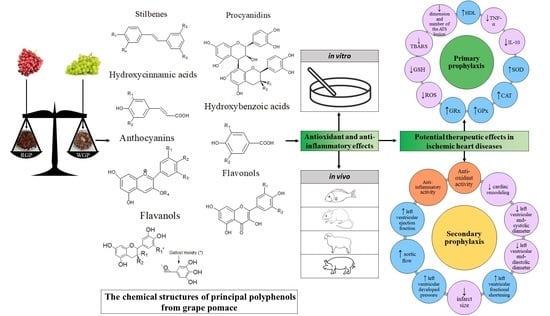
Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases
Abstract
1. Introduction
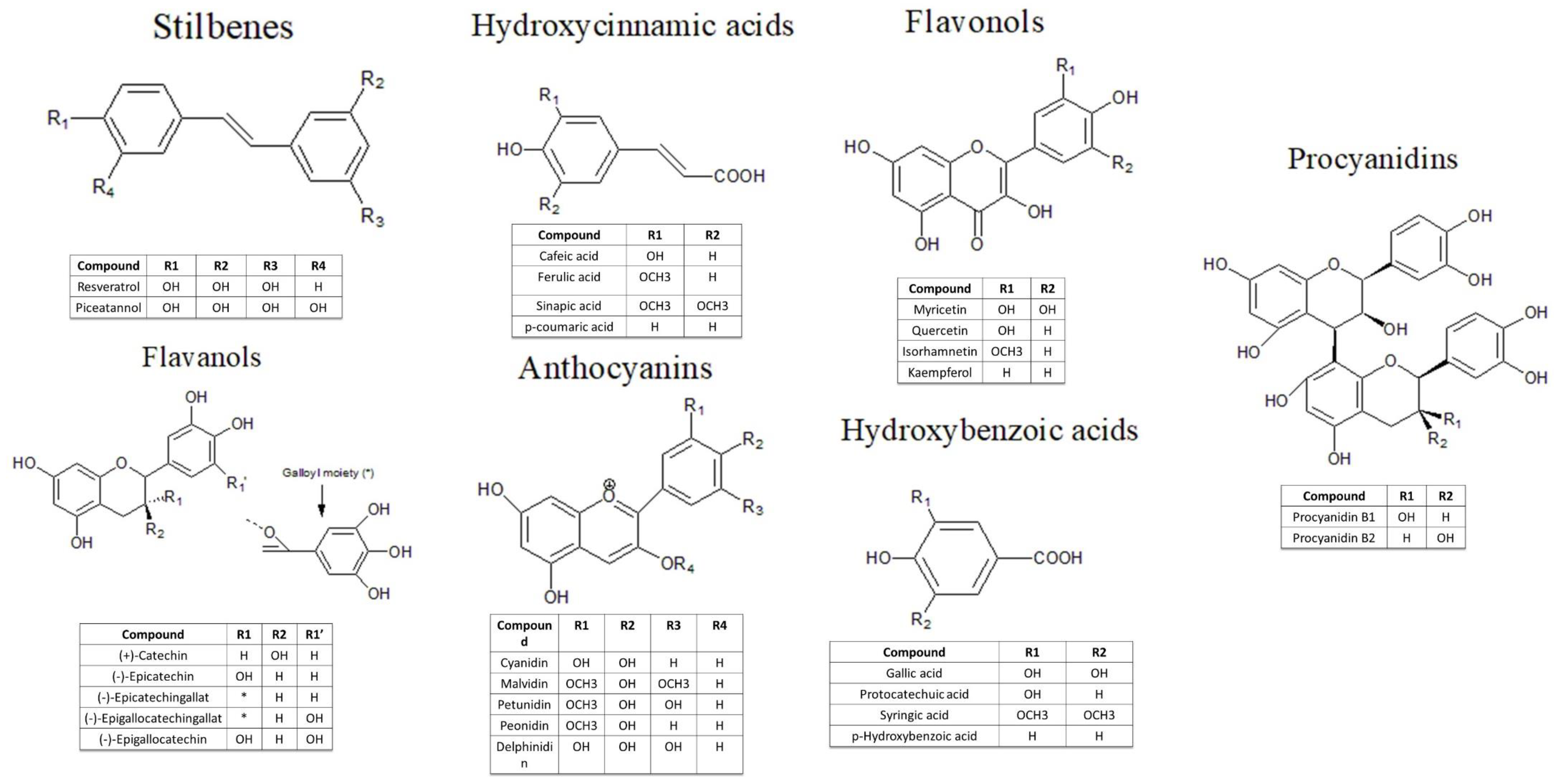
2. Red and White Grape Pomace—Bioactive Compounds
3. Potentially Toxic Effects of Polyphenols from Red and White Grape Pomace
4. Red and White Grape Pomace—Variability of Total Polyphenols Content and Antioxidant Capacity
| Grape Pomace (GP) | TPC (mg GAE */g GP) | Antioxidant Capacity | References | |||
|---|---|---|---|---|---|---|
| DPPH (μmol TE **/g GP) | ABTS (μmol TE/g GP) | FRAP (μmol FeSO4 * 7H2O/g GP) | ||||
| Vitis vinifera sp. Cultivated in Maipo Valley, Chile | [15] | |||||
| White | Sauvignon Blanc | 19 | 120 | - | - | |
| Chardonnay | 17 | 90 | - | - | ||
| Red | Cabernet Sauvignon | 14 | 60 | - | - | |
| Carménère | 13 | 70 | - | - | ||
| Vitis vinifera sp. cultivated in Virginia, USA | [30] | |||||
| White | Vidal Blanc (hybrid variety) | 55.5 | 7.71 | 334 | - | |
| Viognier (Vitis vinifera sp.) | 99.1 | 3.54 | 951 | - | ||
| Red | Cabernet Franc (V. vinifera sp.) | 153.8 | 11.2 | 1013 | - | |
| Chambourcin (hybrid variety) | 92.0 | 28.2 | 378 | - | ||
| Vitis vinifera sp. cultivated in Rhineland-Palatinate, Germany | [32] | |||||
| White | 4 varieties of Pinot Blanc and 6 of Riesling | 48 | - | - | - | |
| Red | 5 varieties of Dornfelder, 5 of Pinot noir and 2 of Portugais bleu | 58 | - | - | - | |
| Vitis vinifera sp. cultivated in Blacksburg, Crozet, Floyd VA, USA | [33] | |||||
| White | Viognier | 11.8 | - | - | - | |
| Vidal Blanc | 12.5 | - | - | - | ||
| Niagara | 24.8 | - | - | - | ||
| Petit Manseng | 32.1 | - | - | - | ||
| Red | Petit Verdot | 64.8 | - | - | - | |
| Merlot | 35.8 | - | - | - | ||
| Cabernet Franc | 36.1 | - | - | - | ||
| Chambourcin | 10.4 | - | - | - | ||
| White | unknown varieties | 90.51 | - | - | 1619 | [34] |
| Red | unknown varieties | 107.40 | - | - | 1886 | |
| Vitis vinifera sp. cultivated in Cappadocia district of Nevsehir province (Emir), Tokat province (Narince), Sarkoy-Murefte district of Trakya region (Gamay), Ankara province (Kalecik Karasi), Elazig province (Okuzgozu), Turkey | [35] | |||||
| White | Emir | 75.5 | - | - | - | |
| Narince | 138.1 | - | - | - | ||
| Red | Gamay | 255.4 | - | - | - | |
| Kalecik Karasi | 205.7 | - | - | - | ||
| Okuzgozu | 281.4 | - | - | - | ||
| Vitis vinifera sp. cultivated in Blackstone, VA, USA | [36] | |||||
| White | Chardonnay | 24.5 | - | - | - | |
| Red | Cabernet Franc | 30.4 | - | - | - | |
