Comparison of End-Tidal Carbon Dioxide Values in ICU Patients with and Without In-Hospital Cardiac Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
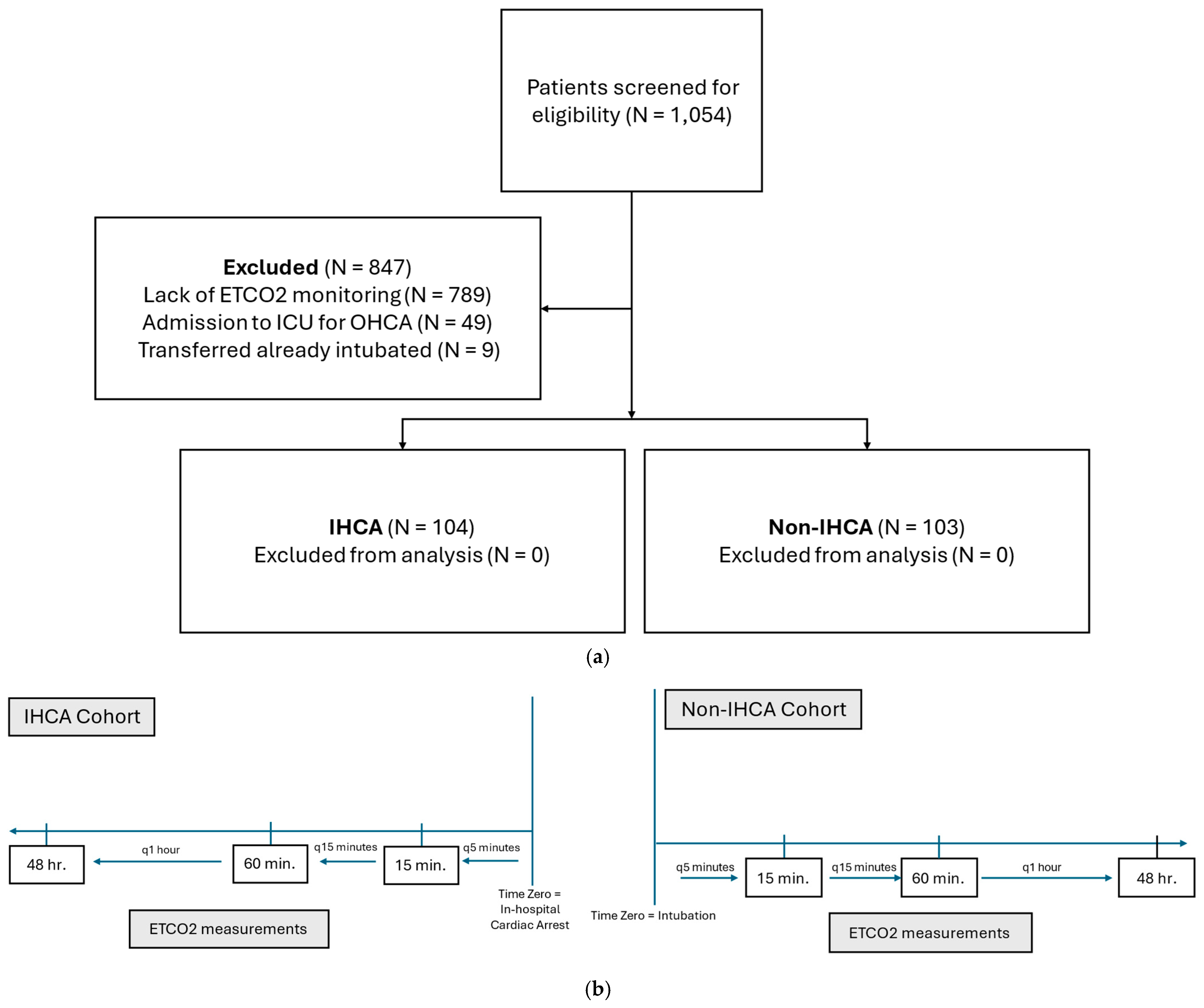
2.2. Study Patients
2.3. Data Collection and Study Outcomes
2.4. Statistical Analysis
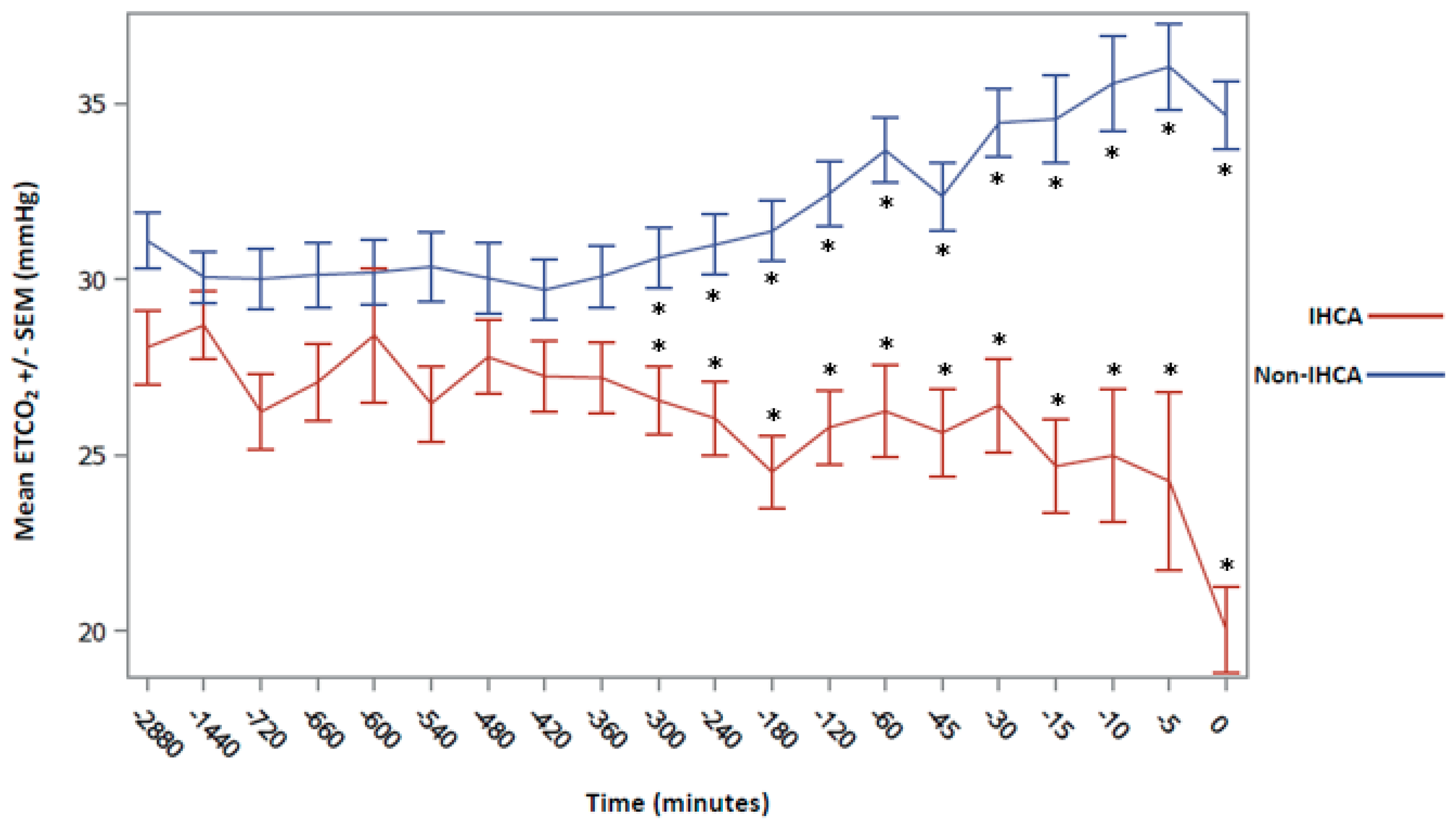
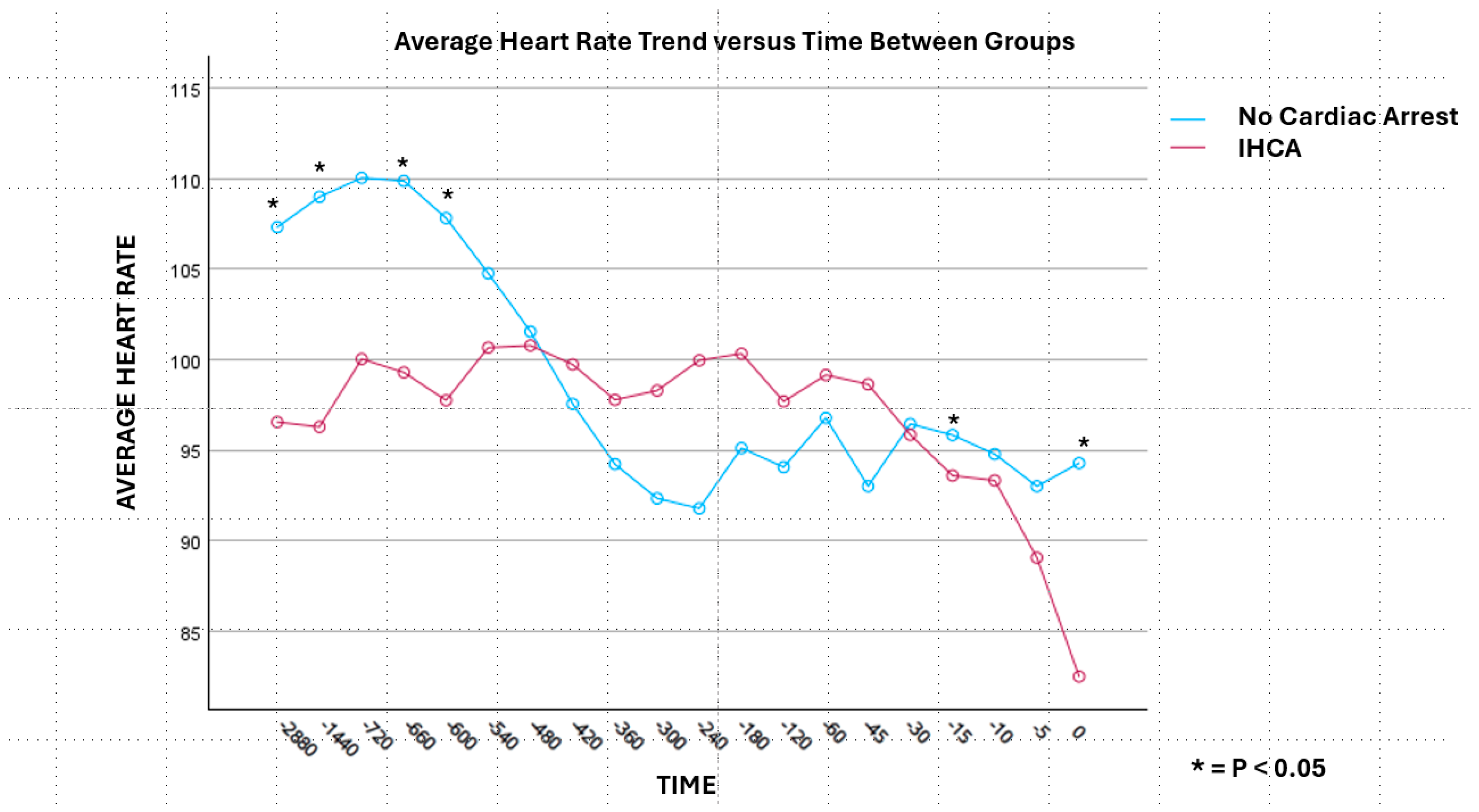
3. Results
| IHCA (n = 104) | Non-IHCA (n = 103) | p-Value | |
|---|---|---|---|
| Age, median years (IQR) | 61.5 (50–70) | 58.0 (49–66) | 0.068 |
| Race, n (%) | <0.001 | ||
| Black | 53 (51) | 35 (34) | |
| White | 19 (18.3) | 28 (27.2) | |
| Hispanic/Latino | 1 (1) | 16 (15.5) | |
| Unknown/Other | 31 (29.8) | 24 (23.3) | |
| Males, n (%) | 62 (59.6) | 55 (53.4) | 0.367 |
| Past medical history, n (%) | |||
| CAD | 30 (28.8) | 14 (13.6) | 0.007 |
| Arrhythmia | 43 (41.3) | 10 (9.7) | <0.001 |
| CKD, n (%) # | 25 (24.0) | 15 (14.6) | 0.113 |
| Stage II | 1 (4) | 0 | 0.317 |
| Stage III | 3 (12) | 2 (13.3) | 0.317 |
| Stage IV | 0 | 2 (13.3) | 0.317 |
| Stage V/dialysis | 21 (84) | 11 (73.3) | 0.392 |
| SOFA score, median (IQR) ^ | 7.0 (4–10) | 9.0 (5–11) | 0.126 |
| Vasopressor therapy, n (%) | 74 (71.2) | 42 (40.8) | <0.001 |
| Antibiotics, n (%) | 85 (81.7) | 93 (90.3) | 0.076 |
| IHCA (n = 104) | Non-IHCA (n = 103) | p-Value | |
|---|---|---|---|
| Arterial blood gas, median (IQR) | |||
| pH | 7.29 (7.12–7.38) | 7.33 (7.23–7.44) | 0.002 |
| pCO2, mmHg | 37 (29–47) | 40 (30–51) | 0.053 |
| pO2, mmHg | 90 (72–117) | 89 (67–122) | 0.436 |
| Laboratory values, median (IQR) | |||
| Potassium, mmol/L | 4.5 (3.9–5.1) | 4.1 (3.7–4.6) | <0.001 |
| Calcium, mg/dL | 7.9 (7.3–8.5) | 8.1 (7.5–8.7) | 0.042 |
| Magnesium, mg/dL | 2.1 (1.9–2.4) | 2.0 (1.8–2.2) | 0.017 |
