Prenatal Low Testosterone Levels Induced by DNAH8 Dysfunction Leads to Urethral Fusion and Male Differentiation Abnormalities
Abstract
1. Introduction
2. Materials and Methods
2.1. DNAH8 Knockout Mouse Model
2.2. Morphological Measurements
2.3. ELISA Testosterone Measurement
2.4. Immunofluorescence Staining
2.5. Label-Free Quantitative Mass Spectrometry-Based Proteomics and LC-MS/MS Analysis
2.6. RT-qPCR
2.7. Single-Cell RNA-Seq
2.8. Quantification and Statistical Analysis
3. Results
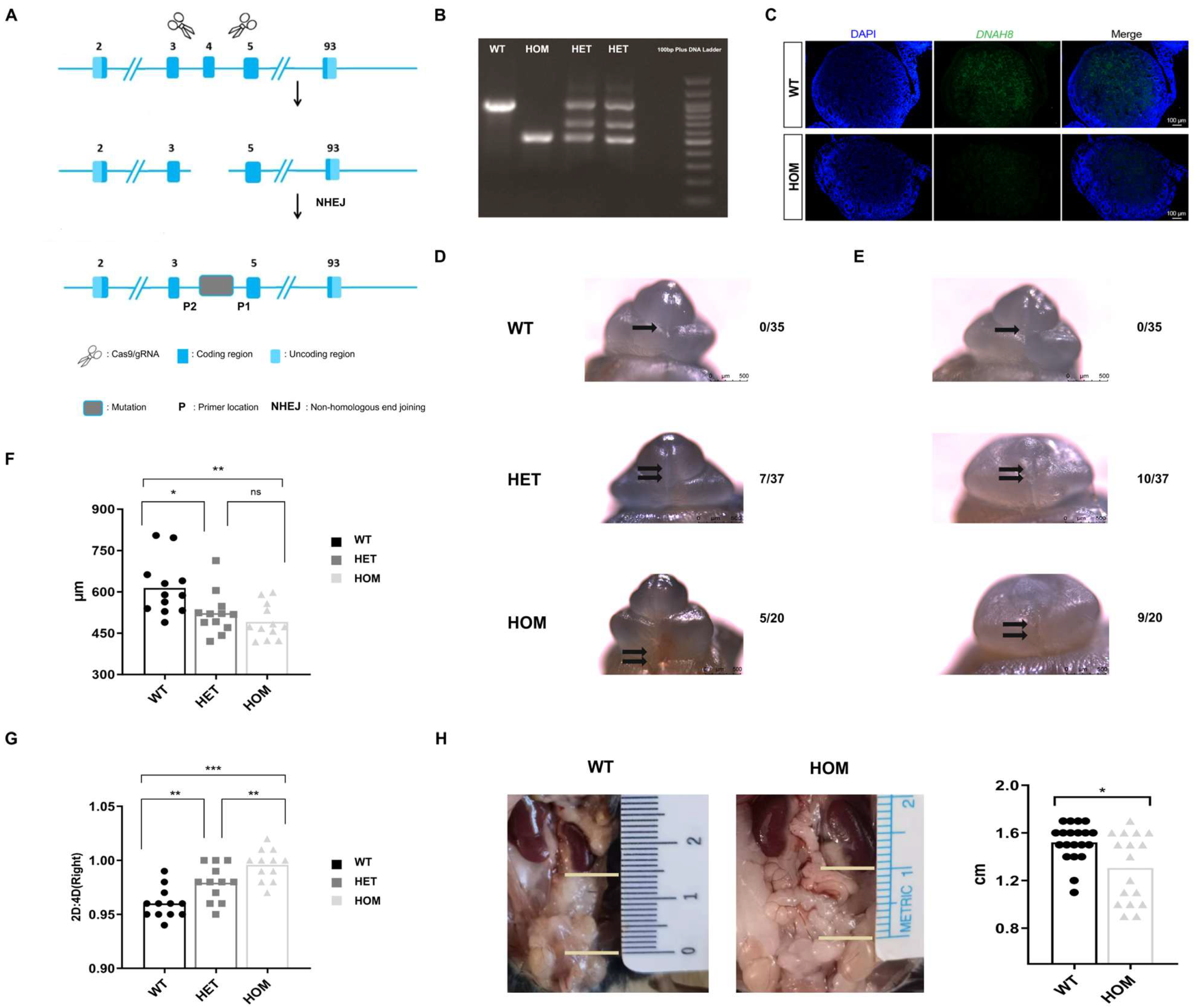
3.1. Generation of the DNAH8 KO Mouse Model
3.2. Loss of DNAH8 Function Leads to Abnormalities in the Development of the Male Urethra and the Masculinization Phenotype
3.3. Overview of the General Cell Populations in the Fetal External Genitalia
3.4. Distal Glans Cell Significant Deficiency Is a Major Pathological Feature in the Early Differentiation Stages of DNAH8 KO Fetal External Genitalia
3.5. DNAH8 Deletion Decreases Prenatal Testosterone Level and Steroid Biosynthesis
3.6. Characterization of the Cellular Composition of the Fetal Testis
3.7. Loss of DNAH8 Delays the Differentiation of Cells in the Sertoli and Steroidogenic Lineages
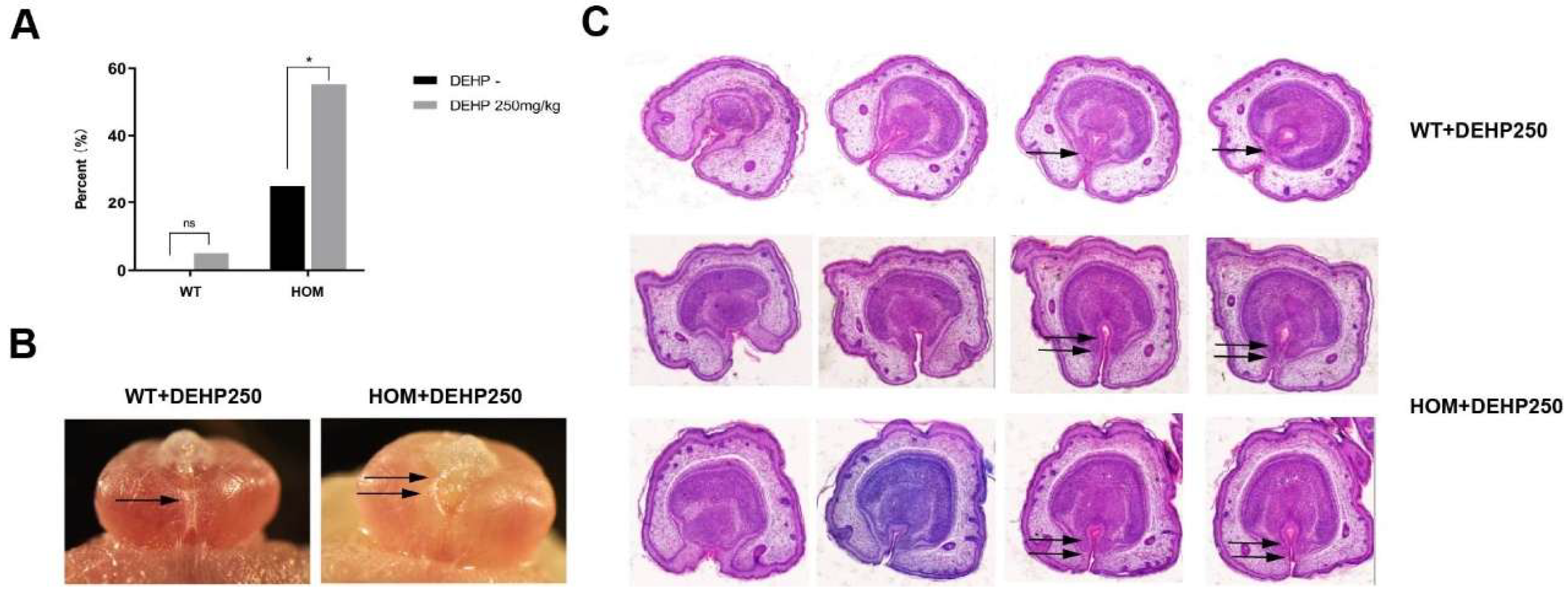
3.8. The Synergistic Effect of DNAH8 Deficiency and Low-Dose DEHP Can Lead to Hypospadias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Nassar, N.; Mastroiacovo, P.; Canfield, M.; Groisman, B.; Bermejo-Sanchez, E.; Ritvanen, A.; Kiuru-Kuhlefelt, S.; Benavides, A.; Sipek, A.; et al. Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980–2010. Eur. Urol. 2019, 76, 482–490. [Google Scholar] [CrossRef]
- Leunbach, T.L.; Berglund, A.; Ernst, A.; Hvistendahl, G.M.; Rawashdeh, Y.F.; Gravholt, C.H. Prevalence, Incidence, and Age at Diagnosis of Boys with Hypospadias: A Nationwide Population-Based Epidemiological Study. J. Urol. 2025, 21, 350–360. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, H.J.; de Wall, L.L. Hypospadias, all there is to know. Eur. J. Pediatr. 2017, 176, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Suzuki, K.; Murashima, A.; Kajioka, D.; Acebedo, A.R.; Miyagawa, S.; Haraguchi, R.; Ogino, Y.; Yamada, G. Regulation of masculinization: Androgen signalling for external genitalia development. Nat. Rev. Urol. 2018, 15, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Albadawi, E.A.; Alzaman, N.S.; Elhassan, Y.H.; Eltahir, H.M.; Abouzied, M.M.; Albadrani, M.S. The Association between Maternal Endocrine-Disrupting Chemical Exposure during Pregnancy and the Incidence of Male Urogenital Defects: A Systematic Review and Meta-Analysis. Metabolites 2024, 14, 477. [Google Scholar] [CrossRef]
