Mitral Valve Transcatheter Edge-to-Edge Repair (MV-TEER) in Patients with Secondary Mitral Regurgitation Improves Hemodynamics, Enhances Renal Function, and Optimizes Quality of Life in Patients with Advanced Renal Insufficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. NICaS® Device and Procedure
2.3. Impact of MV-TEER on Quality of Life (EQ-5D-3L Questionnaire)
2.4. Study Objectives
2.5. Statistical Analysis
3. Results
3.1. Overall Cohort
3.1.1. Baseline Characteristics
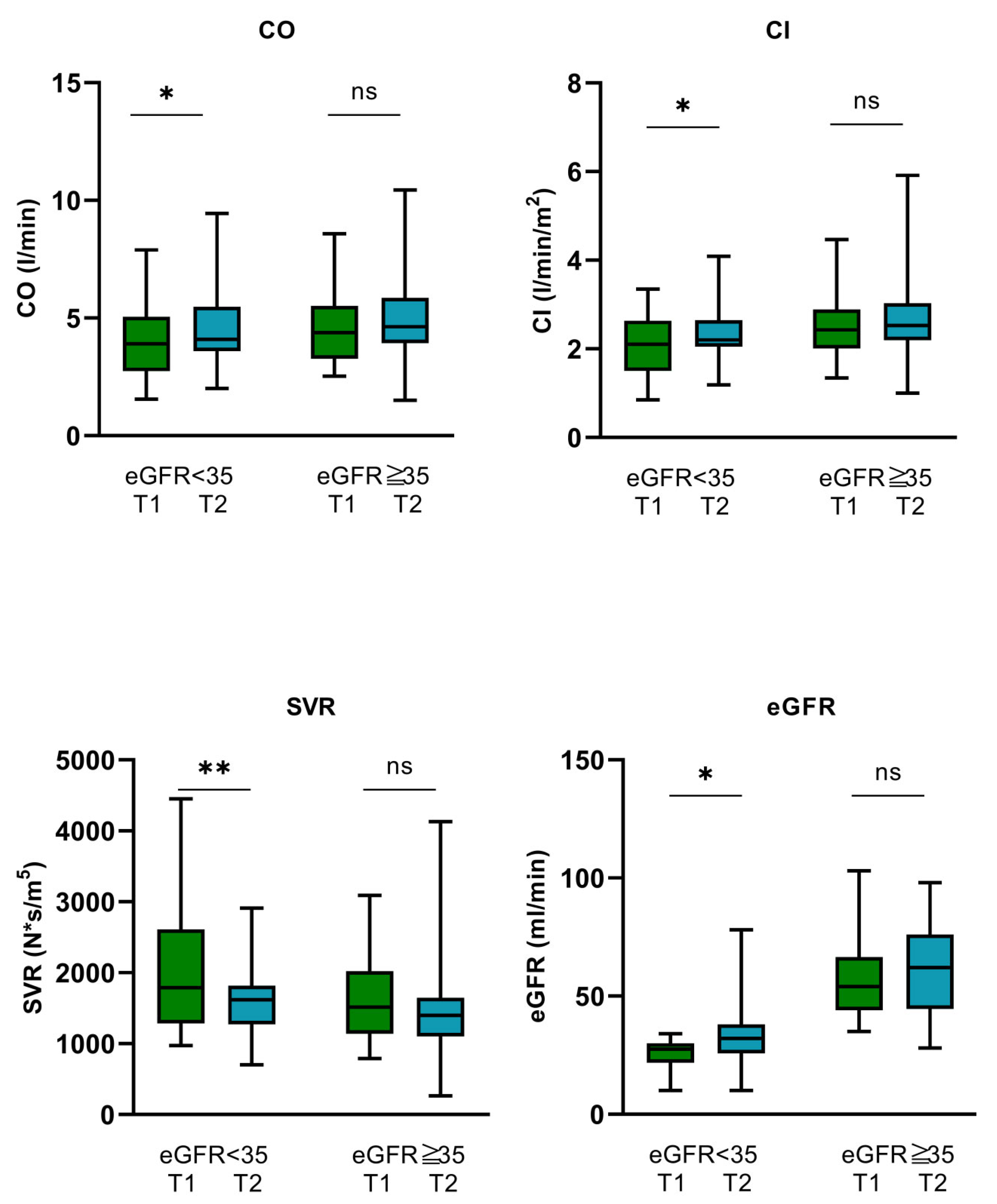
3.1.2. Hemodynamics, Tissue Perfusion, and Renal Function
3.2. Impact of MV-TEER in Relation to Renal Function
3.2.1. Baseline Characteristics in eGFR Subgroups
3.2.2. Hemodynamics, Tissue Perfusion, and Renal Function
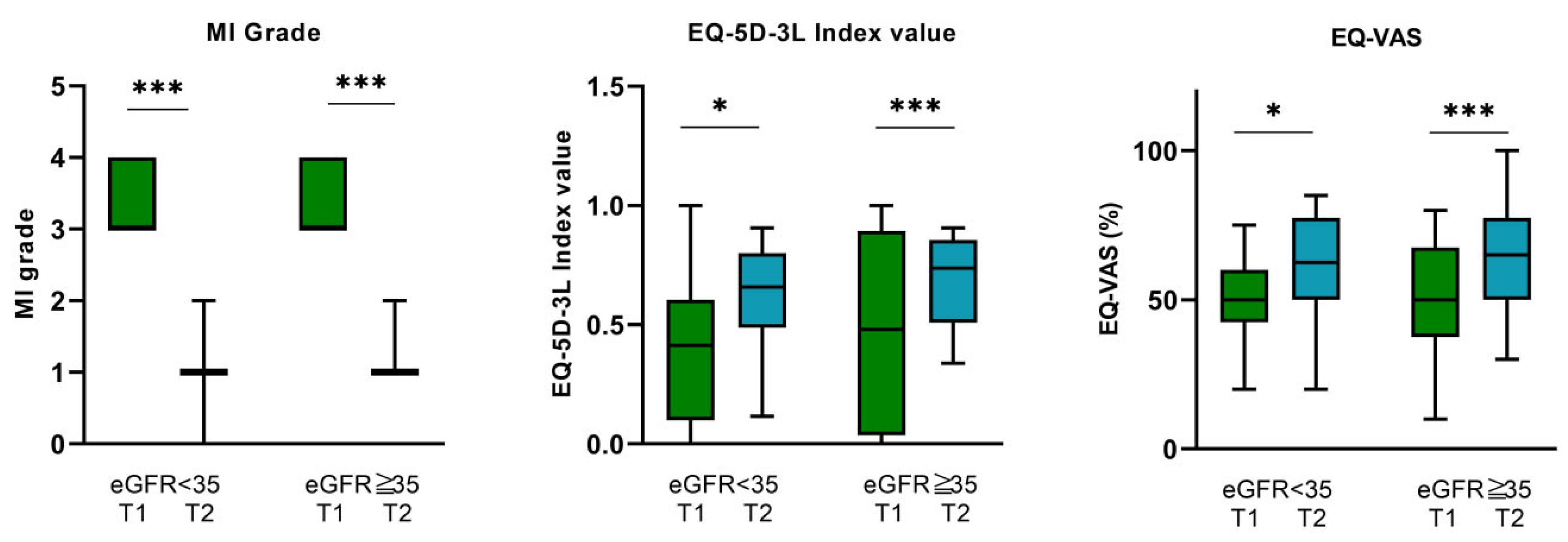
3.3. Impact of MV-TEER on Quality of Life—EQ-5D-3L and EQ-VAS Questionnaire
3.3.1. Overall Cohort
3.3.2. eGFR < 35 mL/min Subgroup
3.3.3. eGFR ≥ 35 mL/min Subgroup
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S. Mitral valve prolapse. Nihon Rinsho Jpn. J. Clin. Med. 2005, 63, 1195–1200. [Google Scholar]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Chokkalingam Mani, B. Mitral Valve Insufficiency; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Coffey, S.; Cairns, B.J.; Iung, B. The modern epidemiology of heart valve disease. Heart 2016, 102, 75–85. [Google Scholar] [CrossRef]
