Potential Regulatory Role of miR-15b, miR-99b, and miR-181a of the Shikonin-Induced MAPK/ERK Apoptotic Signaling Pathway in Renal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Shikonin Treatment of CAKI-2 and A-498 Cells
2.3. RNA Isolation from Cell Lines
2.4. Reverse Transcription PCR (RT-PCR)
2.5. TaqMan microRNA Assay
2.6. In Silico miRNA Analysis for Target and Pathway Prediction
2.7. Ingenuity Pathway Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.9. Protein Isolation and Western Blot
2.10. Statistical Analysis
2.11. Correlation Analysis of the Expression of miRNAs and Their Specific Targets
3. Results
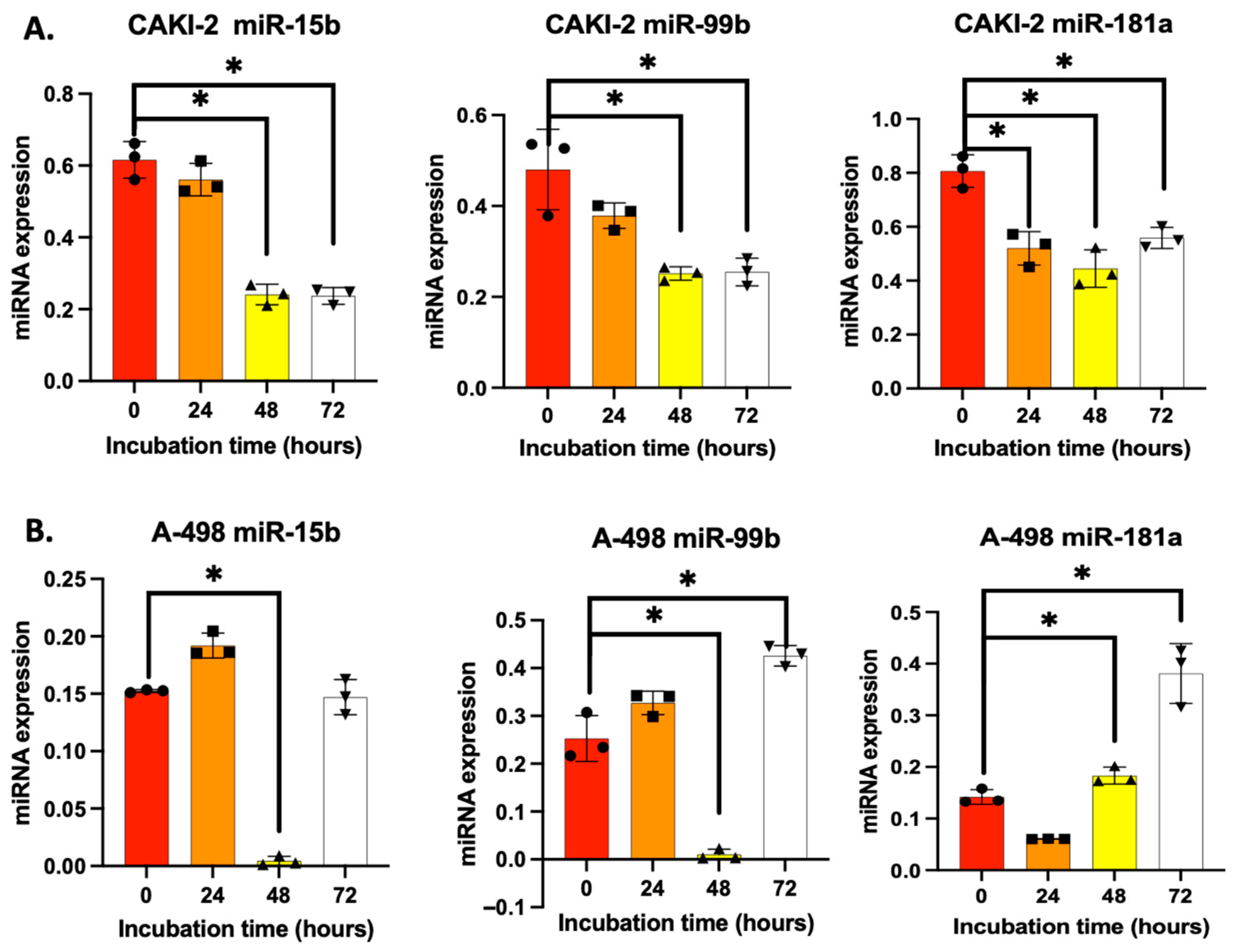
3.1. Shikonin Regulates microRNA Expression in CAKI-2 and A-498 Cell Lines Differently
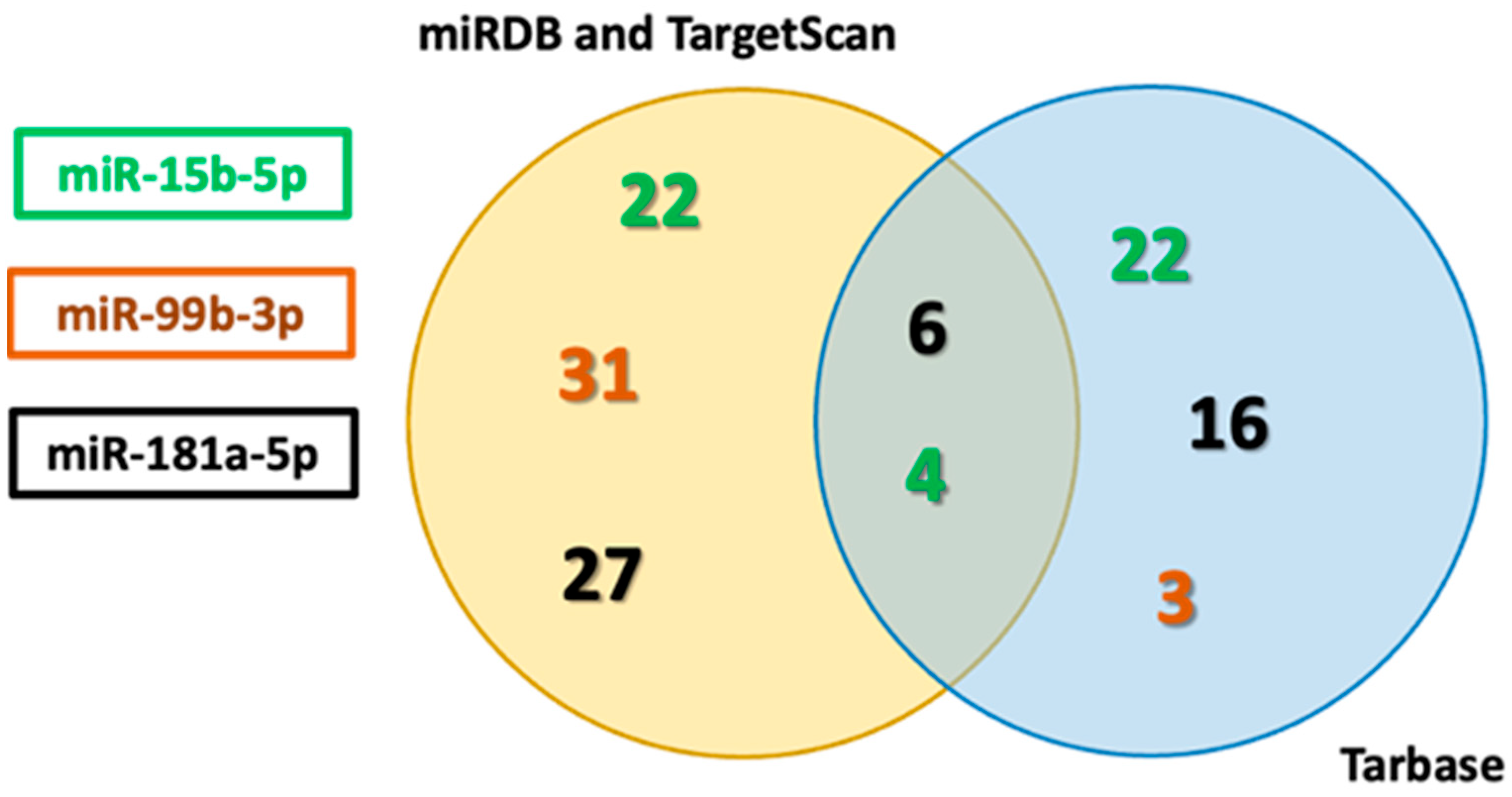
3.2. In Silico Identification of Apoptosis-Related miRNAs Targets
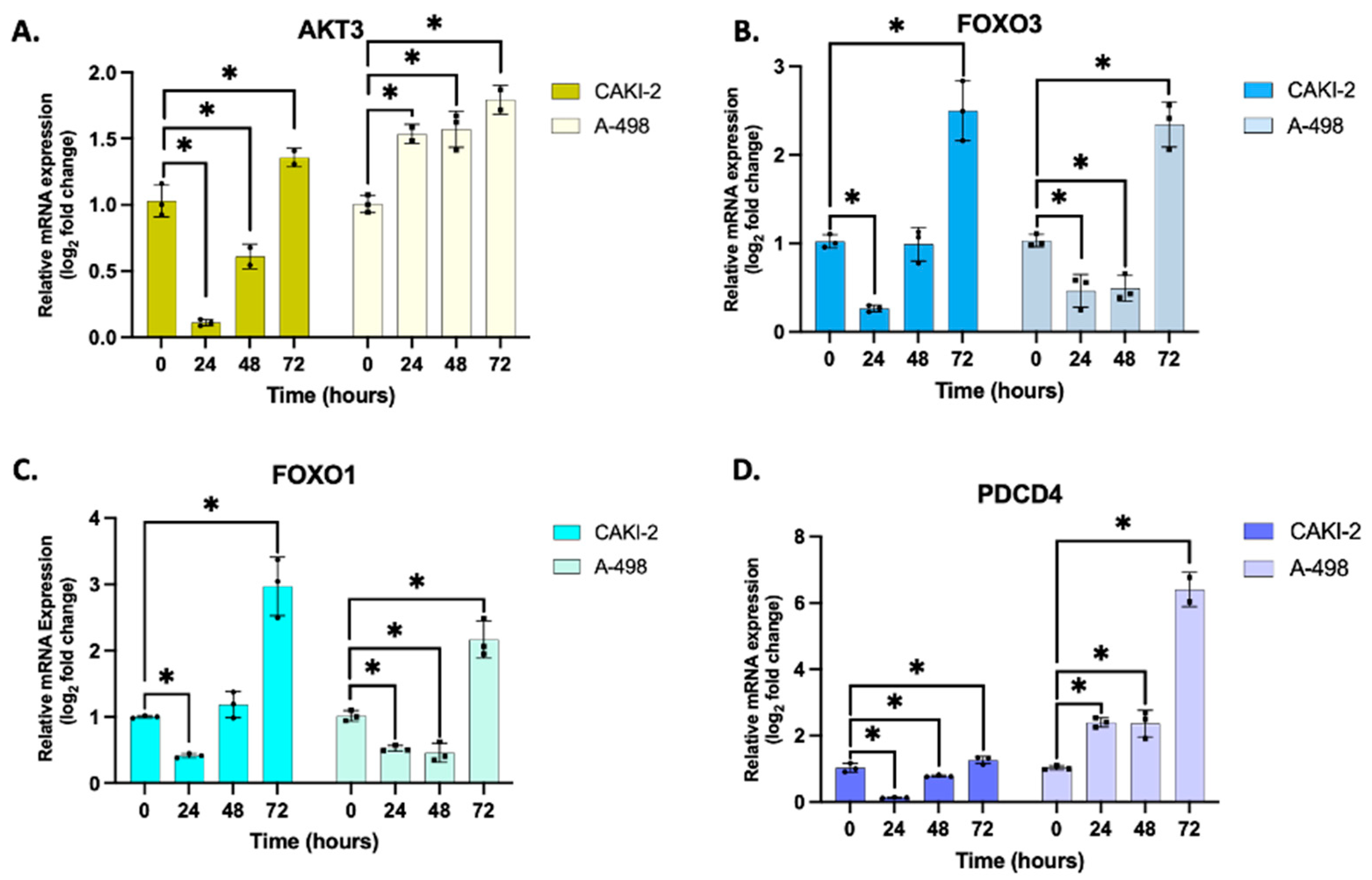
3.3. Validation of Putative Target Gene Expression by qRT-PCR
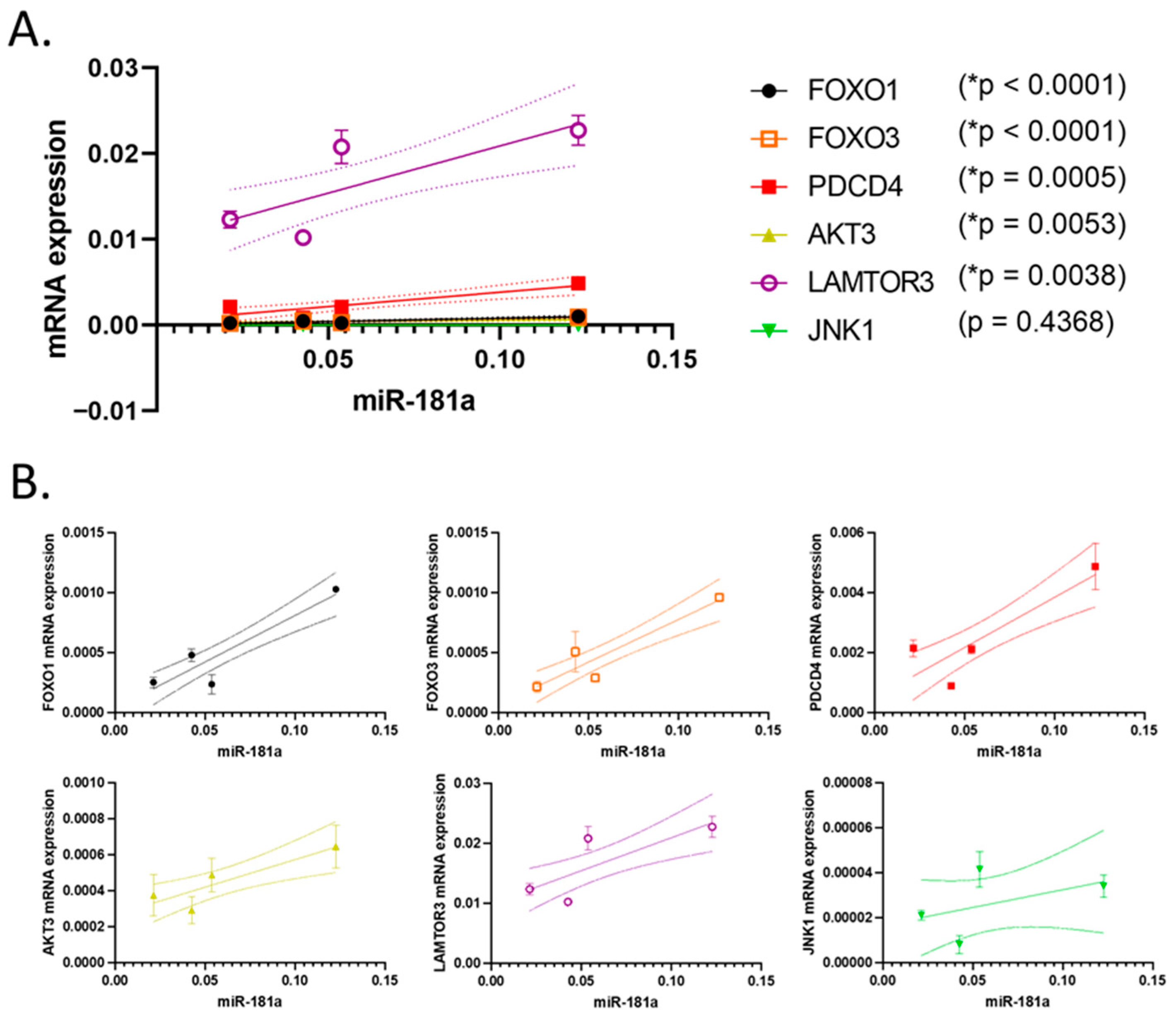
3.4. Correlation of the Expression of the Studied miRNAs and Their Putative Target
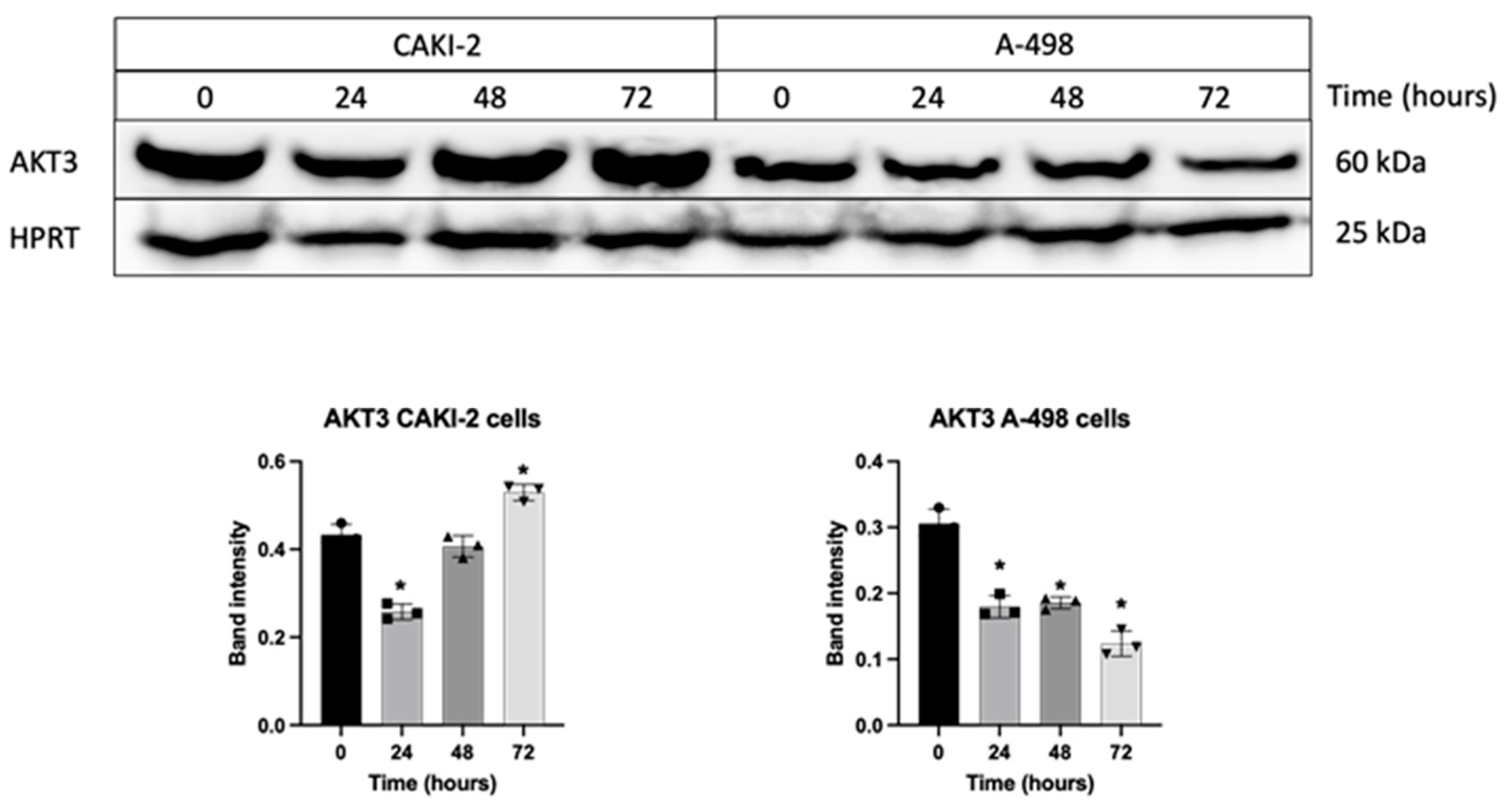
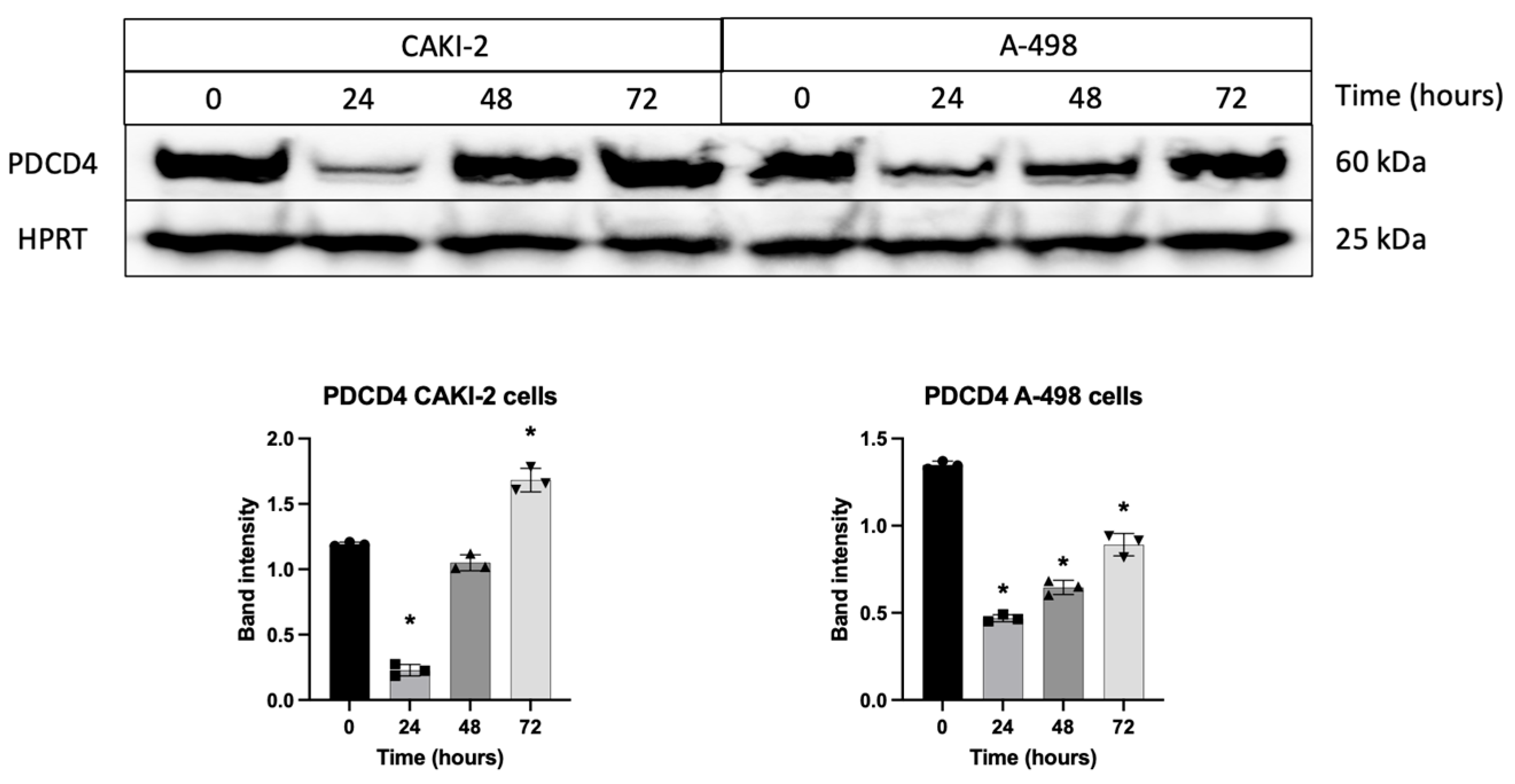
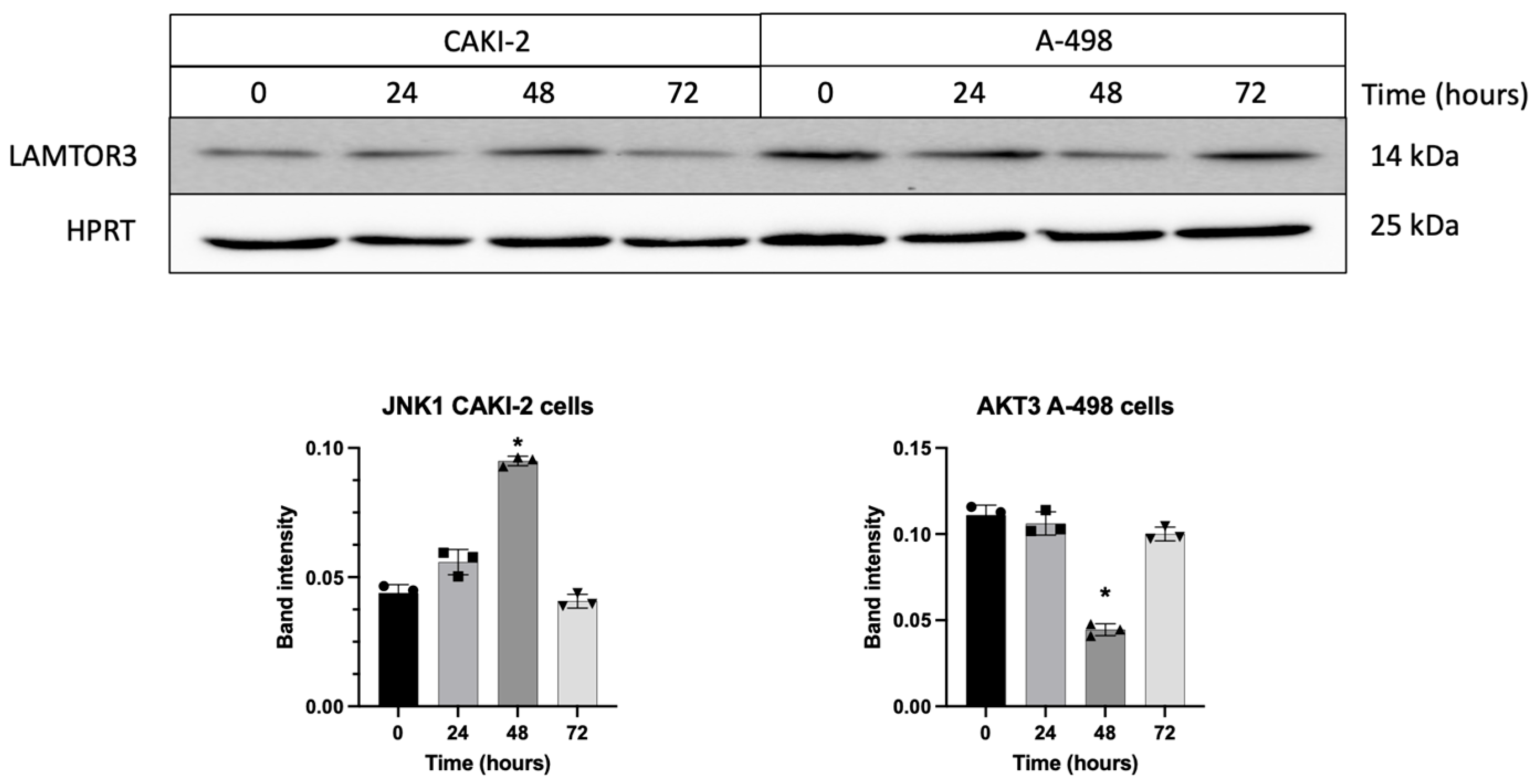
3.5. Shikonin Regulates the Expression of miRNAs’ Specific Target Proteins Related to Apoptosis
3.6. Pathway Analysis Showed Interaction of Shikonin Leading to Apoptosis and Renal Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ras | Reticular Activating System |
| ROS | Reactive oxygen species |
| PI3K | Phosphoinositide 3-kinase |
| Akt (PKB) | Protein kinase B |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| miRNA | MicroRNA |
| BCL-2 | BCL2 apoptosis regulator |
| SIRT1 | Sirtuin 1 |
| MCL1 | MCL1 apoptosis regulator, BCL2 family member |
| MAPK1 | Mitogen-activated protein kinase 1 |
| mTOR | Mammalian target of rapamycin |
| RCC | Renal cell carcinoma |
| ccRCC | Clear cell renal cell carcinoma |
