Clinical Signs at Diagnosis and Comorbidities in a Large Cohort of Patients with Lipedema in Spain
Abstract
1. Introduction
2. Methods
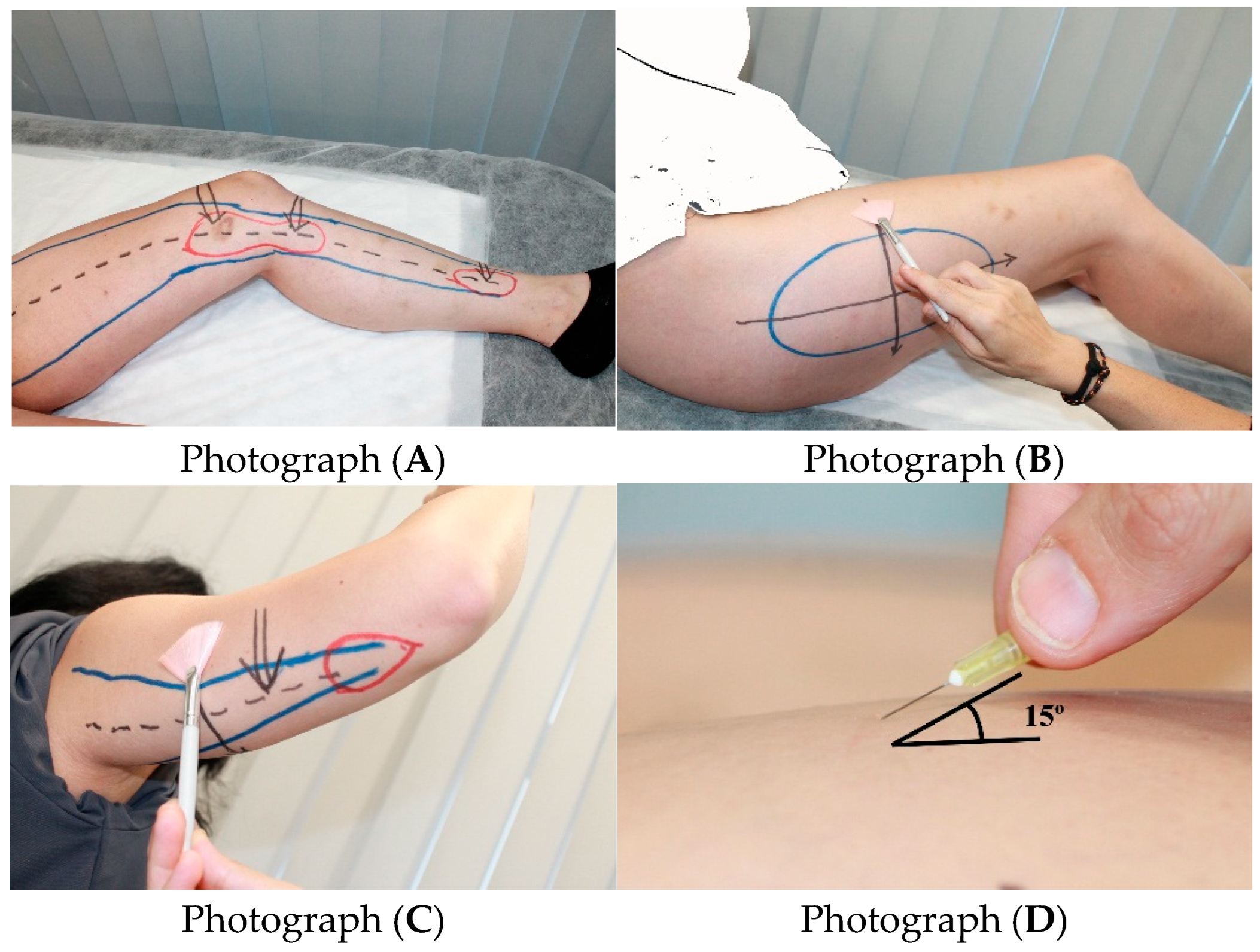
2.1. Variables and Operational Definitions
2.2. Statistical Analysis
3. Results
3.1. General Descriptive Data and Clinical Signs of Lipedema on Physical Examination
3.2. Lipedema Classification and Diagnosis by Anatomical Region Based on Clinical, Ultrasound, and Elastography Findings
3.3. Comorbidities and Lipedema
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcolea, J.M.; Alonso, A.B.; Arroyo, B.A.; Domingo, P.; Galindo, G.A.; Gracia, G.M.; Iglesias, U.C.; Insua, N.E.; Martín, C.E.; Martínez, Á.J.; et al. Consensus Document on Lipedema 2018; Spanish Association of Lymphedema and Lipedema: Barcelona, Spain, 2018. [Google Scholar]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; Mc Donahue, P.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Mortada, H.; Alhithlool, A.W.; AlBattal, N.Z.; Shetty, R.K.; Al-Mekhlafi, G.A.; Hong, J.P.; Alshomer, F. Lipedema: Clinical Features, Diagnosis, and Management. Arch. Plast. Surg. 2025, 52, 185–196. [Google Scholar] [CrossRef]
- Wold, L.E.; Hine, E.A., Jr.; Allen, E.V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (11th Revision). Available online: https://icd.who.int/browse/2025-01/mms/en#1172950828 (accessed on 15 April 2025).
