Efficacy of Stromal Vascular Fraction Treatment for Knee Osteoarthritis: A Single-Arm Experimental Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Intervention
2.4. Outcomes
2.5. Statistical Analysis and Sample Size
3. Results
3.1. Baseline Demographic and Clinical Characteristics
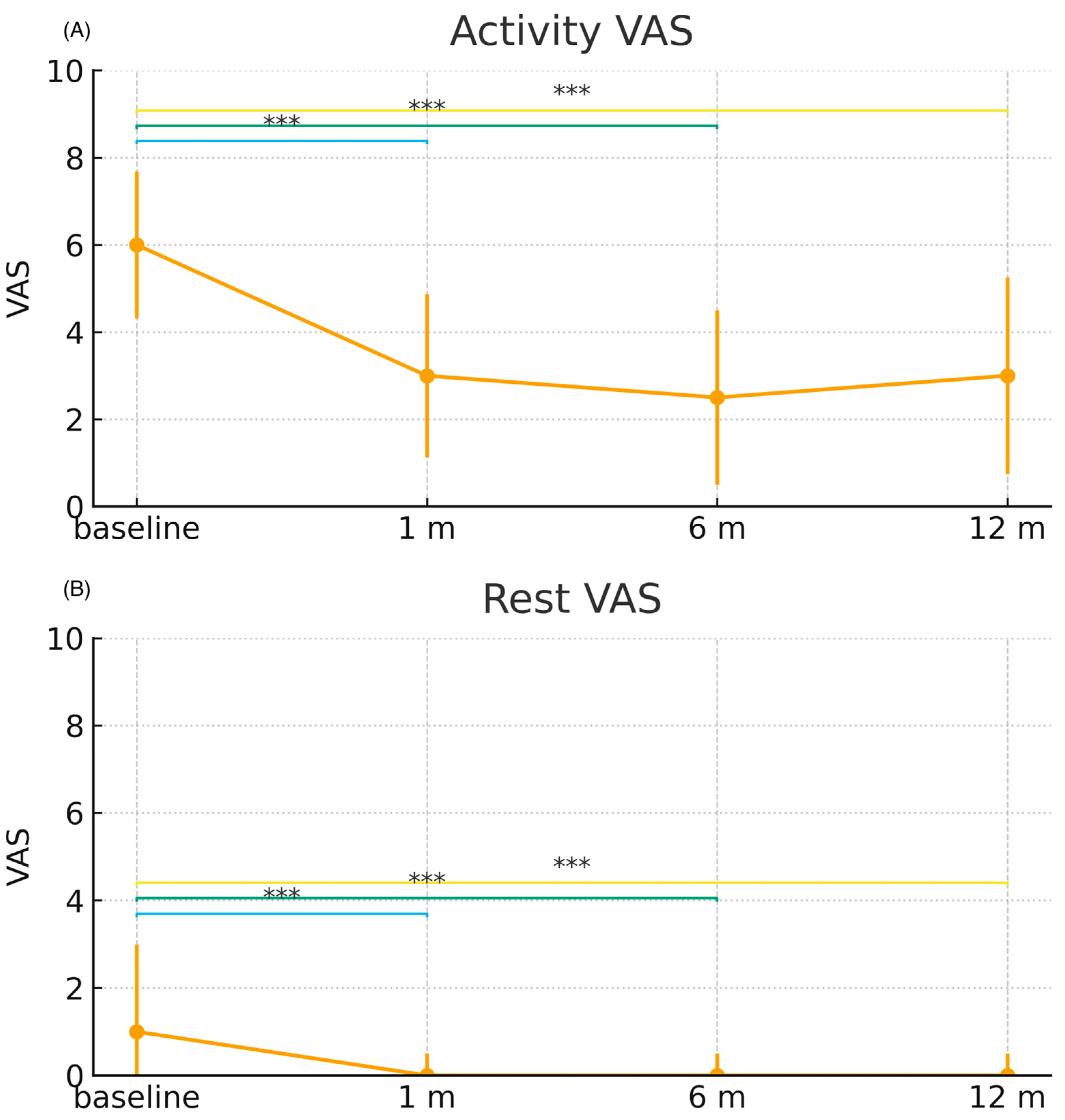
3.2. Pain
3.3. Functionality
3.4. Quality of Life
3.5. Radiologic Cartilage Changes
3.6. Biological Data
3.7. Safety Profile
3.8. Other Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSC | Adipose-derived Mesenchymal Stem Cell |
| BMI | Body Mass Index |
| CONSORT | Consolidated Standards of Reporting Trials |
| HIV | Human Immunodeficiency Virus |
| IQR | Interquartile Range |
| KOA | Knee Osteoarthritis |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| MCID | Minimum Clinically Important Difference |
| MOCART | Magnetic Resonance Observation of Cartilage Repair Tissue |
| MRI | Magnetic Resonance Imaging |
| MSC | Mesenchymal Stromal Cell |
| PI | Principal Investigator |
| PROM | Patient-Related Outcome Measures |
| SD | Standard Deviation |
| SVF | Stromal Vascular Fraction |
| VAS | Visual Analog Scale |
References
- Roubille, C.; Sellam, J.; Pelletier, J.P.; Martel-Pelletier, J. The multifaceted nature of osteoarthritis: Mechanisms, biomarkers, and treatment perspectives. Nat. Rev. Rheumatol. 2023, 19, 97–112. [Google Scholar] [CrossRef]
- Bijlsma, J.W.J.; Berenbaum, F.; Lafeber, F.P.J.G. Clinical aspects and management of osteoarthritis. Lancet Rheumatol. 2023, 5, e193–e204. [Google Scholar] [CrossRef]
- Coggon, D.; Croft, P.; Kellingray, S.; Barrett, D.; McLaren, M.; Cooper, C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000, 43, 1443–1449. [Google Scholar] [CrossRef]
- Duong, V.; Abdel Shaheed, C.; Ferreira, M.L.; Telles, R.W.; Zhang, Y.; Cooper, C. Risk factors for the development of knee osteoarthritis across the lifespan: A systematic review and meta-analysis. Osteoarthr. Cartil. 2025, 33, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.F.; Cleveland, R.J.; Allen, K.D.; Golightly, Y. Racial/Ethnic, Socioeconomic, and Geographic Disparities in the Epidemiology of Knee and Hip Osteoarthritis. Rheum. Dis. Clin. N. Am. 2021, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Wu, C.L.; Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transpl. 2013, 22, 1395–1408. [Google Scholar] [CrossRef]
- Xie, R.H.; Gong, S.G.; Song, J.; Wu, P.P.; Hu, W.L. Effect of mesenchymal stromal cells transplantation on the outcomes of patients with knee osteoarthritis: A systematic review and meta-analysis. J. Orthop. Res. 2024, 42, 753–768. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Yin, L.; Li, Y.; Liu, H. Transplantation of three mesenchymal stem cells for knee osteoarthritis, which cell and type are more beneficial? a systematic review and network meta-analysis. J. Orthop. Surg. Res. 2024, 19, 366. [Google Scholar] [CrossRef]