| ATCC | American Type Culture Collection |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| FBS | Fetal Bovine Serum |
| DMSO | Dimethyl sulfoxide |
| cDNA | Complementary DNA |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| CT | Cycle threshold |
| RNU6 | U6 small nuclear RNA |
| mRNA | messenger RNA |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| PBS | Phosphate-buffered saline |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene fluoride |
| HPRT | Hypoxanthine Phosphoribosyltransferase |
| CI | Confidence interval |
| Akt3 | Serine/threonine kinase 3 |
| LAMTOR3 | Late endosomal/lysosomal adaptor, MAPK and MTOR activator 3 |
| DEDD | Death effector domain-containing protein |
| NIBAN1 | Niban apoptosis regulator 1 |
| SOX4 | SRY-box transcription factor 4 |
| JNK (MAPK8) | c-Jun N-terminal kinase (Mitogen-activated protein kinase 8) |
| TNFSF10 | TNF superfamily member 10 |
| MAPK9 | Mitogen-activated protein kinase 9 |
| FGF12 | Fibroblast growth factor 12 |
| BCL2L11 | BCL2-like 11 |
| FOXO1 | Forkhead box O1 |
| FOXO3 | Forkhead box O3 |
| PDCD4 | Programmed cell death 4 |
| JNK1 (MAPK8) | c-Jun N-terminal kinase 1 (Mitogen-activated protein kinase 8) |
| eIF4A | Eukaryotic initiation factor-4A |
| IPA | Ingenuity Pathway Analysis |
| IGF1R | Insulin-like growth factor 1 receptor |
References
- Ye, X.; Wu, X.; Lian, S.; Cai, R.; Wei, X.; Nan, T.; Cai, Y.; Su, Y.; Wei, J. Recent Advances of Shikonin in the Molecular Mechanisms of Anticancer, Anti-Inflammation and Immunoregulation. Am. J. Chin. Med. 2025, 53, 1093–1118. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, F.; Pozo, F.M.; Tian, D.; Geng, X.; Yao, X.; Zhang, Y.; Tang, J. Shikonin Inhibits Cancer Through P21 Upregulation and Apoptosis Induction. Front. Pharmacol. 2020, 11, 861. [Google Scholar]