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue-derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef]
- Szél, E.; Kemény, L.; Groma, G.; Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 2014, 83, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Redondo Galán, C.; García Bascones, M.; Marquina Valero, M.A. Lipedema: Clínica, diagnóstico y tratamiento. Revisión de la literatura [Lipoedema: Symptoms, diagnosis and treatment. A literature review]. Rehabilitacion 2019, 53, 104–110. [Google Scholar] [CrossRef]
- Falck, J.; Rolander, B.; Nygårdh, A.; Jonasson, L.L.; Mårtensson, J. Women with lipoedema: A national survey on their health, health-related quality of life, and sense of coherence. BMC Womens Health 2022, 22, 457. [Google Scholar] [CrossRef] [PubMed]
- Luta, X.; Buso, G.; Porceddu, E.; Psychogyiou, R.; Keller, S.; Mazzolai, L. Clinical characteristics, comorbidities, and correlation with advanced lipedema stages: A retrospective study from a Swiss referral centre. PLoS ONE 2025, 20, e0319099. [Google Scholar] [CrossRef]
- Falck, J.; Nygårdh, A.; Rolander, B.; Jonasson, L.L.; Mårtensson, J. Dealing with lipoedema: Women’s experiences of healthcare, self-care, and treatments-a mixed-methods study. BMC Womens Health 2025, 25, 171. [Google Scholar] [CrossRef]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.E.; Białaszek, W.; Ostaszewski, P. Quality of life in women with lipoedema: A contextual behavioral approach. Qual. Life Res. 2016, 25, 401–408. [Google Scholar]
- Clarke, C.; Kirby, J.N.; Smidt, T.; Best, T. Stages of lipoedema: Experiences of physical and mental health and health care. Qual. Life Res. 2023, 32, 127–137. [Google Scholar]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med. Genet. A 2010, 152A, 970–976. [Google Scholar] [PubMed]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1 (AKR1C1) as the First Mutated Gene in a Family with Nonsyndromic Primary Lipedema. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar]
- Klimentidis, Y.C.; Chen, Z.; Gonzalez-Garay, M.L.; Grigoriadis, D.; Sackey, E.; Pittman, A.; Ostergaard, P.; Herbst, K.L. Genome-wide association study of a lipedema phenotype among women in the UK Biobank identifies multiple genetic risk factors. Eur. J. Hum. Genet. 2023, 31, 338–344. [Google Scholar] [CrossRef]
- Michelini, S.; Herbst, K.L.; Precone, V.; Manara, E.; Marceddu, G.; Dautaj, A.; Maltese, P.E.; Paolacci, S.; Ceccarini, M.R.; Beccari, T.; et al. A Multi-Gene Panel to Identify Lipedema-Predisposing Genetic Variants by a Next-Generation Sequencing Strategy. J. Pers. Med. 2022, 12, 268. [Google Scholar]
- Halk, A.B.; Damstra, R.J. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebology 2017, 32, 152–159. [Google Scholar] [CrossRef]
- Aerenhouts, D.; Clarys, P.; Taeymans, J.; Van Cauwenberg, J. Estimating Body Composition in Adolescent Sprint Athletes: Comparison of Different Methods in a 3 Years Longitudinal Design. PLoS ONE 2015, 10, e0136788. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep. Med. 2021, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Miglietta, S.; Borghini, R.; Relucenti, M.; Sorrentino, V.; Chen, R.; Li, X.; Fazi, F.; Donato, G.; Familiari, G.; Petrozza, V.; et al. New Insights into Intestinal Permeability in Irritable Bowel Syndrome-Like Disorders: Histological and Ultrastructural Findings of Duodenal Biopsies. Cells 2021, 10, 2593. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, H.; Ji, L.; Song, P.; Ma, X. Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model. Nutrients 2021, 13, 3515. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Fiasca, F.; Minelli, M.; Maio, D.; Mattei, A.; Vergallo, I.; Cifone, M.G.; Cinque, B.; Minelli, M. The Effects of Low-Nickel Diet Combined with Oral Administration of Selected Probiotics on Patients with Systemic Nickel Allergy Syndrome (SNAS) and Gut Dysbiosis. Nutrients 2020, 12, 1040. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Jain, V.; Chodankar, R.R.; Maybin, J.A.; Critchley, H.O.D. Uterine bleeding: How understanding endometrial physiology underpins menstrual health. Nat. Rev. Endocrinol. 2022, 18, 290–308. [Google Scholar] [CrossRef]