| Blood urea nitrogen, mg/dL | 37 (22–52) | 27 (16–50) | 0.051 |
| Serum creatinine, mg/dL | 2.3 (1.4–3.6) | 1.6 (0.9–3.0) | 0.005 |
| Glucose, mg/dL | 130 (100–172) | 128 (107–177) | 0.270 |
| Lactic acid, mmol/L | 4.1 (2.0–9.8) | 2.4 (1.4–3.9) | <0.001 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmberg, M.J.; Ross, C.E.; Fitzmaurice, G.M.; Chan, P.S.; Duval-Arnould, J.; Grossestreuer, A.V.; Yankama, T.; Donnino, M.W.; Andersen, L.W.; for the American Heart Association’s Get With The Guidelines–Resuscitation Investigators; et al. Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005580. [Google Scholar] [CrossRef]
- Girotra, S.; Nallamothu, B.K.; Spertus, J.A.; Li, Y.; Krumholz, H.M.; Chan, P.S. American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N. Engl. J. Med. 2012, 367, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Kaye, W.; Ornato, J.P.; Larkin, G.L.; Nadkarni, V.; Mancini, M.E.; Berg, R.A.; Nichol, G.; Lane-Trultt, T. Cardiopulmonary resuscitation of adults in the hospital: A report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003, 58, 297–308. [Google Scholar] [CrossRef]
- Bircher, N.G.; Chan, P.S.; Xu, Y. American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Delays in Cardiopulmonary Resuscitation, Defibrillation, and Epinephrine Administration All Decrease Survival in In-hospital Cardiac Arrest. Anesthesiology 2019, 130, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Knudson, J.D.; Neish, S.R.; Cabrera, A.G.; Lowry, A.W.; Shamszad, P.; Morales, D.L.S.; Graves, D.E.; Williams, E.A.; Rossano, J.W. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: An analysis of the Kids’ Inpatient Database. Crit. Care Med. 2012, 40, 2940–2944. [Google Scholar] [CrossRef]