- Cui, G.H.; Wang, Y.Y.; Li, C.J.; Shi, C.H.; Wang, W.S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp. Ther. Med. 2016, 12, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Damia, E.; Chicharro, D.; Lopez, S.; Cuervo, B.; Rubio, M.; Sopena, J.J.; Vilar, J.M.; Carrillo, J.M. Adipose-Derived Mesenchymal Stem Cells: Are They a Good Therapeutic Strategy for Osteoarthritis? Int. J. Mol. Sci. 2018, 19, 1926. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, J.P.; Dos-Anjos, S.; Blázquez-Martínez, A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: Hypothesis on the regulatory role of intra-articular adipose tissue. J. Orthop. Surg. Res. 2020, 15, 137. [Google Scholar] [CrossRef]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 207. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Vaish, A.; Chavada, V.; Murrell, W.D.; Vaishya, R. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis-a systematic review. Int. Orthop. 2021, 45, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, B.; Vandenbulcke, F.; Vitale, N.D.; Iacono, F.; Ashmore, K.; Marcacci, M.; Kon, E. Minimally Manipulated Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence. Stem Cells Int. 2019, 2019, 1735242. [Google Scholar] [CrossRef]
- Hurley, E.T.; Yasui, Y.; Gianakos, A.L.; Seow, D.; Shimozono, Y.; Kerkhoffs, G.M.M.J.; Kennedy, J.G. Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal vascular fraction therapy for knee osteoarthritis: A systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef]
- Grimes, D.A.; Hubacher, D.; Nanda, K.; Schulz, K.F.; Moher, D.; Altman, D.G. The Good Clinical Practice guideline: A bronze standard for clinical research. Lancet 2005, 366, 172–174. [Google Scholar] [CrossRef]
- Murray, I.R.; Geeslin, A.G.; Goudie, E.B.; Petrigliano, F.A.; LaPrade, R.F. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): Platelet-Rich Plasma and Mesenchymal Stem Cells. J. Bone Jt. Surg. Am. 2017, 99, 809–819. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.F.; Bjerre, E.; Hansen, M.D.; Tendal, B.; Hilden, J.; Hróbjartsson, A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: Systematic review of empirical studies. J. Clin. Epidemiol. 2018, 101, 87–106.e2. [Google Scholar] [CrossRef]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 2000, 27, 1513–1517. [Google Scholar] [PubMed]
- Fukui, N.; Yamane, S.; Ishida, S.; Tanaka, K.; Masuda, R.; Tanaka, N.; Katsuragawa, Y.; Fukui, S. Relationship between radiographic changes and symptoms or physical examination findings in subjects with symptomatic medial knee osteoarthritis: A three-year prospective study. BMC Musculoskelet. Disord. 2010, 11, 269. [Google Scholar] [CrossRef]