- Chang, I.-C.; Huang, Y.-J.; Chiang, T.-I.; Yeh, C.-W.; Hsu, L.-S. Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol. Pharm. Bull. 2010, 33, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Jeung, Y.-J.; Kim, H.-G.; Ahn, J.; Lee, H.-J.; Lee, S.-B.; Won, M.; Jung, C.-R.; Im, J.-Y.; Kim, B.-K.; Park, S.-K.; et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim. Biophys. Acta 2016, 1863, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Croce, C.M. Role of miR-15/16 in CLL. Cell Death Differ. 2015, 22, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Horita, M.; Farquharson, C.; Stephen, L.A. The role of miR-29 family in disease. J. Cell Biochem. 2021, 122, 696–715. [Google Scholar] [CrossRef]
- Király, J.; Szabó, E.; Fodor, P.; Fejes, Z.; Nagy, B.; Juhász, É.; Vass, A.; Choudhury, M.; Kónya, G.; Halmos, G.; et al. Shikonin Causes an Apoptotic Effect on Human Kidney Cancer Cells through Ras/MAPK and PI3K/AKT Pathways. Molecules 2023, 28, 6725. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, T.; Duan, J.; Mu, N.; Zhang, T. MALAT1/miR-15b-5p/MAPK1 mediates endothelial progenitor cells autophagy and affects coronary atherosclerotic heart disease via mTOR signaling pathway. Aging 2019, 11, 1089–1109. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Cheng, K.; Zhang, G.; Wang, X. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif. 2014, 47, 587–595. [Google Scholar] [CrossRef]
- Li, Q.-J.; Chau, J.; Ebert, P.J.; Sylvester, G.; Min, H.; Liu, G.; Braich, R.; Manoharan, M.; Soutschek, J.; Skare, P.; et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 2007, 129, 147–161. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, G.; Jackson, Z.; Colina, J.; Sekhar, S.; DiFeo, A. miR-181a: Regulatory roles, cancer-associated signaling pathway disruptions, and therapeutic potential. Expert. Opin. Ther. Targets 2024, 28, 1061–1091. [Google Scholar] [CrossRef] [PubMed]