- Joodi, M.; Amerizadeh, F.; Hassanian, S.M.; Erfani, M.; Ghayour-Mobarhan, M.; Ferns, G.A.; Khazaei, M.; Avan, A. The genetic factors contributing to hypospadias and their clinical utility in its diagnosis. J. Cell Physiol. 2019, 234, 5519–5523. [Google Scholar] [CrossRef]
- Sharpe, R.M. Androgens and the masculinization programming window: Human–rodent differences. Biochem. Soc. Trans. 2020, 48, 1725–1735. [Google Scholar] [CrossRef]
- Singal, A.K.; Jain, V.G.; Gazali, Z.; Shekhawat, P. Shorter anogenital distance correlates with the severity of hypospadias in pre-pubertal boys. Hum. Reprod. 2016, 31, 1406–1410. [Google Scholar] [CrossRef]
- Thalluri, V.; Woodman, R.J.; Vollenhoven, B.; Tremellen, K.; Zander-Fox, D. Exposure to corticosteroids in the first trimester is associated with an increased risk of urogenital congenital anomalies. Hum. Reprod. 2022, 37, 2167–2174. [Google Scholar] [CrossRef]
- Wong, Y.S.; Tam, Y.H.; Pang, K.K.Y.; Yau, H.C. Incidence and diagnoses of disorders of sex development in proximal hypospadias. J. Pediatr. Surg. 2018, 53, 2498–2501. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Z.Q.; Pan, W.F.; Chen, H.J.; Wu, M.; Lyu, Y.Q.; Xie, H.; Huang, Y.C.; Chen, Z.Z.; Chen, F. The association and underlying mechanism of the digit ratio (2D:4D) in hypospadias. Asian J. Androl. 2024, 26, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Schnack, T.H.; Zdravkovic, S.; Myrup, C.; Westergaard, T.; Christensen, K.; Wohlfahrt, J.; Melbye, M. Familial aggregation of hypospadias: A cohort study. Am. J. Epidemiol. 2008, 167, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Geller, F.; Feenstra, B.; Carstensen, L.; Pers, T.H.; van Rooij, I.A.; Korberg, I.B.; Choudhry, S.; Karjalainen, J.M.; Schnack, T.H.; Hollegaard, M.V.; et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat. Genet. 2014, 46, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Ea, V.; Bergougnoux, A.; Philibert, P.; Servant-Fauconnet, N.; Faure, A.; Breaud, J.; Gaspari, L.; Sultan, C.; Paris, F.; Kalfa, N. How Far Should We Explore Hypospadias? Next-generation Sequencing Applied to a Large Cohort of Hypospadiac Patients. Eur. Urol. 2021, 79, 507–515. [Google Scholar] [CrossRef]
- Sveinbjornsson, G.; Albrechtsen, A.; Zink, F.; Gudjonsson, S.A.; Oddson, A.; Masson, G.; Holm, H.; Kong, A.; Thorsteinsdottir, U.; Sulem, P.; et al. Weighting sequence variants based on their annotation increases power of whole-genome association studies. Nat. Genet. 2016, 48, 314–317. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, Y.; Finnell, R.H.; Ding, Y.; Su, Z.; Wang, Y.; Xie, H.; Chen, F. Whole-exome sequencing study of hypospadias. iScience 2023, 26, 106663. [Google Scholar] [CrossRef]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef]
