MAPK Pathway Activation Patterns in the Synovium Reveal ERK1/2 and EGFR as Key Players in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tissue Collection and Basic Staining Procedures
2.3. Immunofluorescence Staining
2.4. Data Acquisition and Quantitative Analysis
2.4.1. Synovial Lining Thickness Measurement
2.4.2. Cellularity Quantification
2.5. Statistical Analysis
2.6. Differential Gene Expression Analysis
2.7. KEGG Pathway Enrichment Analysis
3. Results
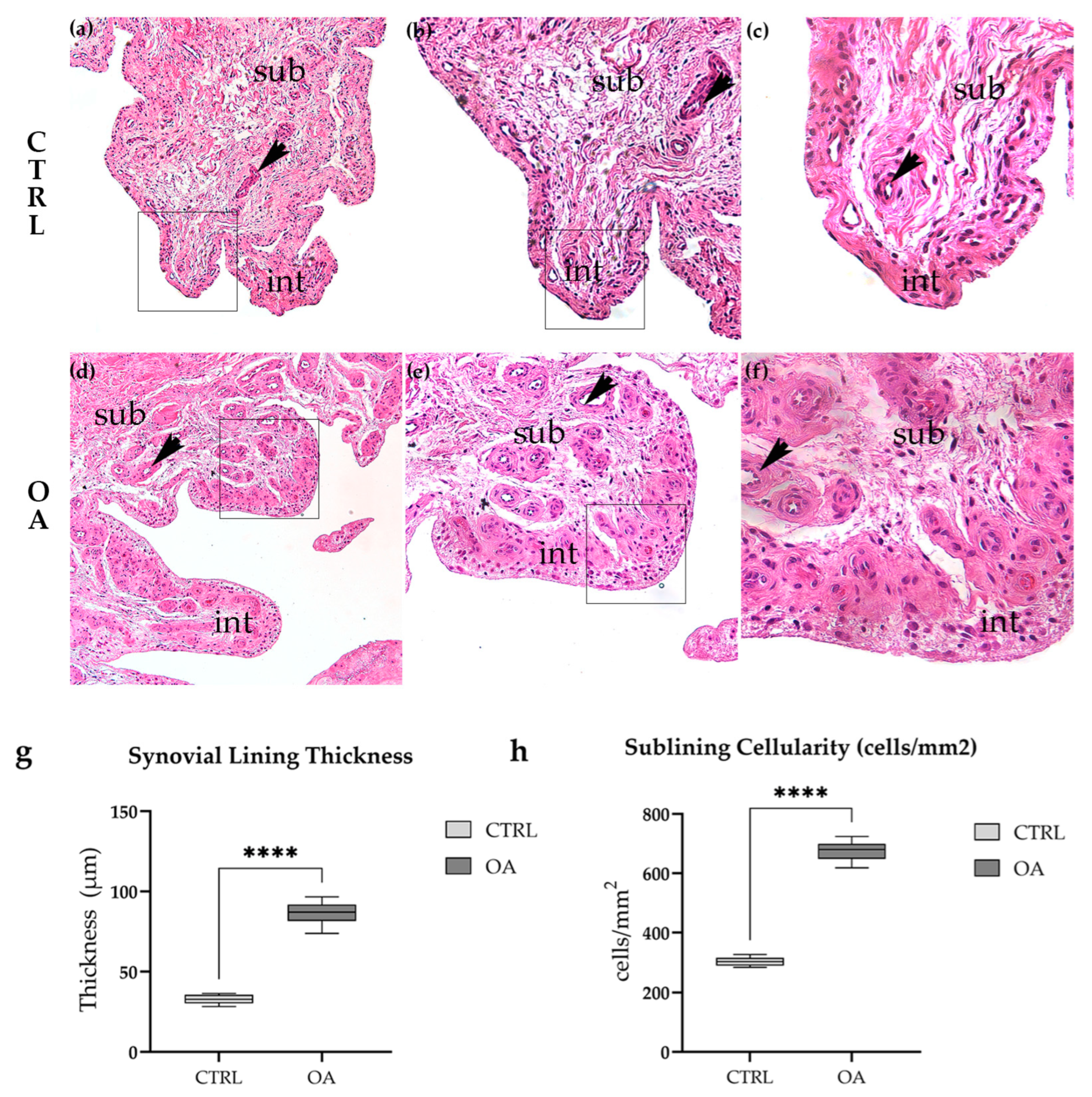
3.1. Histopathological Features of Synovial Tissue in Osteoarthritis
3.1.1. Normal Synovial Architecture in Control Tissue
3.1.2. Synovial Inflammation and Hyperplasia in Osteoarthritis
3.1.3. Quantitative Histological Analysis
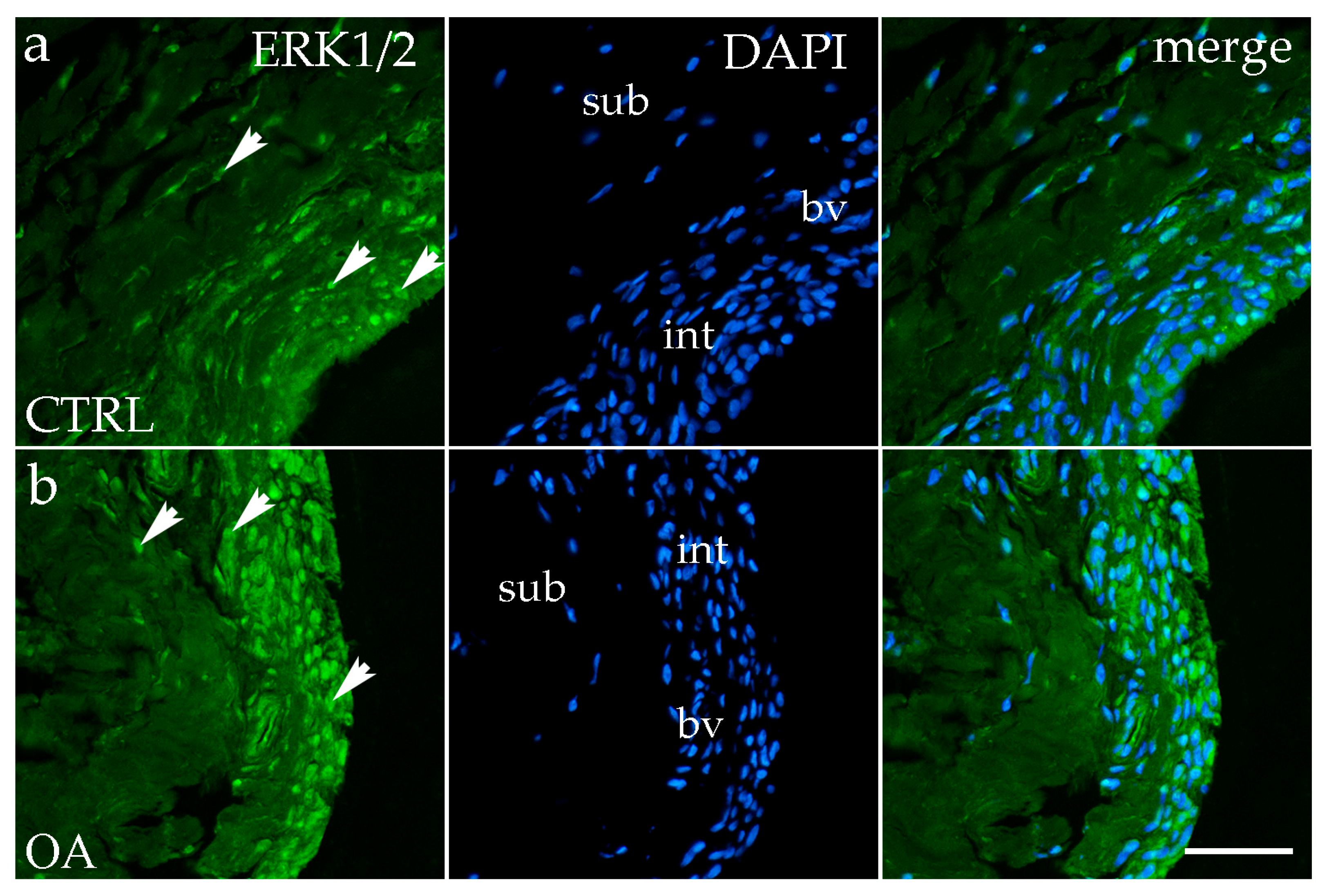
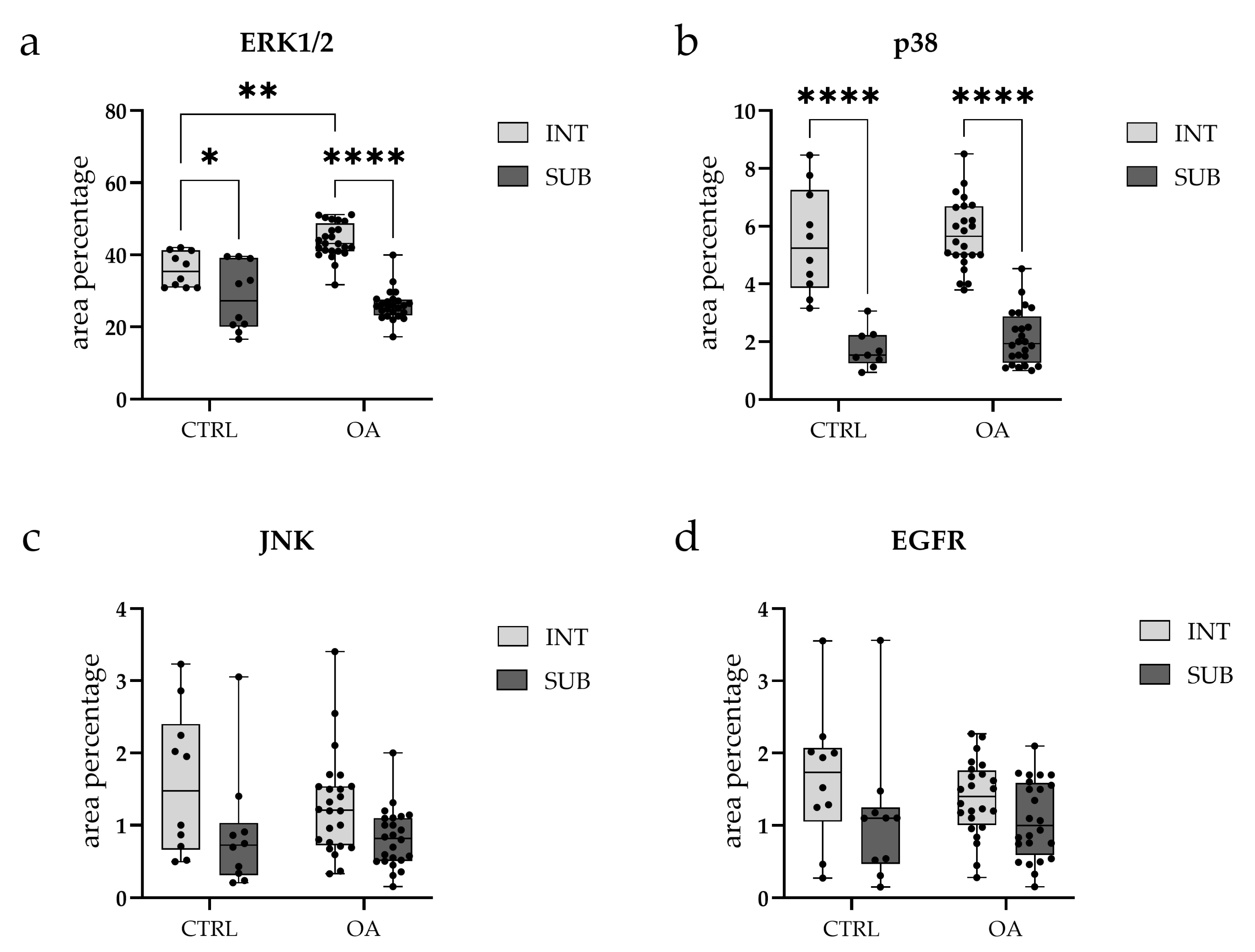
3.2. Immunofluorescence Staining of ERK 1/2
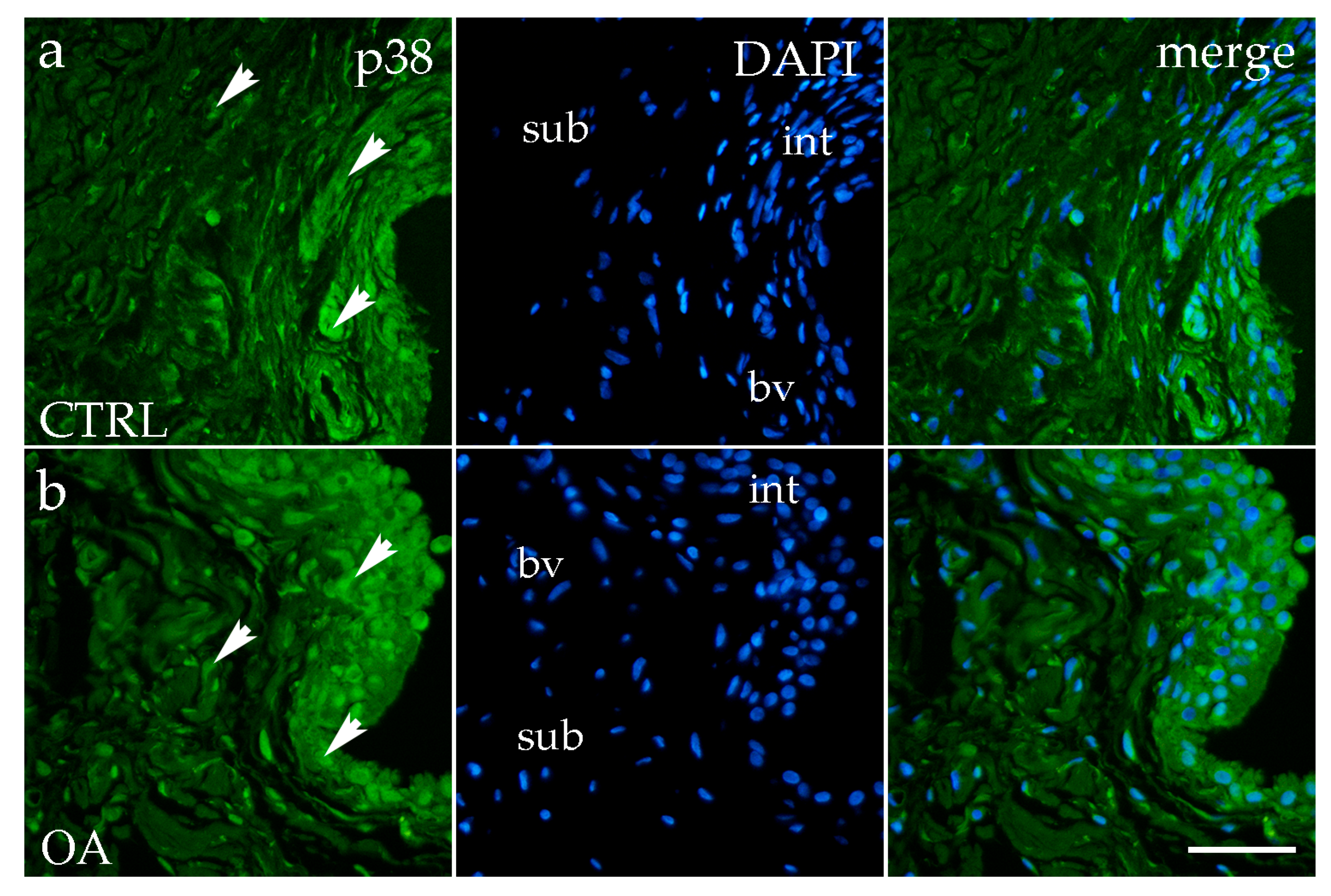
3.3. Immunofluorescence Staining of p38 MAPK
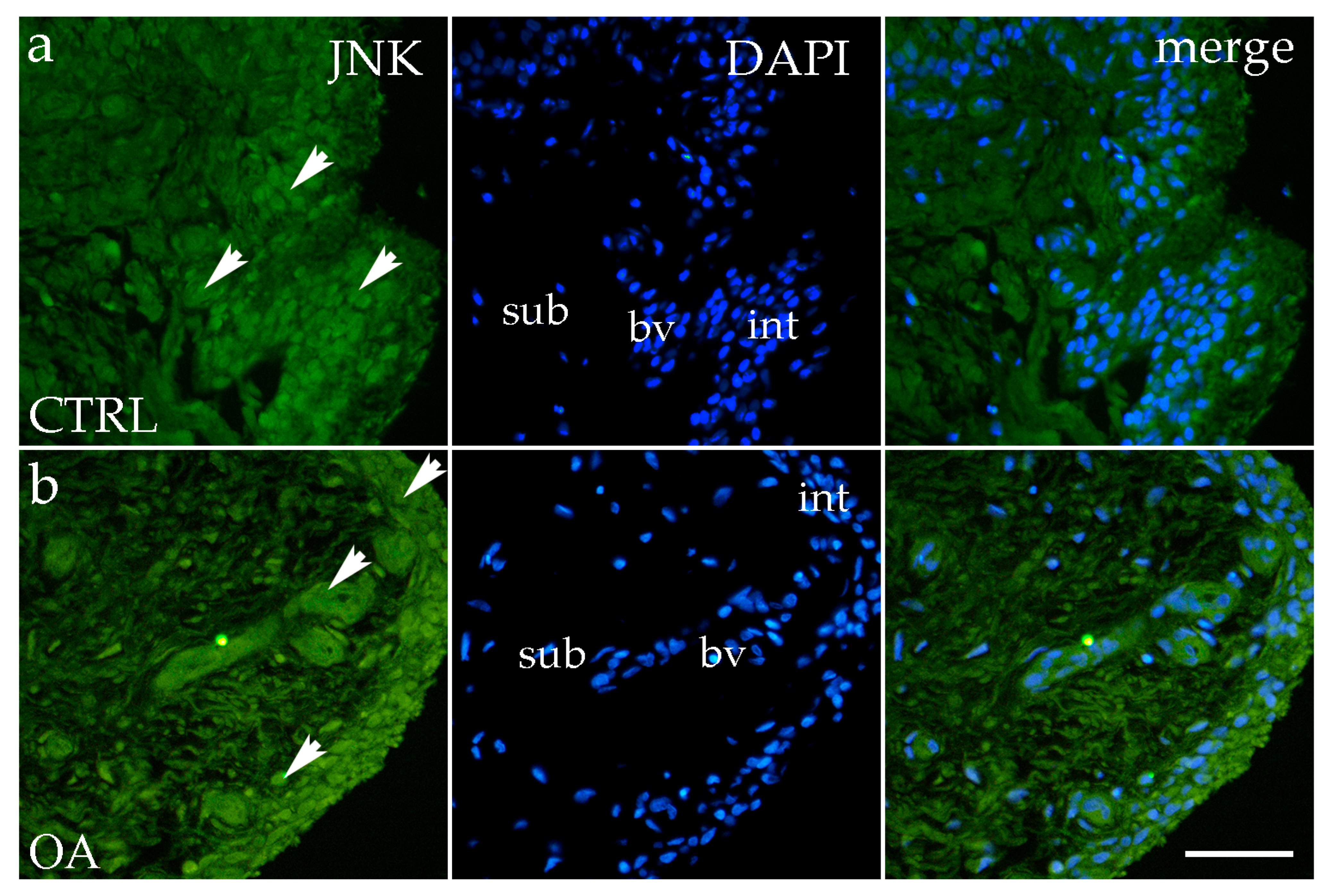
3.4. Immunofluorescence Staining of JNK
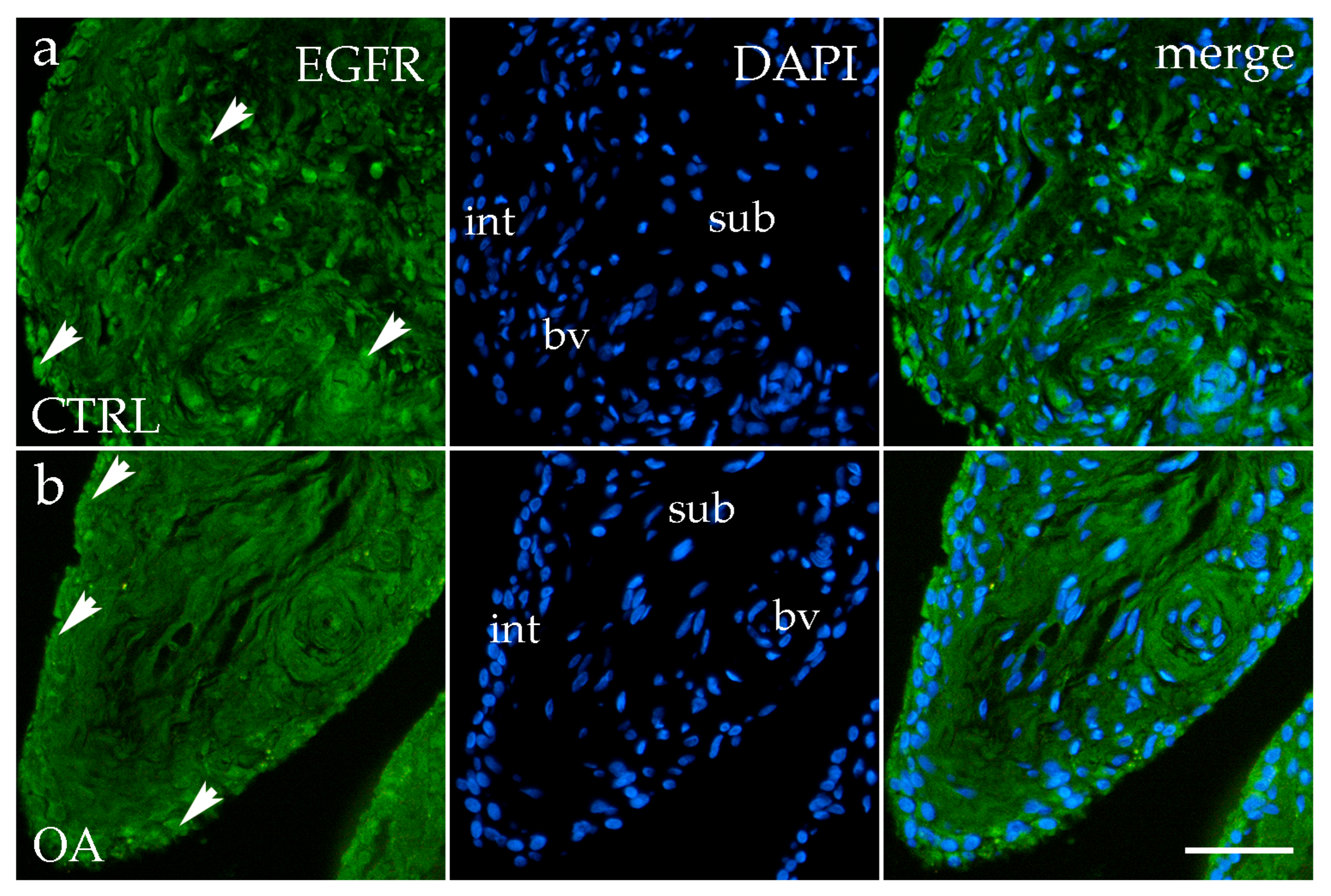
3.5. Immunofluorescence Staining of EGFR
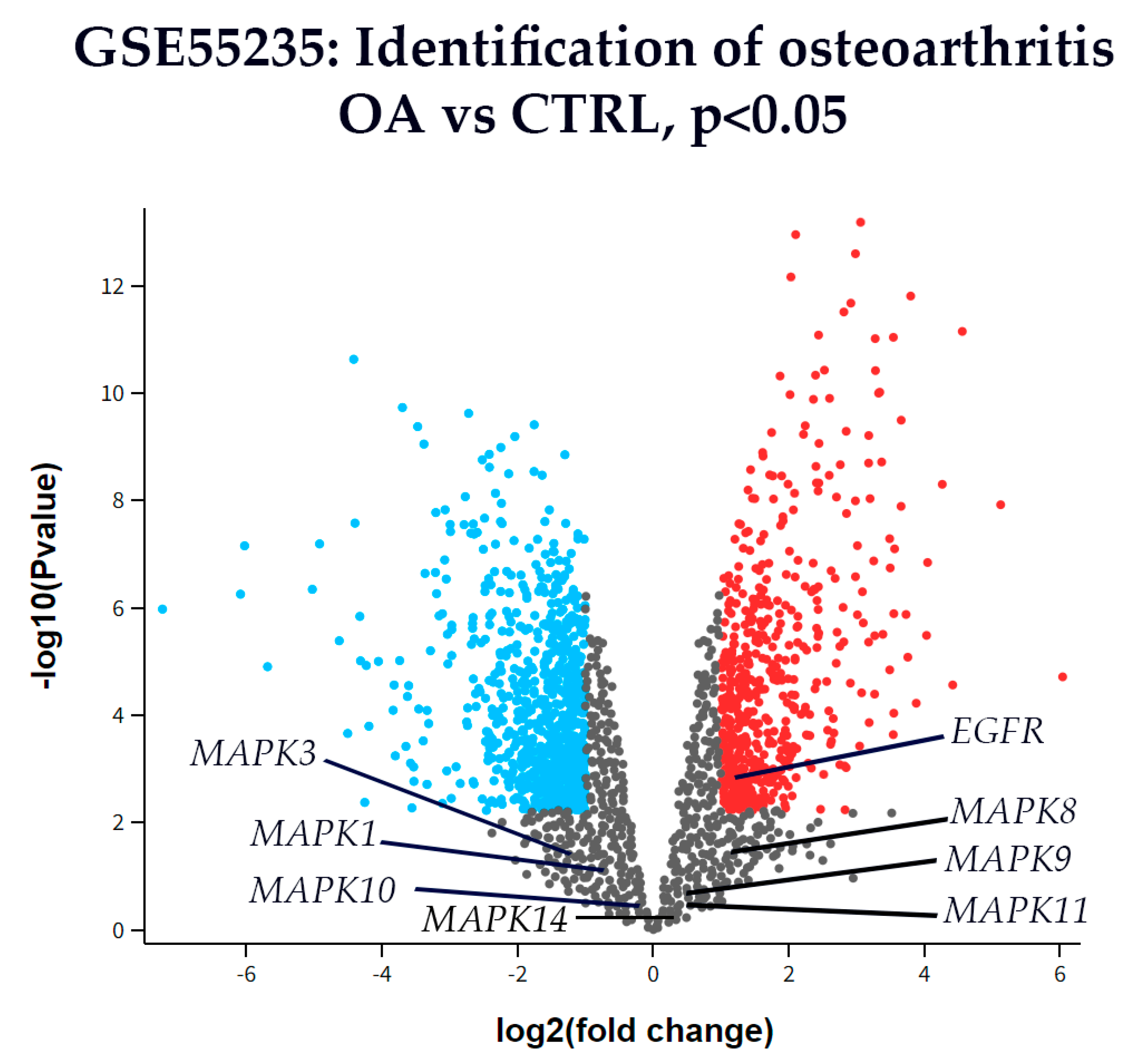
3.6. Differential Gene Expression
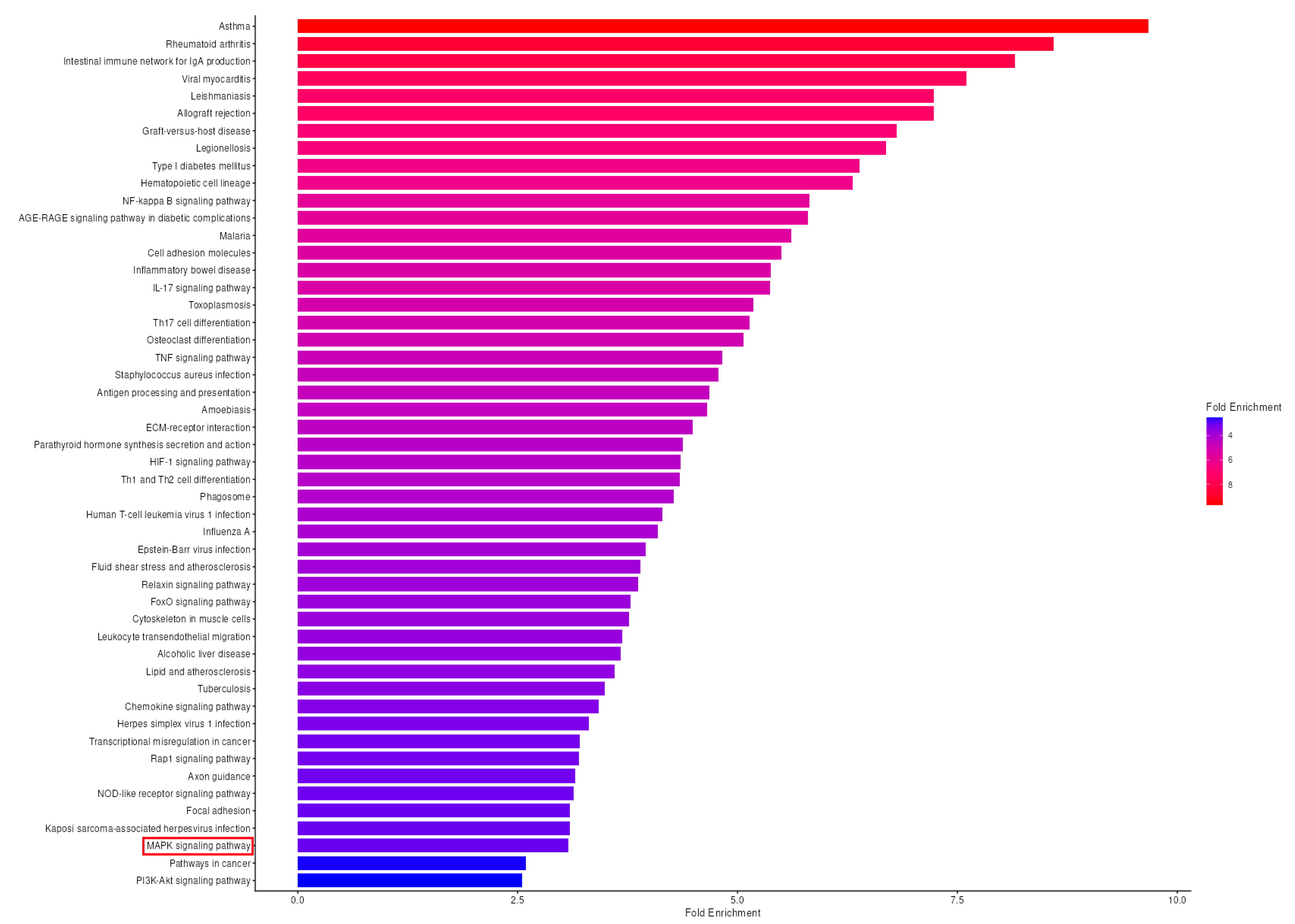
3.7. KEGG Pathway Enrichment Analysis Reveals Significant MAPK Pathway Activation in OA Synovium
4. Discussion
4.1. Principal Findings and Clinical Significance
4.2. ERK1/2: A Key Mediator of Synovial Inflammation in Hip OA
4.3. p38 MAPK: Conserved Regional Expression Without Disease-Specific Changes
4.4. JNK: Context-Dependent Activation in Synovial Inflammation
4.5. EGFR: Dual Roles and Stage-Dependent Expression in OA
4.6. Pathway-Level Integration: KEGG Analysis Reveals Coordinated MAPK Network Dysregulation
4.7. Limitations and Future Directions
4.8. Clinical and Translational Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAMTS5 | A Disintegrin and Metalloproteinase with Thrombospondin Type 1 Motif 5 |
| ANOVA | analysis of variance |
| anti-CCP | anti-cyclic citrullinated peptides |
| BMI | body mass index |
| bv | blood vessel |
| CTRLs | controls |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DEGs | differentially expressed genes |
| DRP1 | Dynamin-Related Protein 1 |
| EGFR | Epidermal Growth Factor Receptor |
| ERK 1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| FDR | False discovery rate |
| GDE | garlic-derived exosomes |
| GEO | Gene Expression Omnibus |
| H&E | Hematoxylin–eosin |
| HHS | Harris Hip Score |
| HSS | higher synovitis score group |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| ICC | intraclass correlation coefficient |
| IHC | Immunohistochemistry |
| IL-1β | Interleukin-1 beta |
| INT | intima |
| IQR | interquartile range |
| JNK | c-Jun N-terminal kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| K-L | Kellgren–Lawrence |
| MAPK | mitogen-activated protein kinase |
| MMP-1 | Matrix Metalloproteinase-1 |
| NCBI | National Center for Biotechnology Information |
| mRNA | messenger RNA |
| OA | Osteoarthritis |
| p38/SAPK | p38 Mitogen-Activated Protein Kinase/stress-activated protein kinase |
| PBS | phosphate-buffered saline |
| qRT-PCR | quantitative real-time reverse transcription polymerase chain reaction |
| RA | rheumatoid arthritis |
| RF | rheumatoid factor |
| RNAseq | RNA sequencing |