- Trichon, B.H.; Felker, G.M.; Shaw, L.K.; Cabell, C.H.; O’Connor, C.M. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am. J. Cardiol. 2003, 91, 538–543. [Google Scholar] [CrossRef]
- Lurz, P.; Besler, C. Mitral Regurgitation in Cardiogenic Shock: A Novel Target for Transcatheter Therapy? JACC Cardiovasc. Interv. 2021, 14, 12–14. [Google Scholar] [CrossRef]
- Chehab, O.; Roberts-Thomson, R.; Ling, C.N.Y.; Marber, M.; Prendergast, B.D.; Rajani, R.; Redwood, S.R. Secondary mitral regurgitation: Pathophysiology, proportionality and prognosis. Heart 2020, 106, 716–723. [Google Scholar] [CrossRef]
- Payen, D.; de Pont, A.C.; Sakr, Y.; Spies, C.; Reinhart, K.; Vincent, J.L. Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators: A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care 2008, 12, R74. [Google Scholar] [CrossRef]
- Sheikh, O.; Nguyen, T.; Bansal, S.; Prasad, A. Acute kidney injury in cardiogenic shock: A comprehensive review. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, E91–E105. [Google Scholar] [CrossRef]
- van den Akker, J.P.C.; Bakker, J.; Groeneveld, A.B.J.; den Uil, C.A. Risk indicators for acute kidney injury in cardiogenic shock. J. Crit. Care 2019, 50, 11–16. [Google Scholar] [CrossRef]
- 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Valvular-Heart-Disease-Guidelines (accessed on 28 August 2021).