- Király, J.; Szabó, E.; Fodor, P.; Vass, A.; Choudhury, M.; Gesztelyi, R.; Szász, C.; Flaskó, T.; Dobos, N.; Zsebik, B.; et al. Expression of hsa-miRNA-15b, -99b, -181a and Their Relationship to Angiogenesis in Renal Cell Carcinoma. Biomedicines 2024, 12, 1441. [Google Scholar] [CrossRef]
- Brodaczewska, K.K.; Szczylik, C.; Fiedorowicz, M.; Porta, C.; Czarnecka, A.M. Choosing the right cell line for renal cell cancer research. Mol. Cancer 2016, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Yates, D.R.; Cancel-Tassin, G.; Cussenot, O.; Rouprêt, M. Genetic pathways involved in carcinogenesis of clear cell renal cell carcinoma: Genomics towards personalized medicine. BJU Int. 2012, 109, 1864–1870. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Shan, Z.-L.; Zhong, L.; Xiao, C.-L.; Gan, L.-G.; Xu, T.; Song, H.; Yang, R.; Li, L.; Liu, B.-Z. Shikonin suppresses proliferation and induces apoptosis in human leukemia NB4 cells through modulation of MAPKs and c-Myc. Mol. Med. Rep. 2017, 16, 3055–3060. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, S.H.; Kim, Y.M.; Kim, S.H.; Yoo, E.S.; Woo, J.S.; Jung, G.H.; Jung, S.H.; Kim, B.S.; Jung, J.Y. Shikonin inhibits proliferation of melanoma cells by MAPK pathway-mediated induction of apoptosis. Biosci. Rep. 2021, 41, BSR20203834. [Google Scholar] [CrossRef]
- Markowitsch, S.D.; Vakhrusheva, O.; Schupp, P.; Akele, Y.; Kitanovic, J.; Slade, K.S.; Efferth, T.; Thomas, A.; Tsaur, I.; Mager, R.; et al. Shikonin Inhibits Cell Growth of Sunitinib-Resistant Renal Cell Carcinoma by Activating the Necrosome Complex and Inhibiting the AKT/mTOR Signaling Pathway. Cancers 2022, 14, 1114. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, T.-J. The role of microRNAs in cell death pathways. Yeungnam Univ. J. Med. 2021, 38, 107–117. [Google Scholar] [CrossRef]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef]