| ROI | regions of interest |
| SD | standard deviations |
| SUB | subintima |
| TNF-α | tumor necrosis factor alpha |
| VAS | visual analog scale |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
References
- McGonagle, D.; Tan, A.L.; Carey, J.; Benjamin, M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J. Anat. 2010, 216, 279–291. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Chen, Y.; Zhang, Y.; Xing, D.; Zhao, L.; Lin, J.; Mei, Y.; Lin, H.-Y.; Zheng, Y. Development and formulation of the classification criteria for osteoarthritis. Ann. Transl. Med. 2020, 8, 1068, Erratum in Ann. Transl. Med. 2020, 8, 1630. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Bai, L. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connect. Tissue Res. 2016, 57, 245–261. [Google Scholar] [CrossRef]
- Das, S.K.; Farooqi, A. Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2008, 22, 657–675. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological perspective of osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, H.; Yuan, Q.; Chen, X.; Li, H. Global, regional, and national epidemiology of osteoarthritis in working-age individuals: Insights from the global burden of disease study 1990–2021. Sci. Rep. 2025, 15, 7907. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Peng, X.; Chen, X.; Zhang, Y.; Tian, Z.; Wang, M.; Chen, Z. Advances in the Pathology and Treatment of Osteoarthritis. J. Adv. Res. 2025, 78, 257–283. [Google Scholar] [CrossRef]
- Shendy, N.A.; Abell, A.N. MAP Kinase Cascades. In Encyclopedia of Molecular Pharmacology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar]
- Liu, X.; Chen, B.; Liu, X.; Zhang, X.; Wu, J. Interplay between MAPK signaling pathway and autophagy in skin aging: Mechanistic insights and therapeutic implications. Front. Cell Dev. Biol. 2025, 13, 1625357. [Google Scholar] [CrossRef]
- Fan, Q.; Zhao, M.; Zhang, X.-D.; Chu, T.-Y.; Kou, Z.-X.; Zhao, Q. Research progress and prospect of MAPK signaling pathway in knee osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 2025, 35, 134. [Google Scholar] [CrossRef]
- Mathien, S.; Tesnière, C.; Meloche, S. Regulation of mitogen-activated protein kinase signaling pathways by the ubiquitin-proteasome system and its pharmacological potential. Pharmacol. Rev. 2021, 73, 1434–1467. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Z.; Jiang, Y.; Liao, T.; Aschner, M.; Wei, Q. Ononin delays the development of osteoarthritis by down-regulating MAPK and NF-κB pathways in rat models. PLoS ONE 2024, 19, e0310293. [Google Scholar] [CrossRef]
- Chowdhury, T.; Salter, D.; Bader, D.; Lee, D. Signal transduction pathways involving p38 MAPK, JNK, NFκB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1β and dynamic compression. Inflamm. Res. 2008, 57, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Chang-Hao Tsao, S.; Weiss, J.; Hudson, C.; Christophi, C.; Cebon, J.; Behren, A.; Dobrovic, A. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci. Rep. 2015, 5, 11198. [Google Scholar] [CrossRef]