- Velez, L.M.; Seldin, M.; Motta, A.B. Inflammation and reproductive function in women with polycystic ovary syndrome. Biol. Reprod. 2021, 104, 1205–1217. [Google Scholar] [CrossRef]
- Castro, J.; Toro, J.; Salamero, M.; Guimerá, E. The Eating Attitudes Test: Validation of the Spanish version. Evaluación Psicológica/Psychol. Assess. 1991, 7, 175–190. [Google Scholar]
- Garcia-Campayo, J.; Sanz-Carrillo, C.; Ibañez, J.A.; Lou, S.; Solano, V.; Alda, M. Validation of the Spanish version of the SCOFF questionnaire for the screening of eating disorders in primary care. J. Psychosom. Res. 2005, 59, 51–55. [Google Scholar] [CrossRef]
- Schingale, F. Lymphödeme—Lipödeme: Diagnose und Therapie. Ein Ratgeber für Betroffene; Schlütersche Verlagsgesellschaft: Hannover, Germany, 2003. [Google Scholar]
- Ricci, V.; Ricci, C.; Gervasoni, F.; Giulio, C.; Farì, G.; Andreoli, A.; Özçakar, L. From physical to ultrasound examination in lymphedema: A novel dynamic approach. J. Ultrasound 2022, 25, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Pathophysiology of cellulite: Possible involvement of selective endotoxemia. Obes. Rev. 2023, 24, e13517. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Is the endotoxin-complement cascade the major driver in lipedema? Trends Endocrinol. Metab. 2024, 35, 769–780. [Google Scholar]
- Wang, T.J.; Stecco, A.; Schleip, R.; Stecco, C.; Pirri, C. Change in gliding properties of the iliotibial tract in hypermobile Ehlers-Danlos Syndrome. J. Ultrasound 2023, 26, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, B.; Castori, M.; Berglund, B.; Cohen, H.; Grahame, R.; Kazkaz, H.; Levy, H. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Reimer, L.C.U.; Jacobsen, J.S.; Mechlenburg, I. Hypermobility among patients with greater trochanteric pain syndrome. Dan. Med. J. 2019, 66, A5539. [Google Scholar] [PubMed]
- Hou, Z.C.; Ao, Y.F.; Hu, Y.L.; Jiao, C.; Guo, Q.W.; Li, N.; Jiang, Y.-F.; Jiang, D. Balance training benefits chronic ankle instability with generalized joint hypermobility: A prospective cohort study. BMC Musculoskelet. Disord. 2023, 24, 7. [Google Scholar] [CrossRef]
- Huang, B.; Kim, Y.T.; Kim, J.U.; Shin, J.H.; Park, Y.W.; Kim, H.N. Modified Broström Procedure for Chronic Ankle Instability With Generalized Joint Hypermobility. Am. J. Sports Med. 2016, 44, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, X.; Xie, Z.; Ma, L.; Zhong, G.; Li, L.; Huang, W.; Zhang, Y. Kinematic alterations of the ankle in subjects with generalized joint hypermobility compared with the controls: A cross-sectional study. J. Orthop. Surg. 2022, 30, 10225536221125951. [Google Scholar] [CrossRef]
- Croy, T.; Saliba, S.A.; Saliba, E.; Anderson, M.W.; Hertel, J. Differences in lateral ankle laxity measured via stress ultrasonography in individuals with chronic ankle instability, ankle sprain copers, and healthy individuals. J. Orthop. Sports Phys. Ther. 2012, 42, 593–600. [Google Scholar] [CrossRef]
- Schook, C.C.; Mulliken, J.B.; Fishman, S.J.; Alomari, A.I.; Grant, F.D.; Greene, A.K. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast. Reconstr. Surg. 2011, 127, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Askar, O.; Kassem, K.A. A radiological study of the effect of the deep fascia on the communicating veins of the leg. Br. J. Radiol. 1963, 36, 583–585. [Google Scholar] [CrossRef]