- Yen, J.-H.; Keak, P.Y.; Wu, C.-L.; Chen, H.-J.; Gao, W.-Y.; Liou, J.-W.; Chen, Y.-R.; Lin, L.-I.; Chen, P.-Y. Shikonin, a natural naphthoquinone phytochemical, exerts anti-leukemia effects in human CBF-AML cell lines and zebrafish xenograft models. Biomed. Pharmacother. 2024, 179, 117395. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Liu, T.-J.; Lai, H.-C. Shikonin Induces Apoptosis, Necrosis, and Premature Senescence of Human A549 Lung Cancer Cells through Upregulation of p53 Expression. Evid.-Based Complement. Altern. Med. 2015, 2015, 620383. [Google Scholar] [CrossRef]
- Uzuner, E.; Ulu, G.T.; Gürler, S.B.; Baran, Y. The Role of MiRNA in Cancer: Pathogenesis, Diagnosis, and Treatment. Methods Mol. Biol. 2022, 2257, 375–422. [Google Scholar] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, Y.-C.D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Yu, S.; Huang, H.-Y.; Lin, Y.-C.; Huang, Y.; Zhang, B.; Xiao, J.; Zuo, H.; Wang, J.; Li, Z.; et al. miRTarBase 2025: Updates to the collection of experimentally validated microRNA–target interactions. Nucleic Acids Res. 2025, 53, D147–D156. [Google Scholar] [CrossRef]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 2011, 1813, 1938–1945. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, D.; Du, R.; Pan, Y.; Zhao, L.; Sun, S.; Hong, L.; Liu, J.; Fan, D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer 2008, 123, 372–379. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Wen, H.; Feng, C. Overexpression of miR-15b Promotes Resistance to Sunitinib in Renal Cell Carcinoma. J. Cancer 2019, 10, 3389–3396. [Google Scholar] [CrossRef]
- Shen, H.; Fang, K.; Guo, H.; Wang, G. High Glucose-Induced Apoptosis in Human Kidney Cells Was Alleviated by miR-15b-5p Mimics. Biol. Pharm. Bull. 2019, 42, 758–763. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Wang, N.; Sun, M. miR-15b, a diagnostic biomarker and therapeutic target, inhibits oesophageal cancer progression by regulating the PI3K/AKT signalling pathway. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mou, Q.; Zhu, Z.; Zhao, L.; Zhu, L. MALAT1 promotes liver fibrosis by sponging miR-181a and activating TLR4-NF-κB signaling. Int. J. Mol. Med. 2021, 48, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, J.; Zhai, Y.; Du, F.; Li, M.; Wu, X.; Chen, Y.; Wang, S.; Xiao, Z.; Wu, Z. Role of miR-181a-5p in cancer (Review). Int. J. Oncol. 2023, 63, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gibson, V.P.; Hardy, P. The Role of MiR-181 Family Members in Endothelial Cell Dysfunction and Tumor Angiogenesis. Cells 2022, 11, 1670. [Google Scholar] [CrossRef]
- Li, Y.; Kuscu, C.; Banach, A.; Zhang, Q.; Pulkoski-Gross, A.; Kim, D.; Liu, J.; Roth, E.; Li, E.; Shroyer, K.R.; et al. miR-181a-5p Inhibits Cancer Cell Migration and Angiogenesis via Downregulation of Matrix Metalloproteinase-14. Cancer Res. 2015, 75, 2674–2685. [Google Scholar] [CrossRef]
- Arnott, M.; Sampilo, N.F.; Song, J.L. Transcription of microRNAs is regulated by developmental signaling pathways and transcription factors. Front. Cell Dev. Biol. 2024, 12, 1356589. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Hu, Y.; Xu, L.; Jiang, Y.; Zhang, C.; Ding, L.; Jiang, R.; Sun, J.; Sun, H.; et al. miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017, 8, e3088. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, H.; Zhao, H.; Zhou, Y.; Xu, R.; Xu, X.; Zheng, L.; Xue, Z.; Xia, W.; Zhang, B.; et al. MicroRNA-99a induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC Cancer 2012, 12, 546, Erratum in BMC Cancer 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Marques, M.; Carvalho, A.; Cavadas, C.; Aveleira, C.A. PI3K/AKT/MTOR and ERK1/2-MAPK signaling pathways are involved in autophagy stimulation induced by caloric restriction or caloric restriction mimetics in cortical neurons. Aging 2021, 13, 7872–7882. [Google Scholar] [CrossRef] [PubMed]
- Furukawa-Hibi, Y.; Kobayashi, Y.; Chen, C.; Motoyama, N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid. Redox Signal. 2005, 7, 752–760. [Google Scholar] [CrossRef]