- Tarvasmäki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.-P.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 2018, 20, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, N.; Resta, M.L.; Somaschini, A.; Campodonico, J.; Lucci, C.; Moltrasio, M.; Bonomi, A.; Cornara, S.; Camporotondo, R.; Demarchi, A.; et al. Acute kidney injury and in-hospital mortality in patients with ST-elevation myocardial infarction of different age groups. Int. J. Cardiol. 2021, 344, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Dunlay, S.M.; Barsness, G.W.; Vallabhajosyula, S.; Vallabhajosyula, S.; Sundaragiri, P.R.; Gersh, B.J.; Jaffe, A.S.; Kashani, K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS ONE 2019, 14, e0222894. [Google Scholar] [CrossRef] [PubMed]
- Mezhonov, E.M.; Vialkina, I.A.; Vakulchik, K.A.; Shalaev, S.V. Acute kidney injury in patients with ST-segment elevation acute myocardial infarction: Predictors and outcomes. Saudi J. Kidney Dis. Transplant. 2021, 32, 318–327. [Google Scholar] [CrossRef]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2021, 162, e183–e353. [Google Scholar] [CrossRef]
- Al Younis, S.M.; Hadjileontiadis, L.J.; Stefanini, C.; Khandoker, A.H. Non-invasive technologies for heart failure, systolic and diastolic dysfunction modeling: A scoping review. Front. Bioeng. Biotechnol. 2023, 11, 1261022. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Milo, O.; Kaluski, E.; Vered, Z.; Cotter, G. Whole-body electrical bio-impendance is accurate in non invasive determination of cardiac output: A thermodilution controlled, prospective, double blind evaluation. J. Card. Fail. 2004, 10, 38–39. [Google Scholar] [CrossRef]
- Paredes, O.L.; Shite, J.; Shinke, T.; Watanabe, S.; Otake, H.; Matsumoto, D.; Imuro, Y.; Ogasawara, D.; Sawada, T.; Yokoyama, M. Impedance cardiography for cardiac output estimation: Reliability of wrist-to-ankle electrode configuration. Circ. J. 2006, 70, 1164–1168. [Google Scholar] [CrossRef]
- Tanino, Y.; Shite, J.; Paredes, O.L.; Shinke, T.; Ogasawara, D.; Sawada, T.; Kawamori, H.; Miyoshi, N.; Kato, H.; Yoshino, N.; et al. Whole body bioimpedance monitoring for outpatient chronic heart failure follow up. Circ. J. 2009, 73, 1074–1079. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Emoto, N.; Miyagawa, K.; Nakayama, K.; Kinutani, H.; Tanaka, H.; Shinke, T.; Hirata, K. Noninvasive and simple assessment of cardiac output and pulmonary vascular resistance with whole-body impedance cardiography is useful for monitoring patients with pulmonary hypertension. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 2383–2389. [Google Scholar] [CrossRef]
- Buchholz, I.; Marten, O.; Janssen, M.F. Feasibility and validity of the EQ-5D-3L in the elderly Europeans: A secondary data analysis using SHARE(d) data. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2022, 31, 3267–3282. [Google Scholar] [CrossRef] [PubMed]