- Chung, J.H.; Heo, S. Varicose Veins and the Diagnosis of Chronic Venous Disease in the Lower Extremities. J. Chest Surg. 2024, 57, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Sannikov, A.B.; Emel’ianenko, V.M.; Solokhin, S.A.; Rachkov, M.A.; Drozdova, I.V.; Shaĭdakov, E.V. Sviaz’ perforantnykh i vnutrimyshechnykh ven goleni pri varikoznoĭ bolezni. Angiol. Sosud. Khir. 2021, 27, 73–81. [Google Scholar] [CrossRef]
- Kachlik, D.; Pechacek, V.; Hnatkova, G.; Hnatek, L.; Musil, V.; Baca, V. The venous perforators of the lower limb—A new terminology. Phlebology 2019, 34, 650–668. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, V.; Tajmir, S.; Hedgire, S.S.; Ganguli, S.; Prabhakar, A.M. Lower extremity venous reflux. Cardiovasc. Diagn. Ther. 2016, 6, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Recek, C. Competent and incompetent calf perforators in primary varicose veins: A resistant myth. Phlebology 2015, 31, 532–540. [Google Scholar] [CrossRef]
- Sarin, S.; Scurr, J.H.; Smith, P.D. Medial calf perforators in venous disease: The significance of outward flow. J. Vasc. Surg. 1992, 16, 40–46. [Google Scholar] [CrossRef][Green Version]
- Smith, P.C. Noninvasive venous investigation. Vasc. Med. Rev. 1990, 1, 139–166. [Google Scholar] [CrossRef]
- Niimi, K.; Hirai, M.; Iwata, H.; Miyazaki, K. Ultrasonographic findings and the clinical results of treatment for lymphedema. Ann. Vasc. Dis. 2014, 7, 369–375. [Google Scholar] [CrossRef]
- Rai, P.; Mahajan, A.; Shukla, S.; Pokar, N. Imaging and management of lymphedema in the era of precision oncology. Br. J. Radiol. 2025, 98, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Dimakakos, P.B.; Stefanopoulos, T.; Antoniades, P.; Antoniou, A.; Gouliamos, A.; Rizos, D. MRI and ultrasonographic findings in the investigation of lymphedema and lipedema. Int. Surg. 1997, 82, 411–416. [Google Scholar]
- Faerber, G.; Cornely, M.; Daubert, C.; Erbacher, G.; Fink, J.; Hirsch, T.; Mendoza, E.; Miller, A.; Rabe, E.; Rapprich, S.; et al. S2k guideline lipedema. J. Dtsch. Dermatol. Ges. 2024, 22, 1303–1315. [Google Scholar] [CrossRef]
- Hirsch, T.; Schleinitz, J.; Marshall, M.; Faerber, G. Is the differential diagnosis of lipoedema by means of high-resolution ultrasonography possible? Phlebologie 2018, 47, 182–187. [Google Scholar]
- Caggiati, A. Fascial relationships of the short saphenous vein. J. Vasc. Surg. 2001, 34, 241–246. [Google Scholar] [CrossRef]
- Ghods, M.; Georgiou, I.; Schmidt, J.; Kruppa, P. Disease progression and comorbidities in lipedema patients: A 10-year retrospective analysis. Dermatol. Ther. 2020, 33, e14534. [Google Scholar] [CrossRef]
- Romeijn, J.R.M.; de Rooij, M.J.M.; Janssen, L.; Martens, H. Exploration of Patient Characteristics and Quality of Life in Patients with Lipoedema Using a Survey. Dermatol. Ther. 2018, 8, 303–311. [Google Scholar] [CrossRef]
- Patton, L.; Ricolfi, L.; Bortolon, M.; Gabriele, G.; Zolesio, P.; Cione, E.; Cannataro, R. Observational study on a large Italian population with lipedema: Biochemical and hormonal profile, anatomical and clinical evaluation, self-reported history. Int. J. Mol. Sci. 2024, 25, 1599. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut as a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Legakis, I.; Chrousos, G.P.; Chatzipanagiotou, S. Thyroid Diseases and Intestinal Microbiome. Horm. Metab. Res. 2023, 55, 813–818. [Google Scholar] [CrossRef]
- Tywanek, E.; Michalak, A.; Świrska, J.; Zwolak, A. Autoimmunity, New Potential Biomarkers and the Thyroid Gland-The Perspective of Hashimoto’s Thyroiditis and Its Treatment. Int. J. Mol. Sci. 2024, 25, 4703. [Google Scholar] [CrossRef]