- Shi, J.; Wang, E.; Zuber, J.; Rappaport, A.; Taylor, M.; Johns, C.; Lowe, S.W.; Vakoc, C.R. The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9; NrasG12D acute myeloid leukemia. Oncogene 2013, 32, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, Q.; Liu, Q.; Pan, D.; Jiang, Y.; Liu, M.; Liu, M.; Xu, H.; Lin, C. Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Rheumatology 2017, 56, 1417–1427. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zhang, F.-J.; Zeng, C.; Luo, W.; Xiao, W.-F.; Gao, S.-G.; Lei, G.-H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, J.; Deng, M.; Fang, Y.; Zhan, D.; Wang, G. Identification of pathways and genes associated with meniscus degeneration using bioinformatics analyses. Am. J. Transl. Res. 2021, 13, 12410. [Google Scholar]
- Zhang, C.; Lin, Y.; Yan, C.H.; Zhang, W. Adipokine signaling pathways in osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 865370. [Google Scholar] [CrossRef]
- Lee, K.-T.; Chen, B.-C.; Liu, S.-C.; Lin, Y.-Y.; Tsai, C.-H.; Ko, C.-Y.; Tang, C.-H.; Tung, K.-C. Nesfatin-1 facilitates IL-1β production in osteoarthritis synovial fibroblasts by suppressing miR-204-5p synthesis through the AP-1 and NF-κB pathways. Aging 2021, 13, 22490. [Google Scholar] [CrossRef]
- Prasadam, I.; Van Gennip, S.; Friis, T.; Shi, W.; Crawford, R.; Xiao, Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010, 62, 1349–1360. [Google Scholar] [CrossRef]
- Djouad, F.; Rackwitz, L.; Song, Y.; Janjanin, S.; Tuan, R.S. ERK1/2 activation induced by inflammatory cytokines compromises effective host tissue integration of engineered cartilage. Tissue Eng. Part A 2009, 15, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Novak, K.; Haqqi, T.M. ERK1/2-mediated activation of DRP1 regulates mitochondrial dynamics and apoptosis in chondrocytes. Osteoarthr. Cartil. 2022, 30, 315–328. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK signaling pathway in osteoarthritis: Pathological and therapeutic aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Hamamura, K.; Goldring, M.B.; Yokota, H. Involvement of p38 MAPK in regulation of MMP13 mRNA in chondrocytes in response to surviving stress to endoplasmic reticulum. Arch. Oral Biol. 2009, 54, 279–286. [Google Scholar] [CrossRef]
- National Collaborating Centre for Chronic Conditions. Osteoarthritis: National Clinical Guidelines for Care and Management in Adults; Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Lu, J.; Yu, M.; Li, J. PKC-δ promotes IL-1β-induced apoptosis of rat chondrocytes and via activating JNK and P38 MAPK pathways. Cartilage 2024, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Radons, J.; Bosserhoff, A.K.; Grässel, S.; Falk, W.; Schubert, T.E. p38MAPK mediates IL-1-induced down-regulation of aggrecan gene expression in human chondrocytes. Int. J. Mol. Med. 2006, 17, 661–668. [Google Scholar] [CrossRef]
- Han, F.; Jiang, H.; Qu, W.; Rui, Y.-J. KLF11 protects chondrocytes via inhibiting p38 MAPK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6505–6516. [Google Scholar] [PubMed]
- Lei, J.; Fu, Y.; Zhuang, Y.; Zhang, K.; Lu, D. LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci. Rep. 2019, 39, BSR20191523. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, W.; Qu, X.; Bi, H.; Sun, X.; Yu, Q.; Cheng, G. miR-296-5p inhibits IL-1β-induced apoptosis and cartilage degradation in human chondrocytes by directly targeting TGF-β1/CTGF/p38MAPK pathway. Cell Cycle 2020, 19, 1443–1453. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, X.; Yao, W.; He, H.; Tang, Z.; Wu, J. MiR-214 inhibits knee osteoarthritis in rats through MAPK signaling pathway. Panminerva Medica 2019. [Google Scholar] [CrossRef]