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ-5D): An instrument for measuring quality of life. Monaldi Arch. Chest Dis. 2012, 78, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Friede, T.; von Bardeleben, R.-S.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Baldus, S.; Doenst, T.; Pfister, R.; Gummert, J.; Kessler, M.; Boekstegers, P.; Lubos, E.; Schröder, J.; Thiele, H.; Walther, T.; et al. Transcatheter Repair versus Mitral-Valve Surgery for Secondary Mitral Regurgitation. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Anker, M.S.; Porthun, J.; Schulze, P.C.; Rassaf, T.; Landmesser, U. Percutaneous Transcatheter Edge-To-Edge Repair for Functional Mitral Regurgitation in Heart Failure: A Meta-Analysis of 3 Randomized Controlled Trials. J. Am. Coll. Cardiol. 2024. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Patsalis, N.; Kreutz, J.; Chatzis, G.; Syntila, S.; Choukeir, M.; Schieffer, B.; Markus, B. Early risk predictors of acute kidney injury and short-term survival during Impella support in cardiogenic shock. Sci. Rep. 2024, 14, 17484. [Google Scholar] [CrossRef]
- McCallum, W.; Sarnak, M.J. Cardiorenal Syndrome in the Hospital. Clin. J. Am. Soc. Nephrol. 2023, 18, 933–945. [Google Scholar] [CrossRef]
- D’ Marco, L. Congestive Nephropathy. Int. J. Environ. Res. Public Health 2022, 19, 2499. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Lombardi, C.; Ruocco, G.; Padeletti, M.; Nuti, R.; Metra, M.; Ronco, C. Chronic kidney disease and worsening renal function in acute heart failure: Different phenotypes with similar prognostic impact? Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 534–548. [Google Scholar] [CrossRef]
- Legrand, M.; Mebazaa, A.; Ronco, C.; Januzzi, J.L. When cardiac failure, kidney dysfunction, and kidney injury intersect in acute conditions: The case of cardiorenal syndrome. Crit. Care Med. 2014, 42, 2109–2117. [Google Scholar] [CrossRef]
| Demographics, Characteristics, and Comorbidities | ||||
| Total (n = 45) | eGFR < 35 mL/min (n = 16) | eGFR ≥ 35 mL/min (n = 29) | p Value | |
| Age (years) | 78.7 ± 6.7 | 78.3 ± 7 | 78.9 ± 6.1 | 0.776 |
| Female n (%) | 18 (40) | 7 (43.8) | 18 (72) | 0.237 |
| Male n (%) | 27 (60) | 9 (56.3) | 11 (37.9) | 0.237 |
| BMI | 26.5 ± 5 | 26.1 ± 4.4 | 26.8 ± 5.3 | 0.041 |
| History of aHTN n (%) | 32 (71.1) | 11 (68.8) | 21 (72.4) | 0.654 |
| History of dyslipidemia n (%) | 22 (48.9) | 9 (56.3) | 13 (44.8) | 0.463 |
| History of DM n (%) | 15 (33.3) | 4 (25) | 11 (37.9) | 0.378 |
| Known history of CKD n (%) | 29 (64.4) | 13 (81.3) | 16 (55.2) | 0.080 |
| Atrial fibrillation n (%) | 35 (77.8) | 11 (68.8) | 24 (82.8) | 0.279 |
| CRT n (%) | 10 (22.2) | 4 (25) | 6 (20.7) | 0.739 |
| ICD n (%) | 5 (11.1) | 4 (25) | 1 (3.4) | 0.028 |