- Vaquero, J.; Longo, U.G.; Forriol, F.; Martinelli, N.; Vethencourt, R.; Denaro, V. Reliability, validity and responsiveness of the Spanish version of the Knee Injury and Osteoarthritis Outcome Score (KOOS) in patients with chondral lesion of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 104–108. [Google Scholar] [CrossRef]
- Alonso, J.; Prieto, L.; Antó, J.M. La versión española del SF-36 Health Survey (Cuestionario de Salud SF-36): Un instrumento para la medida de los resultados clínicos [The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results]. Med. Clin. 1995, 104, 771–776. (In Spanish) [Google Scholar]
- Garratt, A.M.; Ruta, D.A.; Abdalla, M.I.; Buckingham, J.K.; Russell, I.T. The SF36 health survey questionnaire: An outcome measure suitable for routine use within the NHS? BMJ 1993, 306, 1440–1444. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Fodor, P.B.; Paulseth, S.G. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthet. Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.X.; Wu, C.M.; Dubey, N.K.; Deng, Y.H.; Su, C.W.; Pham, T.T.; Thi Le, P.B.; Sestili, P.; Deng, W.P. Time- and Kellgren–Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-articular Injection with Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef]
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino Contreras, R.M.; Scotton, D.; Bibas, M.; Quintero, M.; Monfort, J.; Carné, X.; de Abajo, F.; Oswald, E.; et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Marot, V.; Murgier, J.; Carrozzo, A.; Reina, N.; Monaco, E.; Chiron, P.; Berard, E.; Cavaignac, E. Determination of normal KOOS and WOMAC values in a healthy population. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 541–548. [Google Scholar] [CrossRef]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef]
- Gibbs, N.; Diamond, R.; Sekyere, E.O.; Thomas, W.D. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: A case series. J. Pain Res. 2015, 8, 799–806. [Google Scholar] [CrossRef]
- Biazzo, A.; D’Ambrosi, R.; Masia, F.; Izzo, V.; Verde, F. Autologous adipose stem cell therapy for knee osteoarthritis: Where are we now? Physician Sportsmed. 2020, 48, 392–399. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Muthu, S.; Kartheek, R.R.; Jeyaraman, N.; Rajendran, R.L.; Khanna, M.; Jeyaraman, M.; Packkyarathinam, R.P.; Gangadaran, P.; Ahn, B.C. Is Culture Expansion Necessary in Autologous Mesenchymal Stromal Cell Therapy to Obtain Superior Results in the Management of Knee Osteoarthritis? Meta-Analysis of Randomized Controlled Trials. Bioengineering 2021, 8, 220. [Google Scholar] [CrossRef]
- Güven, S.; Karagianni, M.; Schwalbe, M.; Schreiner, S.; Farhadi, J.; Bula, S.; Bieback, K.; Martin, I.; Scherberich, A. Validation of an automated procedure to isolate human adipose tissue-derived cells by using the Sepax® technology. Tissue Eng. Part C Methods 2012, 18, 575–582. [Google Scholar] [CrossRef]