- Guma, M.; Firestein, G.S. c-Jun N-terminal kinase in inflammation and rheumatic diseases. Open Rheumatol. J. 2012, 6, 220. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tan, T.-H. The c-Jun N-terminal kinase pathway and apoptotic signaling. Int. J. Oncol. 2000, 16, 651–713. [Google Scholar] [CrossRef]
- Ismail, H.M.; Yamamoto, K.; Vincent, T.L.; Nagase, H.; Troeberg, L.; Saklatvala, J. Interleukin-1 acts via the JNK-2 signaling pathway to induce aggrecan degradation by human chondrocytes. Arthritis Rheumatol. 2015, 67, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Valbracht, J.; Lotz, M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J. Clin. Investig. 1996, 98, 2425–2430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosseini, M.; Rose, A.Y.; Song, K.; Bohan, C.; Alexander, J.P.; Kelley, M.J.; Acott, T.S. IL-1 and TNF induction of matrix metalloproteinase-3 by c-Jun N-terminal kinase in trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Kunisch, E.; Kinne, R.W.; Alsalameh, R.J.; Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and-3 m RNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 2016, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.-Y.; Lin, Y.-M.; Liu, S.-C.; Wu, M.-H.; Chung, W.-H.; Tsai, C.-H.; Fong, Y.-C.; Tang, C.-H.; Wang, C.-K. Visfatin increases ICAM-1 expression and monocyte adhesion in human osteoarthritis synovial fibroblasts by reducing miR-320a expression. Aging 2020, 12, 18635. [Google Scholar] [CrossRef]
- Azamar-Llamas, D.; Hernandez-Molina, G.; Ramos-Avalos, B.; Furuzawa-Carballeda, J. Adipokine contribution to the pathogenesis of osteoarthritis. Mediat. Inflamm. 2017, 2017, 5468023. [Google Scholar] [CrossRef]
- Jia, H.; Ma, X.; Tong, W.; Doyran, B.; Sun, Z.; Wang, L.; Zhang, X.; Zhou, Y.; Badar, F.; Chandra, A. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc. Natl. Acad. Sci. USA 2016, 113, 14360–14365. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, X.; Sun, H.; Gui, T.; Li, J.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Nelson, C.L. EGFR signaling is required for maintaining adult cartilage homeostasis and attenuating osteoarthritis progression. J. Bone Miner. Res. 2020, 37, 1012–1023. [Google Scholar] [CrossRef]
- Ceresa, B.P.; Peterson, J.L. Cell and molecular biology of epidermal growth factor receptor. Int. Rev. Cell Mol. Biol. 2014, 313, 145–178. [Google Scholar]
- Zhang, X.; Zhu, J.; Liu, F.; Li, Y.; Chandra, A.; Levin, L.S.; Beier, F.; Enomoto-Iwamoto, M.; Qin, L. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014, 2, 14015. [Google Scholar] [CrossRef]
- Sun, H.; Peng, G.; Chen, K.; Xiong, Z.; Zhuang, Y.; Liu, M.; Ning, X.; Yang, H.; Deng, J. Identification of EGFR as an essential regulator in chondrocytes ferroptosis of osteoarthritis using bioinformatics, in vivo, and in vitro study. Heliyon 2023, 9, e19975. [Google Scholar] [CrossRef]
- Jurić, I.; Kelam, N.; Racetin, A.; Filipović, N.; Čarić, D.; Rošin, M.; Vukojević, K. WNT Signaling Factors as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis. Biomedicines 2025, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Caric, D.; Vukojevic, K. Expression pattern of Syndecan-1 and HSP-70 in hip tissue of patients with osteoarthritis. J. Orthop. 2020, 17, 134–138. [Google Scholar] [CrossRef]