- Orea-Soufi, A.; Paik, J.; Bragança, J.; Donlon, T.A.; Willcox, B.J.; Link, W. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol. Sci. 2022, 43, 1070–1084. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H. The role of Pdcd4 in tumour suppression and protein translation. Biol. Cell 2018, 110, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.-S. The Impact of Pdcd4, a Translation Inhibitor, on Drug Resistance. Pharmaceuticals 2024, 17, 1396. [Google Scholar] [CrossRef]
- Jun, S.; Lee, S.; Kim, H.-C.; Ng, C.; Schneider, A.M.; Ji, H.; Ying, H.; Wang, H.; DePinho, R.A.; Park, J.-I. PAF-mediated MAPK signaling hyperactivation via LAMTOR3 induces pancreatic tumorigenesis. Cell Rep. 2013, 5, 314–322. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef]
- Ji, D.; Chen, Z.; Li, M.; Zhan, T.; Yao, Y.; Zhang, Z.; Xi, J.; Yan, L.; Gu, J. MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Mol. Cancer 2014, 13, 86. [Google Scholar] [CrossRef]
- Rani, M.; Kumari, R.; Singh, S.P.; Devi, A.; Bansal, P.; Siddiqi, A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H.; Alam Rizvi, M.M. MicroRNAs as master regulators of FOXO transcription factors in cancer management. Life Sci. 2023, 321, 121535. [Google Scholar] [CrossRef]
- Han, L. miR-99a inhibits proliferation and migration of cervical cancer cells by targeting IGF1R. J. Buon 2021, 26, 1782–1788. [Google Scholar] [PubMed]
- Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef]
- Pan, C.; Jin, X.; Zhao, Y.; Pan, Y.; Yang, J.; Karnes, R.J.; Zhang, J.; Wang, L.; Huang, H. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. Embo J. 2017, 36, 995–1010. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef]
- Liu, H.; Luo, G.; Xie, B.; Chen, M.; Xie, P.; Zhao, X.; You, Y.; Sun, X. The Role of miR-181a-5p and its Target Genes in the Progression of Clear Cell Renal Cell Carcinoma and Association With the Immune Microenvironment. Clin. Genitourin. Cancer 2025, 23, 102390. [Google Scholar] [CrossRef]