- Nadkarni, V.M.; Larkin, G.L.; Peberdy, M.A.; Carey, S.M.; Kaye, W.; Mancini, M.E.; Nichol, G.; Lane-Truitt, T.; Potts, J.; Ornato, J.P.; et al. National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006, 295, 50–57. [Google Scholar] [CrossRef]
- Kronick, S.L.; Kurz, M.C.; Lin, S.; Edelson, D.P.; Berg, R.A.; Billi, J.E.; Cabanas, J.G.; Cone, D.C.; Diercks, D.B.; Foster, J.; et al. Part 4: Systems of Care and Continuous Quality Improvement. Circulation 2015, 130 (Suppl. S2), S397–S413. [Google Scholar] [CrossRef]
- St. John, R.E. Exhaled gas analysis: Technical and clinical aspects of capnography and oxygen consumption. Crit. Care Nurs. Clin. N. Am. 1989, 20, 363–374. [Google Scholar]
- Ahrens, T. Technology utilization in the cardiac surgical patient: SvO2 and capnography monitoring. Crit. Care Nurs. Q. 1998, 21, 24–40. [Google Scholar] [CrossRef]
- Szaflarski, N.L.; Cohen, N.H. Use of capnography in critically ill adults. Heart Lung. 1991, 20, 363–374. [Google Scholar]
- Aminiahidashti, H.; Shafiee, S.; Zamani Kiasari, A.; Sazgar, M. Applications of End-Tidal Carbon Dioxide (ETCO2) Monitoring in Emergency Department; a Narrative Review. Emergency 2018, 6, e5. [Google Scholar]
- Ahrens, T. Utilization of intensive care unit technology. New Horiz. 1998, 6, 41–51. [Google Scholar]
- Weil, M.H.; Bisera, J.M.; Trevino, R.P.; Rackow, E.C. Cardiac output and end-tidal carbon dioxide. Crit. Care Med. 1985, 13, 907–909. [Google Scholar] [CrossRef]
- Mucksavage, J.J.; He, K.J.P.C.; Chang, J.P.C.; Panlilio-Villanueva, M.M.; Wang, T.; Fraidenburg, D.; Benken, S.T. A Pilot Study of End-Tidal Carbon Dioxide in Prediction of Inhospital Cardiac Arrests. Crit. Care Explor. 2020, 2, e0204. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Carson, S.S.; Lindquist, J.H.; Olsen, M.K.; Govert, J.A.; Chelluri, L.; Quality of Life After Mechanical Ventilation in the Aged (QOL-MV) Investigators. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: A prospective cohort study. Crit. Care 2007, 11, R9. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Amoateng-Adjepong, Y.; Kaufman, D.; Gheorghe, C.; Manthous, C.A. Age, duration of mechanical ventilation, and outcomes of patients who are critically ill. Chest 2009, 136, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Daly, B.J.; Gordon, N.; Brennan, P.F. Survival and quality of life: Short-term versus long-term ventilator patients. Crit. Care Med. 2002, 30, 2655–2662. [Google Scholar] [CrossRef]
- Ahrens, T.; Schallom, L.; Bettorf, K.; Ellner, S.; Hurt, G.; O’Mara, V.; Ludwig, J.; George, W.; Marino, T.; Shannon, W. End-tidal carbon dioxide measurements as a prognostic indicator of outcome in cardiac arrest. Am. J. Crit. Care 2001, 10, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.B.; Kern, K.B.; Otto, C.W.; Milander, M.M.; Ewy, G.A. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation: A prognostic indicator for survival. JAMA 1989, 262, 1347–1351. [Google Scholar] [CrossRef]
- Salen, P.; O’Connor, R.; Sierzenski, P.; Passarello, B.; Pancu, D.; Melanson, S.; Arcona, S.; Reed, J.; Heller, M. Can cardiac sonography and capnography be used independently and in combination to predict resuscitation outcomes? Acad. Emerg. Med. 2001, 8, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified early warning score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef]
- Gardner-Thorpe, J.; Love, N.; Wrightson, J.; Walsh, S.; Keeling, N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: A prospective observational study. Ann. R. Coll. Surg. Engl. 2006, 88, 571–575. [Google Scholar] [CrossRef]