- Rošin, M.; Kelam, N.; Jurić, I.; Racetin, A.; Ogorevc, M.; Corre, B.; Čarić, D.; Filipović, N.; Vukojević, K. Syndecans, Exostosins and Sulfotransferases as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 4557. [Google Scholar] [CrossRef] [PubMed]
- Kelam, J.; Kelam, N.; Filipović, N.; Komić, L.; Racetin, A.; Komić, D.; Kostić, S.; Kuzmić Prusac, I.; Vukojević, K. Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes 2024, 15, 702. [Google Scholar] [CrossRef]
- Kelam, N.; Ogorevc, M.; Gotovac, I.; Kuzmić Prusac, I.; Vukojević, K.; Saraga-Babić, M.; Mardešić, S. Analysis of Kallikrein 6, Acetyl-α-Tubulin, and Aquaporin 1 and 2 Expression Patterns During Normal Human Nephrogenesis and in Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Genes 2025, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Komić, J.; Kelam, N.; Racetin, A.; Filipović, N.; Saraga-Babić, M.; Ihara, D.; Katsuyama, Y.; Vukojević, K. Spatial and Temporal Expression Patterns of EDA2R, PCDH9, and TRAF7 in Yotari (Dab1−/−) Mice: Implicationsfor Understanding CAKUT Pathogenesis. Int. J. Mol. Sci. 2025, 26, 6421. [Google Scholar] [CrossRef]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef]
- Todorović, P.; Kelam, N.; Racetin, A.; Filipović, N.; Katsuyama, Y.; Saraga-Babić, M.; Vukojević, K. Expression Pattern of Dab1, Reelin, PGP9. 5 and Sox2 in the Stomach of Yotari (Dab1−/−) Mice. Genes 2025, 16, 1013. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Woetzel, D.; Huber, R.; Kupfer, P.; Pohlers, D.; Pfaff, M.; Driesch, D.; Häupl, T.; Koczan, D.; Stiehl, P.; Guthke, R. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res. Ther. 2014, 16, R84. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, J.; Shen, N.; Wang, G.; Zhou, G.; Fang, Y.; Lin, J.; Zhao, J. Identification of pathways and genes associated with synovitis in osteoarthritis using bioinformatics analyses. Sci. Rep. 2018, 8, 10050. [Google Scholar] [CrossRef]
- Liao, C.S.; He, F.Z.; Li, X.Y.; Zhang, Y.; Han, P.F. Analysis of common differential gene expression in synovial cells of osteoarthritis and rheumatoid arthritis. PLoS ONE 2024, 19, e0303506. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Fan, Z.; Söder, S.; Oehler, S.; Fundel, K.; Aigner, T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am. J. Pathol. 2007, 171, 938–946. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Fernandes, J.C.; Brunet, J.; Moldovan, F.; Schrier, D.; Flory, C.; Martel-Pelletier, J. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003, 48, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jin, H.; Li, W.; Tong, P.; Yuan, W. Study of the curative effect of Zhang’s Xibi formula and its underlying mechanism involving inhibition of inflammatory responses and delay of knee osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 963. [Google Scholar] [CrossRef]
- Wu, K.; Zhong, Z.; Chen, L.; Luo, H.; Jiang, A.; Tao, L.; Jiang, Y. Integrative analysis of bulk and single-cell RNA sequencing data reveals increased arachidonic acid metabolism in osteoarthritic chondrocytes. Front. Med. 2025, 12, 1552029. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y. Signal Transduction Pathways Involved in Acupuncture-Mediated Inhibition of Synovitis in Knee Osteoarthritis: A Comprehensive Review. Int. J. Gen. Med. 2025, 18, 4105–4117. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Zhang, M.; Xiao, L.; Yang, Y.; Wang, B.; Liu, Y.; Dai, Z.; Zheng, J. Phosphoproteomics reveals the BRAF-ERK1/2 axis as an important pathogenic signaling node in cartilage degeneration. Osteoarthr. Cartil. 2022, 30, 1443–1454. [Google Scholar] [CrossRef]