- García-Rabasco, A.E.; Zaragozá-Ninet, V.; García-Ruíz, R.; de la Cuadra-Oyanguren, J. Allergic contact dermatitis due to nickel: Descriptive study in a tertiary hospital, 2000-2010. Actas Dermosifiliogr. 2014, 105, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.F.; Herbst, K.L. A Young Woman with Excessive Fat in Lower Extremities Develops Disordered Eating and Is Subsequently Diagnosed with Anorexia Nervosa, Lipedema, and Hypermobile Ehlers-Danlos Syndrome. Am. J. Case Rep. 2021, 22, e930840. [Google Scholar] [CrossRef] [PubMed]
- MacLean, J.A., 2nd; Hayashi, K. Progesterone Actions and Resistance in Gynecological Disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef]
- Hon, J.X.; Wahab, N.A.; Karim, A.K.A.; Mokhtar, N.M.; Mokhtar, M.H. MicroRNAs in Endometriosis: Insights into Inflammation and Progesterone Resistance. Int. J. Mol. Sci. 2023, 24, 15001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int. J. Mol. Sci. 2023, 24, 6992. [Google Scholar] [CrossRef]
- Grandi, G.; Mueller, M.D.; Papadia, A.; Kocbek, V.; Bersinger, N.A.; Petraglia, F.; Cagnacci, A.; McKinnon, B. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J. Reprod. Immunol. 2016, 117, 30–38. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, S.; Pan, Z.; Zhao, L.; Yang, C.; Li, C.; Wang, F. Immune and endocrine regulation in endometriosis: What we know. J. Endometr. Uterine Disord. 2023, 4, 100049. [Google Scholar] [CrossRef]
| Areas Affected by Lipedema | According to Clinic | Lipedematous Involvement. Ultrasound Sign of a “Snowstorm” | Elastographic Involvement of the Saphenous Compartment Greater Decrease in Elasticity | Lymphatic Involvement Ultrasound Sign (Stone-Paved Sign) |
|---|---|---|---|---|
| Infraumbilical abdomen | Not applicable | Yes/No | Not applicable | Not applicable |
| Hips | Yes/No | Yes/No | Not applicable | Not applicable |
| Upper half of thigh | Yes/No | Yes/No | Yes/No | Yes/No |
| Lower half of thigh | Yes/No | Yes/No | Yes/No | Yes/No |
| Upper half of the calf | Yes/No | Yes/No | Yes/No | Yes/No |
| Lower half of the calf | Yes/No | Yes/No | Yes/No | Yes/No |
| Proximal half of the arm | Yes/No | Not applicable | Not applicable | Not applicable |
| Distal half of the arm (forearm) | Yes/No | Not applicable | Not applicable | Not applicable |
| Feet below the ankle | Yes/No | Not applicable | Not applicable | Yes/No |
| Variables | Total (n = 1803) |
|---|---|
| Sociodemographic | |
| Age. mean (SD) | 42.9 (11.3) |
| Anthropometric | 15.8 [6–11] |
| Weight (kg), mean (SD) | 75 (16.4) |
| Height (cm), mean (SD) | 162 (6.3) |
| Body Mass Index (kg/m2), mean (SD) | 28.6 (6.2) |
| Obesity | |
| Normal weight, n (%) | 576 (31.9%) |
| Overweight type 1, n (%) | 273 (15.1%) |
| Overweight type 2, n (%) | 288 (16%) |
| Obesity type 1, n (%) | 382 (21.2%) |
| Obesity type 2, n (%) | 182 (10.1%) |
| Obesity type 3 (Morbid), n (%) | 90 (5%) |
| Obesity type 4 (Extreme), n (%) | 8 (0.4%) |
| Underweight, n (%) | 4 (0.2%) |
| Fat distribution pattern | |
| Android, n (%) | 220 (12.2%) |
| Gynoid, n (%) | 1583 (87.8%) |
| Bioimpedance analysis | |
| Total Body Fat Mass (%), mean (SD) | 27.7 (11.8) |
| Percentage of body fat relative to total body weight (%), mean (SD) | 34.4 (9.8) |
| Percentage of fat in the lower right limb (%), mean (SD) | 5.2 (2.1) |
| Percentage of body weight of the lower right limb (%), mean (SD) | 37.5 (9.2) |
| Percentage of fat in the lower left limb (%), mean (SD) | 5.1 (2) |
| Percentage of body weight of the lower left limb (%), mean (SD) | 37.5 (9.3) |
| Physical examination | |
| Deep pressure pain in the Great Saphenous Compartment (upper half), n (%) | 1780 (98.7%) |
| Deep pressure pain in the Great Saphenous Compartment (lower half), n (%) | 1775 (98.4%) |
| Deep pressure pain in the Small Saphenous Compartment, n (%) | 1738 (96.4%) |
| Superficial pressure pain in the Post. Brachial Compartment, n (%) | 1443 (80%) |
| Tender points | |
| Internal supracondylar, n (%) | 1781 (98.8%) |
| Internal infracondylar, n (%) | 1785 (99%) |