- Doi, K.; Tanaka, S.; Iida, H.; Eto, H.; Kato, H.; Aoi, N.; Kuno, S.; Hirohi, T.; Yoshimura, K. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: Bench and bed analysis. J. Tissue Eng. Regen. Med. 2013, 7, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- London, N.J.; Miller, L.E.; Block, J.E. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med. Hypotheses 2011, 76, 887–892. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; de Sire, A.; Migliore, A.; Brandi, M.L.; Piscitelli, P. Early osteoarthritis: How to define, diagnose, and manage. A systematic review. Eur. Geriatr. Med. 2017, 8, 383–396. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Q.; Xia, J.; Chen, X.; Xiong, J.; Yang, L.; Liang, Y. Modification of mesenchymal stem cells for cartilage-targeted therapy. J. Transl. Med. 2022, 20, 515. [Google Scholar] [CrossRef]
- Zipfel, N.; Horreh, B.; Hulshof, C.T.J.; de Boer, A.G.E.M.; van der Burg-Vermeulen, S.J. The relationship between the living lab approach and successful implementation of healthcare innovations: An integrative review. BMJ Open 2022, 12, e058630. [Google Scholar] [CrossRef]
- Borst, J.M.; Ruoss, S.; Palmer, I.; Smith, T.; Kalunian, K.; Ward, S.R. Placebo Effect Sizes in Clinical Trials of Knee Osteoarthritis Using Intra-Articular Injections of Biologic Agents. Arthritis Care Res. 2025, 77, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Schüler, C.; Ginner, N.; Erben, R.G. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci. Rep. 2019, 9, 10153. [Google Scholar] [CrossRef] [PubMed]


| Study Sample (N = 78) | |
|---|---|
| Sex, No. (%) | |
| Female | 32 (41.0) |
| Male | 46 (59.0) |
| Age (years), mean (SD) | 59.12 (11.27) |
| <60 years, No. (%) | 42 (53.8) |
| ≥60 years, No. (%) | 36 (46.2) |
| BMI, mean (SD) | 27.05 (3.95) |
| <30, No. (%) | 60 (76.9) |
| ≥30, No. (%) | 18 (23.1) |
| Affected knee, No. (%) | |
| Left | 39 (50.0) |
| Right | 39 (50.0) |
| Reinfusion, No. (%) | |
| Yes | 8 (10.3) |
| No | 70 (89.7) |
| Previous treatments, No. (%) | |
| Platelet-rich plasma | 23 (29.5) |
| Steroids | 9 (11.5) |
| Hyaluronic acid | 16 (20.5) |
| Surgery a | 27 (77.1) |
| MOCART b, median [range] | |
| Lateral tibiofemoral joint | 30.0 [0.0–50.0] |
| Medial tibiofemoral joint | 15.0 [0.0–50.0] |
| Patellofemoral joint | 20.0 [0.0–50.0] |
| Joint effusion | 0.0 [0.0–5.0] |
| Total | 65.0 [10.0–135.0] |
| Study Sample (N = 78) | |
|---|---|
| VAS a, median [range] | |
| Rest | 1 [0–9] |
| Activity | 6 [1–10] |
| KOOS b, median [range] | |
| Pain | 63.9 [25.0–100] |
| Symptom | 75.0 [28.6–100] |
| Activities of daily living | 75.0 [5.8–98.5] |
| Sport | 30.0 [0–95.0] |
| Quality of life | 31.3 [0–75.0] |
| Total | 63.1 [19.6–91.5] |
| SF-36 c, median [range] | |
| Physical functioning | 57.5 [0–100] |
| Role limitations due to physical health | 50 [0–100] |
| Role limitations due to emotional problems | 100 [0–100] |
| Energy/fatigue | 62.5 [20–100] |
| Emotional well-being | 78.3 [10–100] |