- Armfield, B.A.; Cohn, M.J. Single cell transcriptomic analysis of external genitalia reveals complex and sexually dimorphic cell populations in the early genital tubercle. Dev. Biol. 2021, 477, 145–154. [Google Scholar] [CrossRef]
- Amato, C.M.; Yao, H.H. Developmental and sexual dimorphic atlas of the prenatal mouse external genitalia at the single-cell level. Proc. Natl. Acad. Sci. USA 2021, 118, e2103856118. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.; Shen, J.; Sinclair, A.; Baskin, L.; Cunha, G.R. Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation 2018, 101, 46–64. [Google Scholar] [CrossRef]
- Li, Y.; Sinclair, A.; Cao, M.; Shen, J.; Choudhry, S.; Botta, S.; Cunha, G.; Baskin, L. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: The double zipper hypothesis. J. Urol. 2015, 193, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, G.; Shen, J.; Yue, A.; Isaacson, D.; Sinclair, A.; Cao, M.; Liaw, A.; Cunha, G.R.; Baskin, L. Human glans and preputial development. Differentiation 2018, 103, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Huang, F.; Liu, Y.; Cao, X.; Shen, L.; Long, C.; He, D.; Lin, T.; Wei, G. Epithelial-mesenchymal transformation and apoptosis in rat urethra development. Pediatr. Res. 2017, 82, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Ademi, H.; Djari, C.; Mayere, C.; Neirijnck, Y.; Sararols, P.; Rands, C.M.; Stevant, I.; Conne, B.; Nef, S. Deciphering the origins and fates of steroidogenic lineages in the mouse testis. Cell Rep. 2022, 39, 110935. [Google Scholar] [CrossRef]
- Stevant, I.; Neirijnck, Y.; Borel, C.; Escoffier, J.; Smith, L.B.; Antonarakis, S.E.; Dermitzakis, E.T.; Nef, S. Deciphering Cell Lineage Specification during Male Sex Determination with Single-Cell RNA Sequencing. Cell Rep. 2018, 22, 1589–1599. [Google Scholar] [CrossRef]
- Suen, H.C.; Rao, S.; Luk, A.C.S.; Zhang, R.; Yang, L.; Qi, H.; So, H.C.; Hobbs, R.M.; Lee, T.L.; Liao, J. The single-cell chromatin accessibility landscape in mouse perinatal testis development. Elife 2023, 12, e75624. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; DonorConnect; Plath, K.; Hotaling, J.M.; Stukenborg, J.B.; et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021, 28, 764–778e4. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, M.; Wen, Q.; Li, Y.; Wang, Y.; Wang, Y.; Qin, Y.; Cui, X.; Yang, L.; Huff, V.; et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc. Natl. Acad. Sci. USA 2015, 112, 4003–4008. [Google Scholar] [CrossRef]