| Vitis vinifera sp. cultivated in Cantine Cantele, Apulia Region, Southern Italy | [37] | |||||
| White | Fiano | 127.06 | - | - | - | |
| Red | Negramaro | 127.87 | - | - | - | |
| Vitis vinifera sp. cultivated in Paros, Greece | [38] | |||||
| White | Monemvassia | 4.49 | - | - | 0.32 | |
| Red | Mandilaria | 5.1 | - | - | 0.31 | |
| Aidani mavro | 0.25 | - | - | 0.21 | ||
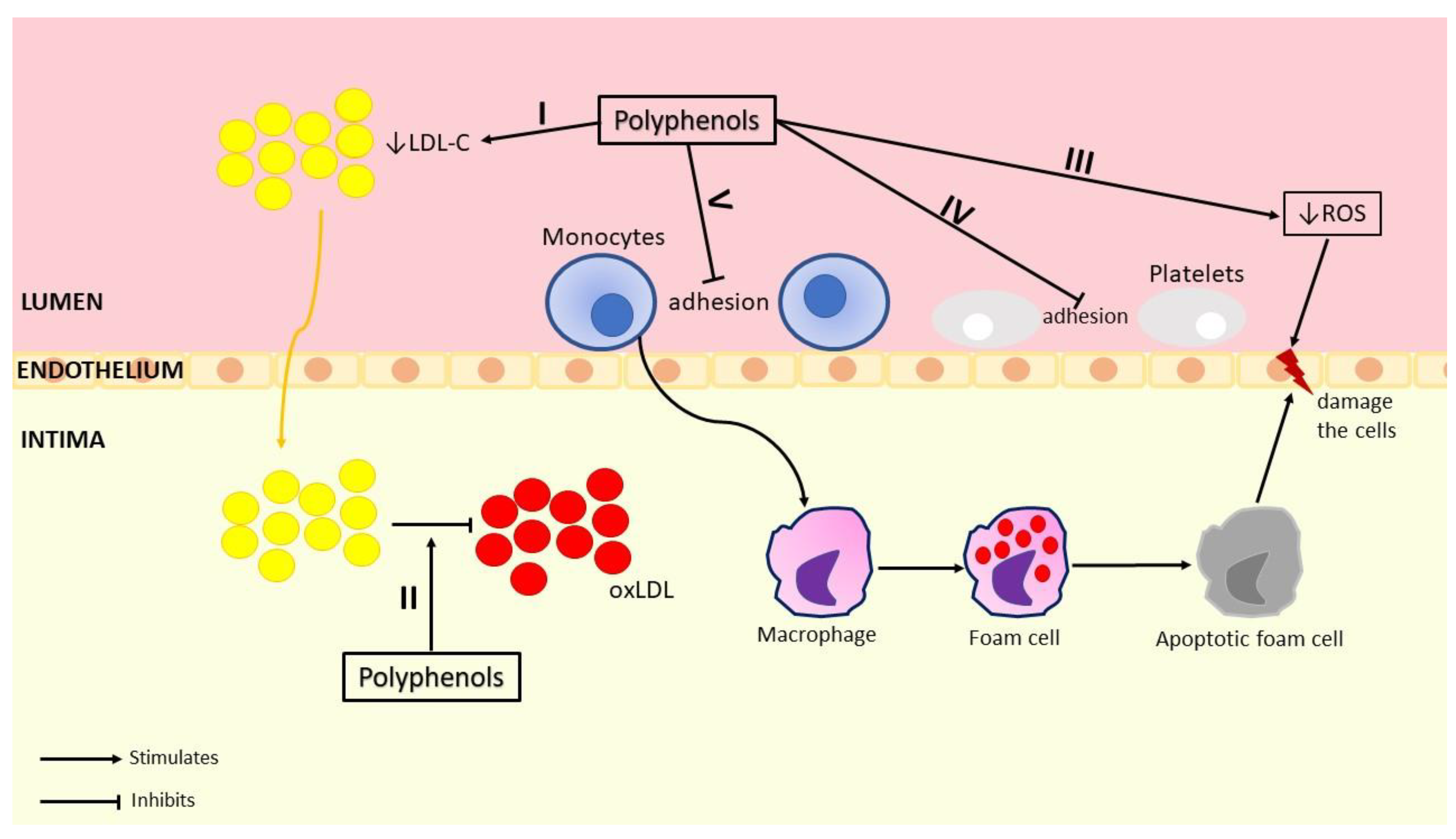
5. Red and White Grape Pomace—In Vitro Antioxidant and Anti-Inflammatory Activities
5.1. Red Grape Pomace Antioxidant Activity
5.2. White Grape Pomace Antioxidant Activity
5.3. Red Grape Pomace Anti-Inflammatory Activity
5.4. White Grape Pomace Anti-Inflammatory Activity
6. Red and White Grape Pomace—In Vivo Antioxidant and Anti-Inflammatory Activities
6.1. Red Grape Pomace Antioxidant Activity
6.2. Red Grapepomace Anti-Inflammatory Activity
6.3. White Grape Pomace Antioxidant and Anti-Inflammatory Activities
7. Ischemic Heart Diseases—What We Know So Far and What Can Be Improved
7.1. Risk Factors
7.2. Diagnostics
7.3. Management
7.4. Potential New Therapy
8. In Vitro and In Vivo Studies—Grape Pomace Antioxidant and Anti-Inflammatory Actions in Ischemic Heart Diseases
| In Vitro Studies | |||
|---|---|---|---|
| Materials | Models | Antioxidant and Anti-Inflammatory Effects | References |
| Resveratrol—100 μmol/L | Neonatal cardiac cells under ischemia/reperfusion exposure |
| [79] |
| Resveratrol—1 mL/2.5 mg/kg food | H2O2 exposed cardiomyocytes sampled from Sprague Dawley rats |
| [80] |
| Resveratrol—3 μM | Human cardiomyocytes’ azidothymidine-induced cardiotoxicity |
| [83] |
| Resveratrol—3 μM | Neonatal human cardiomyocytes in a medium with endotoxin lipopolysaccharide |
| [84] |
| Grape pomace from Vitis vinifera L. cv. Barbera, Carignan, Cabernet Sauvignon, Grenache, Merlot, Petit Verdot, Syrah, Tempranillo and Zinfandel from Baja California, Mexico/methanol extract | Human platelets from 6 healthy persons |
| [86] |
| Grape pomace from Vitis vinifera from Hamburg, Germany | Blood samples |
| [87] |
| 12 GP from white (Italia, Baresana, Beogradska, Autumn Seedless), red (Crimson Seedless, Red Globe, Apulia Rose, Supernova) and black (Autumn Royal, Michele Palieri, Summer Royal) from Turi, Italy | Blood samples from healthy donors Mononuclear cells isolated from the blood samples |
| [88] |
| Resveratrol—25 μM | Neonatal rat cardiac cells in a medium with fractalkine |
| [91] |
| In vivo studies | |||