| Internal supramalleolar, n (%) | 1750 (97.1%) |
| Supraolecranon, n (%) | 1474 (81.8%) |
| False puncture Internal supracondylar, n (%) | 1758 (97.5%) |
| False puncture Internal supramalleolar, n (%) | 1730 (96%) |
| False puncture Supraolecranian, n (%) | 1411 (78.3%) |
| Loss of fine sensation at the pertrochanteric level, n (%) (n = 195) | 176 (90.3%) |
| Kaposi-Stemmer sign | |
| Positive, n (%) | 138 (7.7%) |
| Equivocal, n (%) | 63 (3.5%) |
| Internal supramalleolar edema, n (%) (n = 256) | 229 (89.5) |
| Internal supramalleolar pitting edema, n (%) (n = 1749) | 97 (5.5%) |
| Pretibial edema, n (%) (n = 293) | 259 (88.4%) |
| Pretibial pitting edema, n (%) (n = 270) | 156 (57.8%) |
| Obesity | Fat Distribution Pattern | GSC Pain | ISC Pain | SSC Pain | BC Pain | ISC Point | IIC Point | ISM Point | SO Point | IS False Puncture | IM False Puncture | SO False Puncture | PFS Loss | Weight | Age | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity | r | 1 | −0.315 ** | 0.061 ** | 0.062 ** | 0.087 ** | 0.251 ** | 0.044 | 0.053 * | 0.075 ** | 0.237 ** | 0.048 * | 0.056 * | 0.230 ** | 0.063 | 0.890 ** | 0.181 ** |
| p | 0.000 | 0.009 | 0.008 | 0.000 | 0.000 | 0.062 | 0.024 | 0.001 | 0.000 | 0.043 | 0.017 | 0.000 | 0.380 | 0.000 | 0.000 | ||
| Fat distribution pattern | r | −0.315 ** | 1 | 0.018 | −0.033 | −0.045 | −0.097 ** | 0.020 | −0.003 | −0.006 | −0.105 ** | −0.005 | 0.018 | −0.084 ** | 0.008 | −0.258 ** | −0.311 ** |
| p | 0.000 | 0.440 | 0.161 | 0.054 | 0.000 | 0.385 | 0.891 | 0.811 | 0.000 | 0.827 | 0.438 | 0.000 | 0.906 | 0.000 | 0.000 | ||
| GSC pain | r | 0.061 ** | 0.018 | 1 | 0.545 ** | 0.346 ** | 0.141 ** | 0.708 ** | 0.635 ** | 0.444 ** | 0.125 ** | 0.362 ** | 0.303 ** | 0.096 ** | 0.219 ** | 0.054 * | −0.032 |
| p | 0.009 | 0.440 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.022 | 0.172 | ||
| ISC pain | r | 0.062 ** | −0.033 | 0.545 ** | 1 | 0.525 ** | 0.139 ** | 0.517 ** | 0.619 ** | 0.636 ** | 0.173 ** | 0.354 ** | 0.429 ** | 0.129 ** | −0.024 | 0.033 | 0.010 |
| p | 0.008 | 0.161 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.743 | 0.157 | 0.667 | ||
| SSC pain | r | 0.087 ** | −0.045 | 0.346 ** | 0.525 ** | 1 | 0.264 ** | 0.328 ** | 0.396 ** | 0.503 ** | 0.252 ** | 0.253 ** | 0.350 ** | 0.234 ** | 0.099 | 0.091 ** | 0.008 |
| p | 0.000 | 0.054 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.167 | 0.000 | 0.743 | ||
| BC pain | r | 0.251 ** | −0.097 ** | 0.141 ** | 0.139 ** | 0.264 ** | 1 | 0.083 ** | 0.131 ** | 0.156 ** | 0.885 ** | 0.116 ** | 0.151 ** | 0.857 ** | 0.053 | 0.223 ** | 0.064 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.461 | 0.000 | 0.006 | ||
| ISC point | r | 0.044 | 0.020 | 0.708 ** | 0.517 ** | 0.328 ** | 0.083 ** | 1 | 0.599 ** | 0.514 ** | 0.117 ** | 0.371 ** | 0.259 ** | 0.101 ** | −0.024 | 0.048 * | −0.001 |
| p | 0.062 | 0.385 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.743 | 0.041 | 0.965 | ||
| IIC point | r | 0.053 * | −0.003 | 0.635 ** | 0.619 ** | 0.396 ** | 0.131 ** | 0.599 ** | 1 | 0.506 ** | 0.140 ** | 0.485 ** | 0.404 ** | 0.109 ** | 0.052 * | 0.000 | |
| p | 0.024 | 0.891 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.027 | 0.997 | ||
| ISM point | r | 0.075 ** | −0.006 | 0.444 ** | 0.636 ** | 0.503 ** | 0.156 ** | 0.514 ** | 0.506 ** | 1 | 0.195 ** | 0.306 ** | 0.509 ** | 0.152 ** | 0.196 ** | 0.063 ** | 0.039 |
| p | 0.001 | 0.811 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 | 0.007 | 0.098 | ||
| SO point | r | 0.237 ** | −0.105 ** | 0.125 ** | 0.173 ** | 0.252 ** | 0.885 ** | 0.117 ** | 0.140 ** | 0.195 ** | 1 | 0.099 ** | 0.143 ** | 0.827 ** | 0.029 | 0.210 ** | 0.074 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.682 | 0.000 | 0.002 | ||