- Pakjoo, M.; Ahmadi, S.E.; Zahedi, M.; Jaafari, N.; Khademi, R.; Amini, A.; Safa, M. Interplay between proteasome inhibitors and NF-κB pathway in leukemia and lymphoma: A comprehensive review on challenges ahead of proteasome inhibitors. Cell Commun. Signal. 2024, 22, 105. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Huang, L.; Liu, J.; Zhou, Q.; Du, G.; Lao, S. Chemerin promotes MAPK/ERK activation to induce inflammatory factor production in rat synoviocytes. Exp. Ther. Med. 2022, 24, 684. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yan, X.-J.; Jiang, X.-H.; Lu, F.-L.; Yang, X.-R.; Li, D.-P. Vicenin 3 ameliorates ECM degradation by regulating the MAPK pathway in SW1353 chondrocytes. Exp. Ther. Med. 2021, 22, 1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, M.; Li, X.; Wang, H.; Ren, S.; Zou, D.; Liu, J.; Li, R. Garlic-derived exosomes alleviate osteoarthritis through inhibiting the MAPK signaling pathway. Appl. Biochem. Biotechnol. 2025, 197, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.n.; Ye, L.; Zhang, Y.j.; Fang, J.w.; Yang, T.; Pan, W.z.; Hu, X.y.; Lai, H.h.; Pan, B.; Lou, C. Formononetin improves the inflammatory response and bone destruction in knee joint lesions by regulating the NF-kB and MAPK signaling pathways. Phytother. Res. 2023, 37, 3363–3379. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, X.; Yao, J.; Lin, X.; Weng, Y.; Huang, Y.; Lu, Y.; Shang, J.; Nong, L. Identification and validation of transcriptome-wide association study-derived genes as potential druggable targets for osteoarthritis. Bone Jt. Res. 2025, 14, 224–235. [Google Scholar] [CrossRef]
- Schett, G.; Tohidast-Akrad, M.; Smolen, J.S.; Schmid, B.J.; Steiner, C.W.; Bitzan, P.; Zenz, P.; Redlich, K.; Xu, Q.; Steiner, G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal–regulated kinase, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 2501–2512. [Google Scholar] [CrossRef]
- Görtz, B.; Hayer, S.; Tuerck, B.; Zwerina, J.; Smolen, J.S.; Schett, G. Tumour necrosis factor activates the mitogen-activated protein kinases p38α and ERK in the synovial membrane in vivo. Arthritis Res. Ther. 2005, 7, R1140. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-H.; Wu, C.-H.; Jou, I.-M.; Tu, Y.-K.; Hung, C.-H.; Chou, W.-C.; Chang, Y.-C.; Hsieh, P.-L.; Tsai, K.-L. PKR promotes oxidative stress and apoptosis of human articular chondrocytes by causing mitochondrial dysfunction through p38 MAPK activation—PKR activation causes apoptosis in human chondrocytes. Antioxidants 2019, 8, 370. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Rinotas, V.; Liepouri, F.; Ouzouni, M.-D.; Chalkidi, N.; Papaneophytou, C.; Lampropoulou, M.; Vidali, V.P.; Kontopidis, G.; Couladouros, E.; Eliopoulos, E. Structure-based discovery of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis inhibitors. Int. J. Mol. Sci. 2023, 24, 11290. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, D.J.; Papadakou, E.; Basdra, E.K.; Baltopoulos, P.; Panagiotopoulos, E.; Papavassiliou, A.G. Involvement of the p38 MAPK-NF-κB signal transduction pathway and COX-2 in the pathobiology of meniscus degeneration in humans. Mol. Med. 2008, 14, 160–166. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, Z.; Zhan, W.; Chen, M.; Ruan, J.; Shen, C. VEGFA, MYC, and JUN are abnormally elevated in the synovial tissue of patients with advanced osteoarthritis. Sci. Rep. 2025, 15, 2066. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, C.; Yi, Z.; Lan, C. Explore the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early stage of osteoarthritis. IUBMB Life 2016, 68, 293–302. [Google Scholar] [CrossRef]
- Prasadam, I.; Mao, X.; Wang, Y.; Shi, W.; Crawford, R.; Xiao, Y. Inhibition of p38 pathway leads to OA-like changes in a rat animal model. Rheumatology 2012, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Takebe, K.; Nishiyama, T.; Hayashi, S.; Hashimoto, S.; Fujishiro, T.; Kanzaki, N.; Kawakita, K.; Iwasa, K.; Kuroda, R.; Kurosaka, M. Regulation of p38 MAPK phosphorylation inhibits chondrocyte apoptosis in response to heat stress or mechanical stress. Int. J. Mol. Med. 2011, 27, 329–335. [Google Scholar] [CrossRef][Green Version]
- Clancy, R.; Rediske, J.; Koehne, C.; Stoyanovsky, D.; Amin, A.; Attur, M.; Iyama, K.; Abramson, S. Activation of stress-activated protein kinase in osteoarthritic cartilage: Evidence for nitric oxide dependence. Osteoarthr. Cartil. 2001, 9, 294–299. [Google Scholar] [CrossRef]
- Frisch, S.M.; Ruoslahti, E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997, 9, 701–706. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, Y.; Ji, W.; Dong, Q. Activation of the extracellular-signal-regulated kinase (ERK)/c-Jun N-terminal kinase (JNK) signal pathway and osteogenic factors in subchondral bone of patients with knee osteoarthritis. Ann. Transl. Med. 2021, 9, 663, Erratum in Ann. Transl. Med. 2024, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Kelley, K.L.; Armstrong, A.; Collins, J.A.; Diekman, B.O.; Carlson, C.S. Deletion of JNK enhances senescence in joint tissues and increases the severity of age-related osteoarthritis in mice. Arthritis Rheumatol. 2020, 72, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chang, L.; Yamanishi, Y.; Karin, M.; Firestein, G.S. Joint damage and inflammation in c-Jun N-terminal kinase 2 knockout mice with passive murine collagen-induced arthritis. Arthritis Rheum. 2002, 46, 818–823. [Google Scholar] [CrossRef]
- Ismail, H.; Troeberg, L.; Vincent, T.; Saklatvala, J. JNK-dependent modulation of the protease secretome profile of osteoarthritic cartilage. Osteoarthr. Cartil. 2018, 26, S108–S109. [Google Scholar] [CrossRef]
- De Launay, D.; Van De Sande, M.G.; De Hair, M.J.; Grabiec, A.M.; Van De Sande, G.P.; Lehmann, K.A.; Wijbrandts, C.A.; Van Baarsen, L.G.; Gerlag, D.M.; Tak, P.P. Selective involvement of ERK and JNK mitogen-activated protein kinases in early rheumatoid arthritis (1987 ACR criteria compared to 2010 ACR/EULAR criteria): A prospective study aimed at identification of diagnostic and prognostic biomarkers as well as therapeutic targets. Ann. Rheum. Dis. 2012, 71, 415–423. [Google Scholar]