| Red grape pomace—10% concentration | 60 rats with atherogenic diet-induced ischemic heart disease |
| [78] |
| Grape skin and seed extract from Vitis vinifera cultivated in northern Tunisia—4 g/kg | 24 Wistar male rats with cardiac injury and oxidative stress induced by arsenic |
| [81] |
| Grape seed proanthocyanidins—100 mg/kg, twice a day | Ischemic-induced left ventricle by a 0.09% NaCl–4oC solution from 32 Rattus Norvegicus rats |
| [82] |
| Resveratrol—10 mg/kg IP injection | C57BL/6 mice with endotoxin-induced cardiomyopathy |
| [84] |
| Resveratrol—10 mg/kg/day | L-NAME-induced malignant hypertension mice |
| [85] |
| Resveratrol—20 mg/kg IP injection for 42 days * IP—intraperitoneal injection | C57BL/6 mice with myocardial infarction surgical-induced |
| [91] |
| Resveratrol—2.5 mg/kg for 15 days | Rats under ischemic/reperfusion exposure |
| [92] |
| Methanolic extract from Vitis vinifera seed—125/250 mg/kg | Isoproterenol-induced infarction and streptozotocin-induced diabetes in Wistar mice |
| [93] |
| Resveratrol—30/100 mg/kg for 7 days | Heart sampled from ApoE-KO rats |
| [94] |
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs) Key Facts; WHO: Geneva, Switzerland, 2021; pp. 1–5. [Google Scholar]
- Jensen, R.V.; Hjortbak, M.V.; Bøtker, H.E. Ischemic Heart Disease: An Update. Semin. Nucl. Med. 2020, 50, 195–207. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top 10 Causes of Death—Factsheet; WHO Reports; WHO: Geneva, Switzerland, 2020; pp. 1–9. [Google Scholar]
- Dutheil, F.; Baker, J.S.; Mermillod, M.; De Cesare, M.; Vidal, A.; Moustafa, F.; Pereira, B.; Navel, V. Shift work, and particularly permanent night shifts, promote dyslipidaemia: A systematic review and meta-analysis. Atherosclerosis 2020, 313, 156–169. [Google Scholar] [CrossRef]
- Gow, M.L.; Varley, B.J.; Nasir, R.F.; Skilton, M.R.; Craig, M.E. Aortic intima media thickness in children and adolescents with type 1 diabetes: A systematic review. Pediatr. Diabetes 2022, 23, 489–498. [Google Scholar] [CrossRef] [PubMed]
- EPA. What is a Circular Economy? US EPA: Washington, DC, USA. Available online: https://www.epa.gov/recyclingstrategy/what-circular-economy (accessed on 2 February 2022).