- Liu, C.; Rodriguez, K.; Yao, H.H.C. Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development 2016, 143, 3700–3710. [Google Scholar] [CrossRef]
- Mayere, C.; Regard, V.; Perea-Gomez, A.; Bunce, C.; Neirijnck, Y.; Djari, C.; Bellido-Carreras, N.; Sararols, P.; Reeves, R.; Greenaway, S.; et al. Origin, specification and differentiation of a rare supporting-like lineage in the developing mouse gonad. Sci. Adv. 2022, 8, eabm0972. [Google Scholar] [CrossRef]
- Kothandapani, A.; Lewis, S.R.; Noel, J.L.; Zacharski, A.; Krellwitz, K.; Baines, A.; Winske, S.; Vezina, C.M.; Kaftanovskaya, E.M.; Agoulnik, A.I.; et al. GLI3 resides at the intersection of hedgehog and androgen action to promote male sex differentiation. PLoS Genet. 2020, 16, e1008810. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Li, S.; Zhao, S.; Li, F.; Li, X. Transcriptome and proteome analyses reveal the mechanisms involved in polystyrene nanoplastics disrupt spermatogenesis in mice. Environ. Pollut. 2024, 342, 123086. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Sha, Y.; Zeng, Y.; Huang, N.; Liu, W.; Zhang, X.; Zhou, H. Mutations in DNAH8 contribute to multiple morphological abnormalities of sperm flagella and male infertility. Acta Biochim. Biophys. Sin. 2021, 53, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, C.; Zhang, X.; Liu, X.; Li, J.; Qiao, X.; Liu, H.; Shen, Y. Loss-of-function mutation in DNAH8 induces asthenoteratospermia associated with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2020, 98, 396–401. [Google Scholar] [CrossRef]
- Tang, D.; Sha, Y.; Gao, Y.; Zhang, J.; Cheng, H.; Zhang, J.; Ni, X.; Wang, C.; Xu, C.; Geng, H.; et al. Novel variants in DNAH9 lead to nonsyndromic severe asthenozoospermia. Reprod. Biol. Endocrinol. 2021, 19, 27. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, H.; Khan, T.; Ma, A.; Li, T.; Zhang, H.; Gao, J.; Zhou, J.; Li, Y.; Yu, C.; et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J. Exp. Med. 2020, 217, e20182365. [Google Scholar] [CrossRef]
- Yamada, G.; Suzuki, K.; Haraguchi, R.; Miyagawa, S.; Satoh, Y.; Kamimura, M.; Nakagata, N.; Kataoka, H.; Kuroiwa, A.; Chen, Y. Molecular genetic cascades for external genitalia formation: An emerging organogenesis program. Dev. Dyn. 2006, 235, 1738–1752. [Google Scholar] [CrossRef]
- Miyagawa, S.; Matsumaru, D.; Murashima, A.; Omori, A.; Satoh, Y.; Haraguchi, R.; Motoyama, J.; Iguchi, T.; Nakagata, N.; Hui, C.C.; et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 2011, 152, 2894–2903. [Google Scholar] [CrossRef]
- Miyagawa, S.; Moon, A.; Haraguchi, R.; Inoue, C.; Harada, M.; Nakahara, C.; Suzuki, K.; Matsumaru, D.; Kaneko, T.; Matsuo, I.; et al. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development 2009, 136, 3969–3978. [Google Scholar] [CrossRef]