- Smith, M.E.B.; Chiovaro, J.C.; O’neil, M.; Kansagara, D.; Quiñones, A.R.; Freeman, M.; Motu’apuaka, M.L.; Slatore, C.G. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann. Am. Thorac. Soc. 2014, 11, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.O.; Li, H.; Shahidah, N.; Koh, Z.X.; Sultana, P.; Ong, M.E.H. Poor performance of the modified early warning score for predicting mortality in critically ill patients presenting to an emergency department. World J. Emerg. Med. 2013, 4, 273–278. [Google Scholar] [CrossRef]
- Ong, M.E.H.; Ng, C.H.L.; Goh, K.; Liu, N.; Koh, Z.X.; Shahidah, N.; Zhang, T.T.; Fook-Chong, S.; Lin, Z. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Crit. Care 2012, 16, R108. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, F.; Chen, D. Predictive value of Modified Early Warning Score (MEWS) and Revised Trauma Score (RTS) for the short-term prognosis of emergency trauma patients: A retrospective study. BMJ Open 2021, 11, e041882. [Google Scholar] [CrossRef]
- Reini, K.; Fredrikson, M.; Oscarsson, A. The prognostic value of the Modified Early Warning Score in critically ill patients: A prospective, observational study. Eur. J. Anaesthesiol. 2012, 29, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kia, A.; Timsina, P.; Joshi, H.N.; Klang, E.; Gupta, R.R.; Freeman, R.M.; Reich, D.L.; Tomlinson, M.S.; Dudley, J.T.; Kohli-Seth, R.; et al. MEWS++: Enhancing the Prediction of Clinical Deterioration in Admitted Patients through a Machine Learning Model. J. Clin. Med. 2020, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Blankush, J.M.; Freeman, R.; McIlvaine, J.; Tran, T.; Nassani, S.; Leitman, I.M. Implementation of a novel postoperative monitoring system using automated Modified Early Warning Scores (MEWS) incorporating end-tidal capnography. J. Clin. Monit. Comput. 2017, 31, 1081–1092. [Google Scholar] [CrossRef]