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.; Drăgulinescu, A.-M.; Tomoiagă, L.; Bălăceanu, C.; Iliescu, M. Climate Change and Internet of Things Technologies—Sustainable Premises of Extending the Culture of the Amurg Cultivar in Transylvania—A Use Case for Târnave Vineyard. Sustainability 2021, 13, 8170. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape Phytochemicals and Associated Health Benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine by-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The dose–response effect on polyphenol bioavailability after intake of white and red wine pomace products by Wistar rats. Food Funct. 2020, 11, 1661–1671. [Google Scholar] [CrossRef]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food. Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Fitri, A.; Obitsu, T.; Sugino, T. Effect of ensiling persimmon peel and grape pomace as tannin-rich byproduct feeds on their chemical composition and in vitro rumen fermentation. Anim. Sci. J. 2021, 92, e13524. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Saraci, G.; Sechel, R.; Ciumarnean, L.; Macarie, A.E.; Vlaicu, S.I.; Sava, M.; Vesa, S.C. Grape Pomace Effects and Prevention in Non-alcoholic Steatohepatitis. In Grape Pomace in Health and Disease Prevention; Chedea, V.S., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2022; pp. 209–246. [Google Scholar]
- Neag, M.A.; Mitre, C.I.; Mitre, A.O.; Morhan, V.; Catinean, A.; Botan, E.C.; Melincovici, C.S.; Muntean, D.M.; Buzoianu, A.D. Paradoxical Effect of Grape Pomace Extract on Cisplatin-Induced Acute Kidney Injury in Rats. Pharmaceutics 2019, 11, 656. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols From Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 1522. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Simić, A.; Manojlović, D.; Egan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pop, R.M. Total Polyphenols Content and Antioxidant DPPH Assays on Biological Samples. Polyphen. Plants 2019, 169–183. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and antimicrobial effects of grape pomace extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Marchante, L.; Alonso, S.G.; Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Natural extracts from fresh and oven-dried winemaking by-products as valuable source of antioxidant compounds. Food Sci. Nutr. 2018, 6, 1564–1574. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of polyphenol and crude nutrient content and nutrient digestibility of dried and ensiled white and red grape pomace cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- Jin, Q.; Hair, J.O.; Stewart, A.C.; Keefe, S.F.O.; Neilson, A.P.; Kim, Y.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Industrial White and Red Grape Pomaces in Virginia Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC-DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef] [PubMed]