| IS False puncture | r | 0.048 * | −0.005 | 0.362 ** | 0.354 ** | 0.253 ** | 0.116 ** | 0.371 ** | 0.485 ** | 0.306 ** | 0.099 ** | 1 | 0.544 ** | 0.122 ** | −0.041 | 0.030 | 0.024 |
| p | 0.043 | 0.827 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.569 | 0.204 | 0.311 | ||
| IM False puncture | r | 0.056 * | 0.018 | 0.303 ** | 0.429 ** | 0.350 ** | 0.151 ** | 0.259 ** | 0.404 ** | 0.509 ** | 0.143 ** | 0.544 ** | 1 | 0.199 ** | 0.093 | 0.042 | 0.009 |
| p | 0.017 | 0.438 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.198 | 0.075 | 0.708 | ||
| SO False puncture | r | 0.230 ** | −0.084 ** | 0.096 ** | 0.129 ** | 0.234 ** | 0.857 ** | 0.101 ** | 0.109 ** | 0.152 ** | 0.827 ** | 0.122 ** | 0.199 ** | 1 | 0.049 | 0.202 ** | 0.071 ** |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.498 | 0.000 | 0.003 | ||
| PFS Loss | r | 0.063 | 0.008 | 0.219 ** | −0.024 | 0.099 | 0.053 | −0.024 | --- | 0.196 ** | 0.029 | −0.041 | 0.093 | 0.049 | 1 | 0.120 | 0.006 |
| p | 0.380 | 0.906 | 0.002 | 0.743 | 0.167 | 0.461 | 0.743 | 0.000 | 0.006 | 0.682 | 0.569 | 0.198 | 0.498 | 0.096 | 0.937 | ||
| Weight | r | 0.890 ** | −0.258 ** | 0.054 * | 0.033 | 0.091 ** | 0.223 ** | 0.048 * | 0.052 * | 0.063 ** | 0.210 ** | 0.030 | 0.042 | 0.202 ** | 0.120 | 1 | 0.101 ** |
| p | 0.000 | 0.000 | 0.022 | 0.157 | 0.000 | 0.000 | 0.041 | 0.027 | 0.007 | 0.000 | 0.204 | 0.075 | 0.000 | 0.096 | 0.000 | ||
| Age | r | 0.181 ** | −0.311 ** | −0.032 | 0.010 | 0.008 | 0.064 ** | −0.001 | 0.000 | 0.039 | 0.074 ** | 0.024 | 0.009 | 0.071 ** | 0.006 | 0.101 ** | 1 |
| p | 0.000 | 0.000 | 0.172 | 0.667 | 0.743 | 0.006 | 0.965 | 0.997 | 0.098 | 0.002 | 0.311 | 0.708 | 0.003 | 0.937 | 0.000 | ||
| Variables | Total |
|---|---|
| Classification of Schingale, n (%) (n = 1796) | |
| Type I | 290 (1.6%) |
| Type II | 392 (21.8%) |
| Type III | 539 30%) |
| Type IV | 768 (42.8%) |
| Type V | 68 (3.8%) |
| Classification of Schmeller, n (%) (n = 1792) | |
| Stage I | 799 (44.6%) |
| Stage II | 674 (37.6%) |
| Stage III | 233 (13%) |
| Clinical diagnosis, n (%) (n = 1802) | |
| Hips | 1787 (99.2%) |
| Upper thigh | 1787 (99.2%) |
| Lower thigh | 1780 (98.8%) |
| Upper calf | 1640 (91%) |
| Lower calf | 1557 (86.4%) |
| Proximal arm | 899 (49.9%) |
| Distal arm | 90 (5%) |
| Feet below the ankle, (n = 1693) | 21 (1.2%) |
| Diagnosis based on the “Snowstorm” ultrasound sign, n (%) (n = 1801) | |
| Abdomen | 2 (0.1%) |
| Hips | 11 (0.6%) |
| Upper thigh | 1765 (97.9%) |
| Lower thigh | 1761 (97.7%) |
| Upper calf | 1728 (95.9%) |
| Lower calf | 1700 (94.3%) |
| Diagnosis based on elastographic involvement of the great saphenous vein compartment, n (%) (n = 1802) | |
| Upper thigh | 1789 (99.3%) |
| Lower thigh | 1761 (97.7%) |
| Upper calf | 1356 (75.2%) |
| Lower calf | 1119 (62.1%) |
| Diagnosis based on the “Stone-paved sign” ultrasound sign, n (%) (n = 1802) | |
| Upper thigh | 2 (0.1%) |
| Lower thigh | 1 (0.1%) |
| Upper calf | 164 (9.1%) |
| Lower calf | 851 (47.2%) |
| Feet below the ankle | 125 (6.9%) |
| Stone-Paved Sign UT | Stone-Paved Sign LT | Stone-Paved Sign UC | Stone-Paved Sign LC | Stone-Paved Sign Below the Ankle | Classification of Schingale | Classification of Schmeller | Age | Weight | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Stone-paved sign UT | r | 1 | −0.001 | −0.011 | 0.002 | −0.009 | 0.028 | −0.011 | 0.020 | 0.003 |
| p | 0.973 | 0.655 | 0.937 | 0.699 | 0.237 | 0.640 | 0.408 | 0.893 | ||
| Stone-paved sign LT | r | −0.001 | 1 | −0.007 | 0.025 | −0.006 | 0.020 | 0.032 | −0.023 | 0.055 * |
| p | 0.973 | 0.752 | 0.291 | 0.785 | 0.403 | 0.183 | 0.334 | 0.020 | ||
| Stone-paved sign UC | r | −0.011 | −0.007 | 1 | 0.319 ** | 0.410 ** | 0.221 ** | 0.224 ** | 0.107 ** | 0.284 ** |
| p | 0.655 | 0.752 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Stone-paved sign LC | r | 0.002 | 0.025 | 0.319 ** | 1 | 0.272 ** | 0.376 ** | 0.287 ** | 0.147 ** | 0.353 ** |