- Lin, C.; Yin, Y.; Long, F.; Ma, L. Tissue-specific requirements of beta-catenin in external genitalia development. Development 2008, 135, 2815–2825. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. TGF-beta—an excellent servant but a bad master. J. Transl. Med. 2012, 10, 183. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, F.; Liu, Y.; Li, D.; Zhou, Y.; Shen, L.; Long, C.; Liu, X.; Wei, G. TGF-beta1 relieves epithelial-mesenchymal transition reduction in hypospadias induced by DEHP in rats. Pediatr. Res. 2020, 87, 639–646. [Google Scholar] [CrossRef]
- Aksel, S.; Derpinghaus, A.; Cao, M.; Li, Y.; Cunha, G.; Baskin, L. Neurovascular anatomy of the developing human fetal penis and clitoris. J. Anat. 2024, 245, 35–49. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, J.; Sarila, G.; O’Conner, L.; Menheniott, T.; Hutson, J.M. BDNF is upregulated by androgen in the inguinal fat pad of immature mice and may regulate inguinoscrotal testicular descent. Pediatr. Res. 2021, 91, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Bouldin, C.M.; Choi, K.S.; Harfe, B.D.; Cohn, M.J. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 2009, 136, 3949–3957. [Google Scholar] [CrossRef]
- He, F.; Akbari, P.; Mo, R.; Zhang, J.J.; Hui, C.C.; Kim, P.C.; Farhat, W.A. Adult Gli2+/−;Gli3Δ699/+ Male and Female Mice Display a Spectrum of Genital Malformation. PLoS ONE 2016, 11, e0165958. [Google Scholar] [CrossRef]
- Rodriguez, K.F.; Brown, P.R.; Amato, C.M.; Nicol, B.; Liu, C.F.; Xu, X.; Yao, H.H. Somatic cell fate maintenance in mouse fetal testes via autocrine/paracrine action of AMH and activin B. Nat. Commun. 2022, 13, 4130. [Google Scholar] [CrossRef]
- Wainwright, E.N.; Svingen, T.; Ng, E.T.; Wicking, C.; Koopman, P. Primary cilia function regulates the length of the embryonic trunk axis and urogenital field in mice. Dev. Biol. 2014, 395, 342–354. [Google Scholar] [CrossRef]
- Nygaard, M.B.; Almstrup, K.; Lindbaek, L.; Christensen, S.T.; Svingen, T. Cell context-specific expression of primary cilia in the human testis and ciliary coordination of Hedgehog signalling in mouse Leydig cells. Sci. Rep. 2015, 5, 10364. [Google Scholar] [CrossRef]
- Brooks, E.R.; Wallingford, J.B. Multiciliated cells. Curr. Biol. 2014, 24, R973–R982. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Baskin, L.S. Can we prevent hypospadias? J. Pediatr. Urol. 2007, 3, 420–425. [Google Scholar] [CrossRef][Green Version]
- Kilcoyne, K.R.; Mitchell, R.T. Effect of environmental and pharmaceutical exposures on fetal testis development and function: A systematic review of human experimental data. Hum. Reprod. Update 2019, 25, 397–421. [Google Scholar] [CrossRef]