- Myrtsi, E.; Koulocheri, S.; Iliopoulos, V.; Haroutounian, S. High-Throughput Quantification of 32 Bioactive Antioxidant Phenolic Compounds in Grapes, Wines and Vinification Byproducts by LC–MS/MS. Antioxidants 2021, 10, 1174. [Google Scholar] [CrossRef]
- Posadino, A.M.; Biosa, G.; Zayed, H.; Abou-Saleh, H.; Cossu, A.; Nasrallah, G.K.; Giordo, R.; Pagnozzi, D.; Porcu, M.C.; Pretti, L.; et al. Protective Effect of Cyclically Pressurized Solid–Liquid Extraction Polyphenols from Cagnulari Grape Pomace on Oxidative Endothelial Cell Death. Molecules 2018, 23, 2105. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Demertzis, N.; Mavridou, P.; Karterolioti, H.; Georgadakis, S.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, L.; Statiri, A.; et al. Effects of polyphenolic grape extract on the oxidative status of muscle and endothelial cells. Hum. Exp. Toxicol. 2014, 33, 1099–1112. [Google Scholar] [CrossRef]
- Decean, H.; Fischer-Fodor, E.; Tatomir, C.; Perde-Schrepler, M.; Somfelean, L.; Burz, C.; Hodor, T.; Orasan, R.; Virag, P. Vitis vinifera seeds extract for the modulation of cytosolic factors BAX-α and NF-kB involved in UVB-induced oxidative stress and apoptosis of human skin cells. Clujul Med. 2016, 89, 72. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Dragomir, C.; Taranu, I. Synbiotic combination of prebiotic grape pomace extract and probiotic Lactobacillus sp. reduced important intestinal inflammatory markers and in-depth signalling mediators in lipopolysaccharide-treated Caco-2 cells. Br. J. Nutr. 2019, 121, 291–305. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Bravo, J.; Beltrán-Lissabet, J.F.; Saavedra, K.; Saavedra, N.; Hevia, M.; Alvear, M.; Lanas, F.; Salazar, L.A. Protective effect of Pinot noir pomace extract against the cytotoxicity induced by polycyclic aromatic hydrocarbons on endothelial cells. Food Chem. Toxicol. 2021, 148, 111947. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Kowalski, R.J.; Zhang, S.; Ganjyal, G.M.; Zhu, M.J. Stability and Functionality of Grape Pomace Used as a Nutritive Additive During Extrusion Process. J. Food Process. Technol. 2017, 8, 1–9. Available online: https://www.omicsonline.org/open-access/stability-and-functionality-of-grape-pomace-used-as-a-nutritive-additiveduring-extrusion-process-2157-7110-1000680.php?aid=91452 (accessed on 17 April 2019).