| p | 0.937 | 0.291 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Stone-paved sign below the ankle | r | −0.009 | −0.006 | 0.410 ** | 0.272 ** | 1 | 0.123 ** | 0.173 ** | 0.027 | 0.176 ** |
| p | 0.699 | 0.785 | 0.000 | 0.000 | 0.000 | 0.000 | 0.257 | 0.000 | ||
| Classification of Schingale | r | 0.028 | 0.020 | 0.221 ** | 0.376 ** | 0.123 ** | 1 | 0.388 ** | 0.167 ** | 0.487 ** |
| p | 0.237 | 0.403 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Classification of Schmeller | r | −0.011 | 0.032 | 0.224 ** | 0.287 ** | 0.173 ** | 0.388 ** | 1 | 0.198 ** | 0.541 ** |
| p | 0.640 | 0.183 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Age | r | 0.020 | −0.023 | 0.107 ** | 0.147 ** | 00.027 | 0.167 ** | 0.198 ** | 1 | 0.094 ** |
| p | 0.408 | 0.334 | 0.000 | 0.000 | 0.257 | 0.000 | 0.000 | 0.000 | ||
| Weight | r | 0.003 | 0.055 * | 0.284 ** | 0.353 ** | 0.176 ** | 0.487 ** | 0.541 ** | 0.094 ** | 1 |
| p | 0.893 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Variables | Total |
|---|---|
| Suspected high intestinal permeability, n (%) | 1785 (99%) |
| Ligamentous hyperlaxity syndrome, n (%) (n = 1801) | 1726 (95.8%) |
| Beighton test (0–9), media (DE) | 7.5 (1.6) |
| Bilateral trochanteric pain region, n (%) (n = 1799) | 1753 (97.4%) |
| Iliotibial band involvement (Tensor fascia lata), n (%) (n = 1799) | 1737 (96.6%) |
| Non-dislocated, recurrent ankle sprains due to mechanical instability, n (%) (n = 1798) | 1106 (61.5%) |
| Nickel allergy, n (%) (n = 130) | 88 (67.7%) |
| Thyroid pathology, n (%) (n = 1801) | 1073 (59.5%) |
| Type of thyroid pathology, n (%) (n = 533) | |
| Thyroid nodules | 473 (88.7%) |
| Multinodular goitre | 46 (8.6%) |
| Hypothyroidism | 13(2.4%) |
| Hashimoto’s syndrome | 1 (0.2%) |
| Inflammatory ovarian dysfunction, n (%) (n = 1801) | 1368 (76%) |
| Great saphenous vein, on Doppler ultrasound observation, n (%) (n = 538) | 46 (9.1%) |
| Pain in paratibial perforators, n (%) (n = 543) | 519 (95.6%) |
| Pain in post-tibial perforators, n (%) (n = 538) | 516 (95.9%) |
| Treatment with psychologist, n (%) (n = 531) | 329 (62%) |
| Scoff test (0–5), median [p25–p75] | 2 [1–3] |
| Test EAT-40 (0–120), median [p25–p75] | 21 [14–30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simarro Blasco, J.L.; Michelini, S.; Andrés-Gasco, M.; Lebrero García, A.; Ortega Abad, D.; Margalejo Lombardo, J.; Buj Vargas, J.; Sanchéz-Costa, J.T.; Martín Martínez, M.A. Clinical Signs at Diagnosis and Comorbidities in a Large Cohort of Patients with Lipedema in Spain. Biomedicines 2025, 13, 3049. https://doi.org/10.3390/biomedicines13123049
Simarro Blasco JL, Michelini S, Andrés-Gasco M, Lebrero García A, Ortega Abad D, Margalejo Lombardo J, Buj Vargas J, Sanchéz-Costa JT, Martín Martínez MA. Clinical Signs at Diagnosis and Comorbidities in a Large Cohort of Patients with Lipedema in Spain. Biomedicines. 2025; 13(12):3049. https://doi.org/10.3390/biomedicines13123049
Chicago/Turabian StyleSimarro Blasco, José Luis, Sandro Michelini, Miguel Andrés-Gasco, Alberto Lebrero García, Desirée Ortega Abad, José Margalejo Lombardo, Julian Buj Vargas, Jesús Tomás Sanchéz-Costa, and María Auxiliadora Martín Martínez. 2025. "Clinical Signs at Diagnosis and Comorbidities in a Large Cohort of Patients with Lipedema in Spain" Biomedicines 13, no. 12: 3049. https://doi.org/10.3390/biomedicines13123049
APA StyleSimarro Blasco, J. L., Michelini, S., Andrés-Gasco, M., Lebrero García, A., Ortega Abad, D., Margalejo Lombardo, J., Buj Vargas, J., Sanchéz-Costa, J. T., & Martín Martínez, M. A. (2025). Clinical Signs at Diagnosis and Comorbidities in a Large Cohort of Patients with Lipedema in Spain. Biomedicines, 13(12), 3049. https://doi.org/10.3390/biomedicines13123049