| CHD n (%) | 33 (73.3) | 12 (75) | 21 (72.4) | 0.851 |
| COPD n (%) | 12 (26.7) | 7 (43.8) | 5 (17.2) | 0.054 |
| LVEF (%) median [IQR] mean (SD) | 55 [39–56] 47.5 ± 11.2 | 52 [35–56] 47.5 ± 11.5 | 55 [39–56] 47.4 ± 11.1 | 0.6 |
| MR grade 3 n (%) 4 n (%) | 25 (55.6) 20 (44.4) | 9 (56.3) 7 (43.8) | 16 (55.2) 13 (44.8) | 0.095 |
| MR regurgitation volume (mL) | 59.5 [40–72.5] | 53.5 [40.25–77.5] | 59 [38–71.5] | 0.924 |
| MR EROA (cm2) | 0.36 [0.23–0.59] | 0,40 [0.22–0.68] | 0.33 [0.24–0.54] | 0.553 |
| TAPSE (cm) | 21 [19–22.5] | 22 [20–23] | 21 [18.5–22] | 0.443 |
| TKS’ lateral (cm/s) | 12 [10.2–13] | 12 [11.14.5] | 11 [10–12.3] | 0.184 |
| Hemoglobin (g/L) | 115.4 ± 191 | 111.9 ± 18.5 | 117.7 ± 18.1 | 0.007 |
| Hematocrit (L/L) | 0.36 [0.32–0.38] | 0.35 [0.32–0.38] | 0.36 [0.32–0.39] | 0.537 |
| Sodium (mmol/L) | 139.3 ± 3.2 | 139.4 ± 3.03 | 139 ± 3.3 | 0.543 |
| Potassium (mmol/L) | 4.05 [3.65–4.35] | 4.3 [3.8–4.9] | 4.0 [3.6–4.1] | 0.063 |
| NT-proBNP (pg/mL) | 3782 [1725–8513] | 8513 [3859–15,356] | 2046 [1378–4805] | <0.001 |
| Medication | ||||
| Torasemide (mg/d) n = 45 | 15 [10–15] | 15 [10–15] | 15 [10–15] | 1.0 |
| Eplerenone (mg/d) n = 38 | 25 [25–25] | 25 [25–25] | 25 [25–25] | 1.0 |
| Bisoprolol (mg/d) n = 38 | 5 [2.5–5] | 5 [2.5–5] | 5 [2.5–5] | 0.81 |
| Ramipril (mg/d) n = 22 | 5 [5–10] | 5 [5–10] | 5 [5–10] | 0.868 |
| Candesartan (mg/d) n = 23 | 16 [16–24] | 16 [16–22] | 16 [16–32] | 0.506 |
| Dapagliflozine (mg/d) n = 36 | 10 [10–10] | 10 [10–10] | 10 [10–10] | 1.0 |
| Amlodipine (mg/L) n = 37 | 10 [5–10] | 10 [5–10] | 10 [5–10] | 0.15 |
| Hemodynamics | ||||
| CO (L/min) | 4.38 ± 1.58 | 3.94 ± 1.6 | 4.63 ± 1.5 | 0.165 |
| CI (L/min/m2) | 2.36 ± 2.36 | 2.52 ± 0.82 | 2.07 ± 0.69 | 0.055 |
| SVR (N × s/m5) | 1596 [1177–2132] | 1791 [1285–2612] | 1514 [1138–2022] | 0.129 |
| T1 (Pre-Procedure) (n = 45) | T2 (3–5 d Post-Procedure) (n = 45) | p Value | |
|---|---|---|---|
| SAP (mmHg) | 118.89 ± 18.86 | 114.02 ± 18.79 | 0.135 |
| DAP (mmHg) | 67.2 ± 11.65 | 66.09 ± 11.54 | 0.601 |
| MAP (mmHg) | 84.07 ± 12.59 | 81.84 ± 10.81 | 0.284 |
| HR (bpm) | 77.1 ± 26.8 | 80.1 ± 24.1 | 0.549 |
| CO (L/min) | 4.13 [3.09–5.38] | 4.3 [3.86–5.69] | 0.025 |
| CI (L/min/m2) | 2.31 [1.82–2.78] | 2.44 [2.11–2.82] | 0.032 |
| SVR (N × s/m5) | 1765 [1177–2132] | 1427 [2226–3876] | 0.003 |
| eGFR (mL/min) | 46.26 ± 21.56 | 50.38 ± 21.34 | 0.03 |
| MR Grade | 3 [3–4] | 1 [1–1] | <0.001 |
| eGFR < 35 (T1) n = 16 | eGFR < 35 (T2) n = 16 | p Value | eGFR ≥ 35 (T1) n = 29 | eGFR ≥ 35 (T2) n = 29 | p Value | |
|---|---|---|---|---|---|---|
| SAP (mmHg) | 119.56 ± 14.36 | 117.13 ± 16.5 | 0.599 | 118.52 ± 21.18 | 112.21 ± 20.01 | 0.161 |
| DAP (mmHg) | 68.89 ± 7.53 | 67.44 ± 9.85 | 0.579 | 66.28 ± 13.43 | 65.34 ± 12.47 | 0.758 |
| MAP (mmHg) | 85.5 ± 6.82 | 84 ± 8.85 | 0.551 | 83.28 ± 14.92 | 80.66 ± 11.73 | 0.375 |