- Domínguez-Perles, R.; Guedes, A.; Queiroz, M.; Silva, A.M.; Barros, A.I. Oxidative stress prevention and anti-apoptosis activity of grape (Vitis vinifera L.) stems in human keratinocytes. Food Res. Int. 2016, 87, 92–102. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. Available online: https://scielo.conicyt.cl/pdf/bres/v48/49.pdf (accessed on 22 March 2022). [CrossRef]
- Pérez-Ramírez, I.F.; De Diego, E.H.; Riomoros-Arranz, M.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Effects of acute intake of grape/pomegranate pomace dietary supplement on glucose metabolism and oxidative stress in adults with abdominal obesity. Int. J. Food Sci. Nutr. 2020, 71, 94–105. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Zapatera, B.; Gallego-Narbón, A.; Vaquero, M.P.; Saura-Calixto, F.; Pérez-Jiménez, J. A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers. Food Funct. 2018, 9, 6010–6019. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Léniz, A.; Martínez-Maqueda, D.; Amézqueta, S.; Fernández-Quintela, A.; Hereu, M.; Torres, J.L.; Portillo, M.P.; Pérez-Jiménez, J. Inter-Individual Variability in Insulin Response after Grape Pomace Supplementation in Subjects at High Cardiometabolic Risk: Role of Microbiota and miRNA. Mol. Nutr. Food Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo® ) in reducing tmao serum levels in humans: Preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Taladrid, D.; de Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension- and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Baldissera, M.D.; Descovi, S.N.; Zeppenfeld, C.C.; Verdi, C.M.; Santos, R.C.; da Silva, A.S.; Baldisserotto, B. Grape pomace flour alleviates Pseudomonas aeruginosa-induced hepatic oxidative stress in grass carp by improving antioxidant defense. Microb. Pathog. 2019, 129, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red grape pomace rich in polyphenols diet increases the antioxidant status in key organs— kidneys, liver, and spleen of piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef]
- Kerasioti, E.; Terzopoulou, Z.; Komini, O.; Kafantaris, I.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Anisimov, N.Y.; Tsatsakis, A.M.; Kouretas, D. Tissue specific effects of feeds supplemented with grape pomace or olive oil mill wastewater on detoxification enzymes in sheep. Toxicol. Rep. 2017, 4, 364–372. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Choi, C.-S.; Chung, H.-K.; Choi, M.-K.; Kang, M.-H. Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Nutr. Res. Pr. 2010, 4, 114–120. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20461199 (accessed on 16 April 2019). [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29747456 (accessed on 17 April 2019). [CrossRef]
- Boussenna, A.; Joubert-Zakeyh, J.; Fraisse, D.; Pereira, B.; Vasson, M.-P.; Texier, O.; Felgines, C. Dietary Supplementation with a Low Dose of Polyphenol-Rich Grape Pomace Extract Prevents Dextran Sulfate Sodium-Induced Colitis in Rats. J. Med. Food 2016, 19, 755–758. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Mukai, R.; Ichiyanagi, T.; Ashida, H. Suppression of Lipopolysaccharide and Galactosamine-Induced Hepatic Inflammation by Red Grape Pomace. J. Agric. Food Chem. 2012, 60, 9315–9320. [Google Scholar] [CrossRef]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of Grape Pomace Antioxidant Extract on Oxidative Stress and Inflammation in Diet Induced Obese Mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Șoica, C.; Iuga, M.; Mironeasa, S. Effects of Supplementing Grape Pomace to Broilers Fed Polyunsaturated Fatty Acids Enriched Diets on Meat Quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- WHO. Tobacco; WHO: Geneva, Switzerland, 2021; pp. 1–8. [Google Scholar]
- WHO. Obesity and Overweight; WHO: Geneva, Switzerland, 2021; pp. 1–6. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Ward, S.; Jones, M.L.; Pandor, A.; Holmes, M.; Ara, R.; Ryan, A.; Yeo, W.; Payne, N. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol. Assess. 2007, 11, 1–160, iii. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Gloviczki, P.; Antignani, P.L.; Comerota, A.J.; Dardik, A.; Davies, A.H.; Eckstein, H.-H.; Faggioli, G.; e Fernandes, J.F.; Fraedrich, G.; et al. Benefits and drawbacks of statins and non-statin lipid lowering agents in carotid artery disease. Prog. Cardiovasc. Dis. 2022, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wu, R.-X.; Zhao, L.; Li, J.; Guo, H.-T.; Fan, R.; Cui, Y.; Wang, Y.-M.; Yue, S.-Q.; Pei, J.-M. Resveratrol Attenuates Ischemia/Reperfusion Injury in Neonatal Cardiomyocytes and Its Underlying Mechanism. PLoS ONE 2012, 7, e51223. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.; Netticadan, T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef]
