---|---|---|
| M1/ICA ratio (mean ± SD) | |||
| Initial | 0.546 ± 0.0963 | 0.539 ± 0.116 | 0.655 |
| At POD #7 | 0.525 ± 0.121 | 0.302 ± 0.154 | <0.001 |
| TCD Mean velocity (mean ± SD) | |||
| Initial | 51.848 ± 18.974 | 50.276 ± 23.110 | 0.680 |
| At POD #7 | 58.286 ± 18.153 | 187.477 ± 46.699 | <0.001 |
| At POD #10 | 58.672 ± 21.888 | 191.572 ± 43.621 | <0.001 |
| TCD Peak velocity (mean ± SD) | |||
| Initial | 79.315 ± 34.577 | 70.563 ± 37.853 | 0.185 |
| At POD #7 | 89.466 ± 37.760 | 222.321 ± 67.201 | 0.002 |
| At POD #10 | 86.466 ± 37.760 | 225.094 ± 61.352 | <0.001 |
| Factors | Univariate | p-Value | Multivariate | p-Value |
|---|---|---|---|---|
| Odd Ratio (95% CI) | Odd Ratio (95% CI) | |||
| Age | 0.968 (0.944–0.993) | 0.011 | 0.963 (0.938–0.989) | 0.006 |
| Sex | 0.923 (0.487–1.747) | 0.805 | ||
| Smoking | 1.784 (0.928–3.430) | 0.083 | 1.552 (0.773–3.116) | 0.217 |
| HTN | 0.522 (0.254–1.072) | 0.076 | 1.322 (0.473–3.699) | 0.595 |
| Magnesium | 0.756 (0.123–4.651) | 0.763 | ||
| Statin use | 0.873 (0.384–1.984) | 0.747 | ||
| ARB | 0.267 (0.088–0.805) | 0.019 | 0.246 (0.079–0.771) | 0.016 |
| Additional CCB | 0.394 (0.153–1.018) | 0.054 | 0.515 (0.189–1.405) | 0.195 |
| Fisher grade (III–IV) | 1.870 (0.976–3.583) | 0.059 | 2.732 (1.343–5.560) | 0.006 |
| HH grade | 1.497 (0.805–2.783) | 0.202 | ||
| Tx. Modality (clip) | 1.130 (0.374–3.413) | 0.828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.U.; Byoun, H.S.; Cho, M.J.; Lim, J.-W.; Kim, C.H.; Bang, J.-S. Preventative Effect of Angiotensin Receptor Blockers on Moderate-to-Severe Cerebral Vasospasm Among Patients Who Received Interventions for Aneurysmal Subarachnoid Hemorrhage. Biomedicines 2025, 13, 442. https://doi.org/10.3390/biomedicines13020442
Lee SU, Byoun HS, Cho MJ, Lim J-W, Kim CH, Bang J-S. Preventative Effect of Angiotensin Receptor Blockers on Moderate-to-Severe Cerebral Vasospasm Among Patients Who Received Interventions for Aneurysmal Subarachnoid Hemorrhage. Biomedicines. 2025; 13(2):442. https://doi.org/10.3390/biomedicines13020442
Chicago/Turabian StyleLee, Si Un, Hyoung Soo Byoun, Min Jai Cho, Jeong-Wook Lim, Chang Hyeun Kim, and Jae-Seung Bang. 2025. "Preventative Effect of Angiotensin Receptor Blockers on Moderate-to-Severe Cerebral Vasospasm Among Patients Who Received Interventions for Aneurysmal Subarachnoid Hemorrhage" Biomedicines 13, no. 2: 442. https://doi.org/10.3390/biomedicines13020442
APA StyleLee, S. U., Byoun, H. S., Cho, M. J., Lim, J.-W., Kim, C. H., & Bang, J.-S. (2025). Preventative Effect of Angiotensin Receptor Blockers on Moderate-to-Severe Cerebral Vasospasm Among Patients Who Received Interventions for Aneurysmal Subarachnoid Hemorrhage. Biomedicines, 13(2), 442. https://doi.org/10.3390/biomedicines13020442