| HR (bpm) | 74.6 ± 18 | 74.7 ± 13.3 | 0.972 | 78.48 ± 30.77 | 83.07 ± 28.19 | 0.546 |
| CO (L/min) | 3.94 ± 1.6 | 4.47 ± 1.72 | 0.035 | 4.38 [3.27–5.52] | 4.63 [3.93–5.86] | 0.247 |
| CI (L/min/m2) | 2.07 ± 0.69 | 2.34 ± 0.68 | 0.031 | 2.43 [2.01–2.89] | 2.53 [2.2–3.039] | 0.294 |
| SVR (N × s/m5) | 1791 [1285–2612] | 1618 [2205–5654] | 0.007 | 1514 [1138–2022] | 1397 [2233–3793] | 0.061 |
| eGFR (mL/min) | 25.63 ± 6.54 | 33.81 ± 16.05 | 0.018 | 57.66 ± 18.15 | 59.51 ± 18.38 | 0.408 |
| MR Grade | 3 [3–4] | 1 [1–1] | <0.001 | 3 [3–4] | 1 [1–1] | <0.001 |
| Overall Cohort | |||
|---|---|---|---|
| T1 | 3M-FU | p Value | |
| EQ-VAS (%) | 51.7 ± 0.18 | 62.9 ± 0.17 | p < 0.001 |
| EQ-5D-3L index value | 0.44 ± 0.39 | 0.66 ± 0.20 | p < 0.001 |
| eGFR < 35 mL/min | |||
| EQ-VAS (%) | 51.6 ± 0.14 | 60.6 ± 0.18 | 0.015 |
| EQ-5D-3L index value | 0.527 ± 0.13 | 0.61 ± 0.19 | 0.034 |
| eGFR ≥ 35 mL/min | |||
| EQ-VAS (%) | 51.8 ± 0.17 | 64 ± 0.17 | <0.001 |
| EQ-5D-3L index value | 0.475 ± 0.4 | 0.68 ± 0.19 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markus, B.; Kreutz, J.; Chatzis, G.; Syntila, S.; Kuchenbuch, J.; Mueller, C.; Choukeir, M.; Schieffer, B.; Patsalis, N. Mitral Valve Transcatheter Edge-to-Edge Repair (MV-TEER) in Patients with Secondary Mitral Regurgitation Improves Hemodynamics, Enhances Renal Function, and Optimizes Quality of Life in Patients with Advanced Renal Insufficiency. Biomedicines 2024, 12, 2648. https://doi.org/10.3390/biomedicines12112648
Markus B, Kreutz J, Chatzis G, Syntila S, Kuchenbuch J, Mueller C, Choukeir M, Schieffer B, Patsalis N. Mitral Valve Transcatheter Edge-to-Edge Repair (MV-TEER) in Patients with Secondary Mitral Regurgitation Improves Hemodynamics, Enhances Renal Function, and Optimizes Quality of Life in Patients with Advanced Renal Insufficiency. Biomedicines. 2024; 12(11):2648. https://doi.org/10.3390/biomedicines12112648
Chicago/Turabian StyleMarkus, Birgit, Julian Kreutz, Giorgios Chatzis, Styliani Syntila, Jannis Kuchenbuch, Charlotte Mueller, Maryana Choukeir, Bernhard Schieffer, and Nikolaos Patsalis. 2024. "Mitral Valve Transcatheter Edge-to-Edge Repair (MV-TEER) in Patients with Secondary Mitral Regurgitation Improves Hemodynamics, Enhances Renal Function, and Optimizes Quality of Life in Patients with Advanced Renal Insufficiency" Biomedicines 12, no. 11: 2648. https://doi.org/10.3390/biomedicines12112648
APA StyleMarkus, B., Kreutz, J., Chatzis, G., Syntila, S., Kuchenbuch, J., Mueller, C., Choukeir, M., Schieffer, B., & Patsalis, N. (2024). Mitral Valve Transcatheter Edge-to-Edge Repair (MV-TEER) in Patients with Secondary Mitral Regurgitation Improves Hemodynamics, Enhances Renal Function, and Optimizes Quality of Life in Patients with Advanced Renal Insufficiency. Biomedicines, 12(11), 2648. https://doi.org/10.3390/biomedicines12112648