- Sfaxi, I.; Charradi, K.; Limam, F.; El May, M.V.; Aouani, E. Grape seed and skin extract protects against arsenic trioxide induced oxidative stress in rat heart. Can. J. Physiol. Pharmacol. 2016, 94, 168–176. [Google Scholar] [CrossRef]
- Guler, A.; Sahin, M.A.; Yucel, O.; Yokusoglu, M.; Gamsizkan, M.; Ozal, E.; Demirkilic, U.; Arslan, M. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med. Sci. Monit. 2011, 17, BR326–BR331. [Google Scholar] [CrossRef]
- Pacher, P.; Gao, R.Y.; Mukhopadhyay, P.; Mohanraj, R.; Wang, H.; Horvath, B.; Yin, S. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 2010, 4, 151–155. [Google Scholar] [CrossRef]
- Hao, E.; Lang, F.; Chen, Y.; Zhang, H.; Cong, X.; Shen, X.; Su, G. Resveratrol Alleviates Endotoxin-Induced Myocardial Toxicity via the Nrf2 Transcription Factor. PLoS ONE 2013, 8, e69452. [Google Scholar] [CrossRef]
- Grujić-Milanović, J.; Jaćević, V.; Miloradović, Z.; Jovović, D.; Milosavljević, I.; Milanović, S.D.; Mihailović-Stanojević, N. Resveratrol protects cardiac tissue in experimental malignant hypertension due to antioxidant, anti-inflammatory, and anti-apoptotic properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef]
- Muñoz-Bernal, A.; de la Rosa, L.; Rodrigo-García, J.; Martínez-Ruiz, N.; Sáyago-Ayerdi, S.; Rodriguez, L.; Fuentes, E.; Alvarez-Parrilla, E. Phytochemical Characterization and Antiplatelet Activity of Mexican Red Wines and Their By-products. South Afr. J. Enol. Vitic. 2021, 42, 77–90. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2224-79042021000100009&lng=en&nrm=iso&tlng=en (accessed on 31 August 2022). [CrossRef]
- Bijak, M.; Sut, A.; Kosiorek, A.; Saluk-Bijak, J.; Golanski, J. Dual Anticoagulant/Antiplatelet Activity of Polyphenolic Grape Seeds Extract. Nutrients 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, C.; Milella, R.A.; Incampo, F.; Crupi, P.; Antonacci, D.; Semeraro, N.; Colucci, M. Antithrombotic activity of 12 table grape varieties. Relationship with polyphenolic profile. Food Chem. 2013, 140, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Collen, D. Molecules in focus Tissue factor. Int. J. Biochem. Cell Biol. 1998, 30, 661–667. [Google Scholar] [CrossRef]
- Saha, D.; Sergeeva, E.G.; Ionova, Z.; Gorbach, A. Tissue Factor and Atherothrombosis. Curr. Pharm. Des. 2015, 21, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Wu, B.; Chen, C.; Chen, B.; Zhang, W.; Xu, D.; Bin, J.; Liao, Y. Resveratrol improves myocardial ischemia and ischemic heart failure in mice by antagonizing the detrimental effects of fractalkine. Crit. Care Med. 2012, 40, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mitrovsky, G.; Vasanthi, H.R.; Das, D.K. Antiaging Properties of a Grape-Derived Antioxidant Are Regulated by Mitochondrial Balance of Fusion and Fission Leading to Mitophagy Triggered by a Signaling Network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid. Med. Cell. Longev. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Giribabu, N.; Roslan, J.; Rekha, S.S.; Salleh, N. Methanolic seed extract of Vitis vinifera ameliorates oxidative stress, inflammation and ATPase dysfunction in infarcted and non-infarcted heart of streptozotocin–nicotinamide induced male diabetic rats. Int. J. Cardiol. 2016, 222, 850–865. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol Reverses Endothelial Nitric-Oxide Synthase Uncoupling in Apolipoprotein E Knockout Mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef]

| Materials | Polyphenols Extracts | Models | Antioxidant and Anti-Inflammatory Activity | References |
|---|---|---|---|---|
| Grape pomace from different red Vitis vinifera species | ||||
| GP from Vitis vinifera L. Cagnulari cv. from Santa Maria La Palma, Alghero, Italy | Water/ethanol (60:40, v/v) extract containing:
| H2O2-induced oxidative damage in human umbilical vein endothelial cells |
| [39] |
| GP from Vitis vinifera L. Batiki Tyrnavou cv. from Greece | Ethanol extract containing:
| Tert-butyl hydroperoxide-induced oxidative damage in muscle cells (C2C12) |
| [40] |
| Tert-butyl hydroperoxide-induced oxidative damage in endothelial cells (EA.hy926) |
| |||
| GP from Vitis vinifera seeds | - | UV radiation-induced oxidative stress in human keratinocytes cells (HaCaT cells) |
| [41] |
| GP from Vitis vinifera from Valea Calugareasca | Acetone extract containing:
* higher concentration for procyanidin dimer and epicatechin | Intestinal inflammation model: LPS-inflammation induced in Caco-2 intestinal cells Symbiotic combination with Lactobacillus sp. as probiotic |