- He, H.; Chen, W.; Wei, Y.; Zhang, T.; Geng, W.; Zhai, J. Effects of perinatal exposure to endocrine-disrupting chemicals on the reproductive system of F3 generation male rodents: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 33218–33229. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, X.; Abulimiti, G.; Wang, Y.; Zhang, Q.; Cong, R.; Ji, C.; Luan, J.; Yao, L.; et al. Identification of endocrine-disrupting chemicals targeting the genes and pathways of genital anomalies in males. Ecotoxicol. Environ. Saf. 2022, 247, 114241. [Google Scholar] [CrossRef]
- Shi, B.; He, E.; Chang, K.; Xu, G.; Meng, Q.; Xu, H.; Chen, Z.; Wang, X.; Jia, M.; Sun, W.; et al. Genistein prevents the production of hypospadias induced by Di-(2-ethylhexyl) phthalate through androgen signaling and antioxidant response in rats. J. Hazard. Mater. 2024, 466, 133537. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Yan, W.; Zhang, Z.; Luo, S.; Wen, Q.; Wang, S.; Zhou, N.; Chen, Q.; Xu, Y. Exposure to di-2-ethylhexyl phthalate and breast neoplasm incidence: A cohort study. Sci. Total Environ. 2024, 926, 171819. [Google Scholar] [CrossRef]
- Sui, H.X.; Zhang, L.; Wu, P.G.; Song, Y.; Yong, L.; Yang, D.J.; Jiang, D.G.; Liu, Z.P. Concentration of di(2-ethylhexyl) phthalate (DEHP) in foods and its dietary exposure in China. Int. J. Hyg. Environ. Health 2014, 217, 695–701. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Li, Y.; Wang, Y.; Yu, M.; Lyu, Y.; Chen, F.; Huang, Y.; Ding, Y. Prenatal Low Testosterone Levels Induced by DNAH8 Dysfunction Leads to Urethral Fusion and Male Differentiation Abnormalities. Biomedicines 2025, 13, 3032. https://doi.org/10.3390/biomedicines13123032
Peng Z, Li Y, Wang Y, Yu M, Lyu Y, Chen F, Huang Y, Ding Y. Prenatal Low Testosterone Levels Induced by DNAH8 Dysfunction Leads to Urethral Fusion and Male Differentiation Abnormalities. Biomedicines. 2025; 13(12):3032. https://doi.org/10.3390/biomedicines13123032
Chicago/Turabian StylePeng, Zhiwei, Yao Li, Yaping Wang, Mingming Yu, Yiqing Lyu, Fang Chen, Yichen Huang, and Yu Ding. 2025. "Prenatal Low Testosterone Levels Induced by DNAH8 Dysfunction Leads to Urethral Fusion and Male Differentiation Abnormalities" Biomedicines 13, no. 12: 3032. https://doi.org/10.3390/biomedicines13123032
APA StylePeng, Z., Li, Y., Wang, Y., Yu, M., Lyu, Y., Chen, F., Huang, Y., & Ding, Y. (2025). Prenatal Low Testosterone Levels Induced by DNAH8 Dysfunction Leads to Urethral Fusion and Male Differentiation Abnormalities. Biomedicines, 13(12), 3032. https://doi.org/10.3390/biomedicines13123032