| Social functioning | 87.5 [12.5–100] |
| Pain | 55 [0–100] |
| General health | 72.5 [15–100] |
| Health change | 50 [0–100] |
| BS | 1 m | p-Value a | 6 m | p-Value a | 12 m | p-Value a | |
|---|---|---|---|---|---|---|---|
| Physical functioning | 57.5 [50–71.25] | 72.5 [50–85] | 0.007 | 75 [50–87.5] | <0.001 | 75 [50–87.5] | 0.021 |
| Role limitations due to physical health | 50 [0–100] | 50 [0–100] | 0.350 | 100 [50–100] | <0.001 | 100 [18.75–100] | 0.023 |
| Role limitations due to emotional problems | 100 [66.67–100] | 100 [100–100] | <0.001 | 100 [100–100] | <0.001 | 100 [100–100] | 0.017 |
| Energy/Fatigue | 62.5 [45–80] | 67.5 [55–80] | 0.509 | 70 [60–82.5] | 0.026 | 77.5 [60–85] | 0.012 |
| Emotional well-being | 78.33 [52–90] | 80 [65–89.5] | 1 | 80 [70–90] | 1 | 80 [70–90] | 1 |
| Social Functioning | 87.5 [72–100] | 100 [75–100] | 0.094 | 100 [87.5–100] | <0.001 | 100 [75–100] | <0.001 |
| Pain | 55 [35–70] | 67.5 [45–78] | 0.117 | 77.5 [45–90] | <0.001 | 77.5 [45–90] | 0.023 |
| General Health | 72.5 [56.2–83.7] | 75 [55–83.3] | 1 | 75 [56.2–83.3] | 1 | 75 [56.2–83.3] | 1 |
| Health change | 50 [25–50] | 50 [50–75] | 0.021 | 75 [50–75] | <0.001 | 50 [50–75] | 0.007 |
| Initial Sample, No. (%) | |
|---|---|
| Fresh | 76 (97.4) |
| Frozen | 2 (2.6) |
| Initial adipose tissue (mL), median [range] | 175 [15–500] |
| Concentrated adipose tissue (mL), median [range] | 50 [10–162] |
| Total dose (log-10 scale), mean (SD) | 5.97 (0.53) |
| Cell yield (log-10 scale), mean (SD) | 4.26 (0.53) |
| Viability (%), mean (SD) | 88.67 (9.59) |
| Stromal vascular fraction (%), median [range] | 11.71 [0.36–33.20] |
| Sterility, No. (%) | |
| At extraction | 50 (64.1) |
| After manufacturing | 20 (25.6) |
| Adipose tissue | 27 (43.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boada-Pladellorens, A.; Avellanet, M.; Veiga, A.; Pages-Bolibar, E. Efficacy of Stromal Vascular Fraction Treatment for Knee Osteoarthritis: A Single-Arm Experimental Trial. Biomedicines 2025, 13, 2913. https://doi.org/10.3390/biomedicines13122913
Boada-Pladellorens A, Avellanet M, Veiga A, Pages-Bolibar E. Efficacy of Stromal Vascular Fraction Treatment for Knee Osteoarthritis: A Single-Arm Experimental Trial. Biomedicines. 2025; 13(12):2913. https://doi.org/10.3390/biomedicines13122913
Chicago/Turabian StyleBoada-Pladellorens, Anna, Merce Avellanet, Anna Veiga, and Esther Pages-Bolibar. 2025. "Efficacy of Stromal Vascular Fraction Treatment for Knee Osteoarthritis: A Single-Arm Experimental Trial" Biomedicines 13, no. 12: 2913. https://doi.org/10.3390/biomedicines13122913
APA StyleBoada-Pladellorens, A., Avellanet, M., Veiga, A., & Pages-Bolibar, E. (2025). Efficacy of Stromal Vascular Fraction Treatment for Knee Osteoarthritis: A Single-Arm Experimental Trial. Biomedicines, 13(12), 2913. https://doi.org/10.3390/biomedicines13122913