- Sun, H.; Wu, Y.; Pan, Z.; Yu, D.; Chen, P.; Zhang, X.; Wu, H.; Zhang, X.; An, C.; Chen, Y. Gefitinib for epidermal growth factor receptor activated osteoarthritis subpopulation treatment. EBioMedicine 2018, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Wei, Y.; Luo, L.; Li, J.; Zhong, L.; Yao, L.; Beier, F.; Nelson, C.L.; Tsourkas, A.; Liu, X.S. Activating EGFR signaling attenuates osteoarthritis development following loading injury in mice. J. Bone Miner. Res. 2020, 37, 2498–2511. [Google Scholar] [CrossRef]
- Li, Z.; Xu, M.; Li, R.; Zhu, Z.; Liu, Y.; Du, Z.; Zhang, G.; Song, Y. Identification of biomarkers associated with synovitis in rheumatoid arthritis by bioinformatics analyses. Biosci. Rep. 2020, 40, BSR20201713. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.D.; Akama-Garren, E.H.; Stein, E.A.; Petralia, J.D.; Ruiz, P.J.; Edalati, A.; Lindstrom, T.M.; Robinson, W.H. Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis. J. Immunol. 2012, 188, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
| Control Group | Krenn Score of Synovitis in OA (0–2) | Krenn Score of Synovitis in OA ≥ 3 | * p-Value | |
|---|---|---|---|---|
| Age in years: Median ± IQR | 72.9 (64–77) | 73.3 (70–82) | 69.9 (58–82) | 0.884 |
| Sex: Male/Female | (6/4) | (7/5) | (6/6) | 0.732 |
| BMI (Median ± IQR, kg/m2) | 25.87 (23.97–26.6) | 24.7 (23.25–25.82) | 26.7 (25.5–29.43) | 0.054 |
| K-L grade (median ± IQR) | 0.5 (0–1) | 2 (2–2) | 4 (3–4) | <0.0001 |
| Krenn Score (Median ± IQR) | 0 (0–0) | 6.4 (5.6–9) | 9 (7–9) | <0.0001 |
| HHS (Median ± IQR) | - | 48.7 (43.58–56.8) | 41 (33.48–49.6) | 0.272 |
| VAS (Median ± IQR) | - | 6 (4.6–6.8) | 6 (5–7) | 0.784 |
| WOMAC (Median ± IQR) | - | 46.2 (40.2–56.4) | 47.3 (36.1–55.3) | 0.918 |
| Antibodies | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | p44/42 MAPK (Erk1/2) (137F5) | 4695S | Rabbit | 1:300 | Cell Signaling Technology, Inc. (CST) Danvers, MA, USA |
| EGF Receptor (D38B1) XP® | 4267S | Rabbit | 1:100 | ||
| p38 MAPK Antibody | 9212S | Rabbit | 1:100 | ||
| SAPK/JNK Antibody | 9252S | Rabbit | 1:100 | ||
| Secondary | Alexa Fluor® 488 AffiniPure® Donkey Anti-Rabbit IgG (H + L) | 711-545-152 | Donkey | 1:300 | Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurić, I.; Todorović, P.; Kelam, N.; Boban, D.; Bajt, P.; Racetin, A.; Rošin, M.; Čarić, D.; Vukojević, K. MAPK Pathway Activation Patterns in the Synovium Reveal ERK1/2 and EGFR as Key Players in Osteoarthritis. Biomedicines 2025, 13, 2992. https://doi.org/10.3390/biomedicines13122992
Jurić I, Todorović P, Kelam N, Boban D, Bajt P, Racetin A, Rošin M, Čarić D, Vukojević K. MAPK Pathway Activation Patterns in the Synovium Reveal ERK1/2 and EGFR as Key Players in Osteoarthritis. Biomedicines. 2025; 13(12):2992. https://doi.org/10.3390/biomedicines13122992
Chicago/Turabian StyleJurić, Ivana, Petar Todorović, Nela Kelam, Danica Boban, Patricija Bajt, Anita Racetin, Matko Rošin, Davor Čarić, and Katarina Vukojević. 2025. "MAPK Pathway Activation Patterns in the Synovium Reveal ERK1/2 and EGFR as Key Players in Osteoarthritis" Biomedicines 13, no. 12: 2992. https://doi.org/10.3390/biomedicines13122992
APA StyleJurić, I., Todorović, P., Kelam, N., Boban, D., Bajt, P., Racetin, A., Rošin, M., Čarić, D., & Vukojević, K. (2025). MAPK Pathway Activation Patterns in the Synovium Reveal ERK1/2 and EGFR as Key Players in Osteoarthritis. Biomedicines, 13(12), 2992. https://doi.org/10.3390/biomedicines13122992