- Hay, N. Interplay between FOXO, TOR, and Akt. Biochim. Biophys. Acta 2011, 1813, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wen, Y.; Liu, J.; Hao, J.; Peng, Y.; Zhang, M.; Xie, Y.; Yang, Z.; Yin, X.; Shi, Y.; et al. The miR-15b-5p/miR-379-3p-FOXO axis regulates cell cycle and apoptosis in scleral remodeling during experimental myopia. J. Transl. Med. 2024, 22, 710. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vass, A.; Király, J.; Szabó, E.; Shree, N.; Ramos, D.; Choudhury, M.; Fodor, P.; Szegedi, K.; Halmos, G.; Szabó, Z. Potential Regulatory Role of miR-15b, miR-99b, and miR-181a of the Shikonin-Induced MAPK/ERK Apoptotic Signaling Pathway in Renal Carcinoma. Biomedicines 2025, 13, 2898. https://doi.org/10.3390/biomedicines13122898
Vass A, Király J, Szabó E, Shree N, Ramos D, Choudhury M, Fodor P, Szegedi K, Halmos G, Szabó Z. Potential Regulatory Role of miR-15b, miR-99b, and miR-181a of the Shikonin-Induced MAPK/ERK Apoptotic Signaling Pathway in Renal Carcinoma. Biomedicines. 2025; 13(12):2898. https://doi.org/10.3390/biomedicines13122898
Chicago/Turabian StyleVass, Anna, József Király, Erzsébet Szabó, Nitya Shree, Deisy Ramos, Mahua Choudhury, Petra Fodor, Krisztián Szegedi, Gábor Halmos, and Zsuzsanna Szabó. 2025. "Potential Regulatory Role of miR-15b, miR-99b, and miR-181a of the Shikonin-Induced MAPK/ERK Apoptotic Signaling Pathway in Renal Carcinoma" Biomedicines 13, no. 12: 2898. https://doi.org/10.3390/biomedicines13122898
APA StyleVass, A., Király, J., Szabó, E., Shree, N., Ramos, D., Choudhury, M., Fodor, P., Szegedi, K., Halmos, G., & Szabó, Z. (2025). Potential Regulatory Role of miR-15b, miR-99b, and miR-181a of the Shikonin-Induced MAPK/ERK Apoptotic Signaling Pathway in Renal Carcinoma. Biomedicines, 13(12), 2898. https://doi.org/10.3390/biomedicines13122898