- Roberts, B.W.; Kilgannon, J.H.; Chansky, M.E.; Mittal, N.; Wooden, J.; Trzeciak, S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post–cardiac arrest syndrome. Circulation 2013, 127, 2107–2113. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalton, K.; Mucksavage, J.J.; Fraidenburg, D.R.; He, K.; Chang, J.; Panlilio-Villanueva, M.; Wang, T.; Benken, S.T. Comparison of End-Tidal Carbon Dioxide Values in ICU Patients with and Without In-Hospital Cardiac Arrest. Biomedicines 2025, 13, 412. https://doi.org/10.3390/biomedicines13020412
Dalton K, Mucksavage JJ, Fraidenburg DR, He K, Chang J, Panlilio-Villanueva M, Wang T, Benken ST. Comparison of End-Tidal Carbon Dioxide Values in ICU Patients with and Without In-Hospital Cardiac Arrest. Biomedicines. 2025; 13(2):412. https://doi.org/10.3390/biomedicines13020412
Chicago/Turabian StyleDalton, Kaitlyn, Jeffrey J. Mucksavage, Dustin R. Fraidenburg, Kevin He, James Chang, Maria Panlilio-Villanueva, Tianxiu Wang, and Scott T. Benken. 2025. "Comparison of End-Tidal Carbon Dioxide Values in ICU Patients with and Without In-Hospital Cardiac Arrest" Biomedicines 13, no. 2: 412. https://doi.org/10.3390/biomedicines13020412
APA StyleDalton, K., Mucksavage, J. J., Fraidenburg, D. R., He, K., Chang, J., Panlilio-Villanueva, M., Wang, T., & Benken, S. T. (2025). Comparison of End-Tidal Carbon Dioxide Values in ICU Patients with and Without In-Hospital Cardiac Arrest. Biomedicines, 13(2), 412. https://doi.org/10.3390/biomedicines13020412