| [42] |
| GP from Vitis vinifera variety Montepulciano from Chieti, Italy | Water extract containing:
| H2O2-induced oxidative damage in HypoE22 rat hypothalamus cells |
| [43] |
| GP from Vitis vinifera L. varieties from Emilia Romagna region, Italy | Natural deep eutectic solvents (NaDESs) extract containing:
| Menadione-induced oxidative damage in keratinocyte cells from human skin (HaCaT cells) |
| [44] |
| GP from Vitis vinifera L., cv Negramaro from Azienda Agricola Cantele, Guagnano, Lecce, Italy | Methanol/ethanol (80:20, v/v) extract containing:
| LPS and TNF-α-induced inflammation in human colorectal adenocarcinoma-derived intestinal epithelial cells (Caco-2 cells) and human microvascular endothelial cells (HMEC-1 cells) |
| [45] |
| GP from Vitis vinifera cv Pinot noir from Cautín valley, La Araucanía Region, Chile | Ethanol extract containing:
| Polycyclic aromatic hydrocarbons-induced cytotoxicity in endothelial cells |
| [46] |
| Grape pomace from different white Vitis vinifera species | ||||
| GP from Vitis vinifera cv Chardonnay from Lowden, WA, USA | - | H2O2-induced oxidative damage in human colonic epithelial cells (Caco-2 cells) |
| [47] |
| Red grape pomace versus White grape pomace | ||||
| GP from Vitis vinifera varieties from Quinta da Cavadinha, Pinhão, Portugal Red: Tinto Cão, Tinta Barroca White: Malvasia Fina, Moscatel Branco | Methanol/distilled water (70:30, v/v) extract containing:
| H2O2-induced oxidative damage in human keratinocytes (HaCaT cells) |
| [48] |
| Materials | Polyphenols Extracts | Models | Antioxidant and Anti-Inflammatory Activity | References |
|---|---|---|---|---|
| Grape pomace from different red Vitis vinifera cultivars | ||||
| Vitis vinifera sp. Cabernet Franc from Blackstone, VA, USA | Ethanol extract | Streptozotocin-induced type 2 diabetes in 6-week-old C57BL/6J male mice |
| [36] |
| Vitis vinifera from Uva’Só, Garibaldi, Rio Grande do Sul state, Brazil | - | Pseudomonas aeruginosa-induced hepatic lesion in juvenile grass carps |
| [58] |
| Vitis vinifera from Valea Calugareasca, Romania | Methanol/Acetone extracts | Organs (liver, spleen, kidney) sampled from 20 crossbred TOPIG hybrid (Landrace & Large White with Duroc & Pietrain) pigs |
| [59] |
| Vitis vinifera L. var. Moschato from Tyrnavos (Larissa, Greece) | Water extract | 36 Chios breed male sheep |
| [60] |
| Vitis labruscana L. from Korea | Methanol and ethanol extract | Diet-induced hypercholesterolemia in 48 New Zealand white male rabbits |
| [63] |
| Vitis vinifera from Valea Călugaărească, România | Water extract containing:
| Duodenum and Colon sampled from 20 crossbred TOPIG hybrid (Landrace & Large White with Duroc & Pietrain) pigs |
| [64] |
| Vitis vinifera from the Rhône valley, France | Ethanol extract containing:
| Dextran sodium sulfate-induced inflammatory bowel disease in 5-week-old Wistar male mice |
| [65] |
| Vitis vinifera from Kobe City, Japan | 1% acetic acid in methanol extract | Galactosamine and lipopolysaccharide-induced inflammation in 6-week-old Sprague-Dawley male |
| [69] |
| Vitis vinifera from Virginia vineyard (Blackstone, VA, USA) | Ethanol 1:10 ratio (m/v) extract containing:
| Obese 6 -week-old C57BLK/6J male mice |
| [70] |
| Red grape pomace versus White grape pomace | ||||
| Vitis vinifera from Kobe City, Japan | 1% acetic acid in methanol extract | Galactosamine and lipopolysaccharide-induced inflammation in 6-week-old Sprague-Dawley male |
| [69] |
| Vitis vinifera from Pietroasa, Buzău county, România PUFA enriched * WGP from Tămâioasă Românească variety * RGP from Merlot variety | 3% and 6% dried GP diet | Breast and thigh meat sampled from broilers fed with 3% and 6% grape pomace |
| [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. https://doi.org/10.3390/biomedicines10102337
Bocsan IC, Măgureanu DC, Pop RM, Levai AM, Macovei ȘO, Pătrașca IM, Chedea VS, Buzoianu AD. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines. 2022; 10(10):2337. https://doi.org/10.3390/biomedicines10102337
Chicago/Turabian StyleBocsan, Ioana Corina, Dan Claudiu Măgureanu, Raluca Maria Pop, Antonia Mihaela Levai, Ștefan Octavian Macovei, Ioana Maria Pătrașca, Veronica Sanda Chedea, and Anca Dana Buzoianu. 2022. "Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases" Biomedicines 10, no. 10: 2337. https://doi.org/10.3390/biomedicines10102337
APA StyleBocsan, I. C., Măgureanu, D. C., Pop, R. M., Levai, A. M., Macovei, Ș. O., Pătrașca, I. M., Chedea, V. S., & Buzoianu, A. D. (2022). Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines, 10(10), 2337. https://doi.org/10.3390/biomedicines10102337