Molecular Profile and Clinical Associations of Androgen Receptor Coactivators and Structural Genes in Benign Prostatic Hyperplasia and Metabolic Syndrome
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Extraction of RNA and Quantitative Real-Time Polymerase Chain Reaction
2.3. Statistical Analysis
3. Results
3.1. BPH Genetic Profile
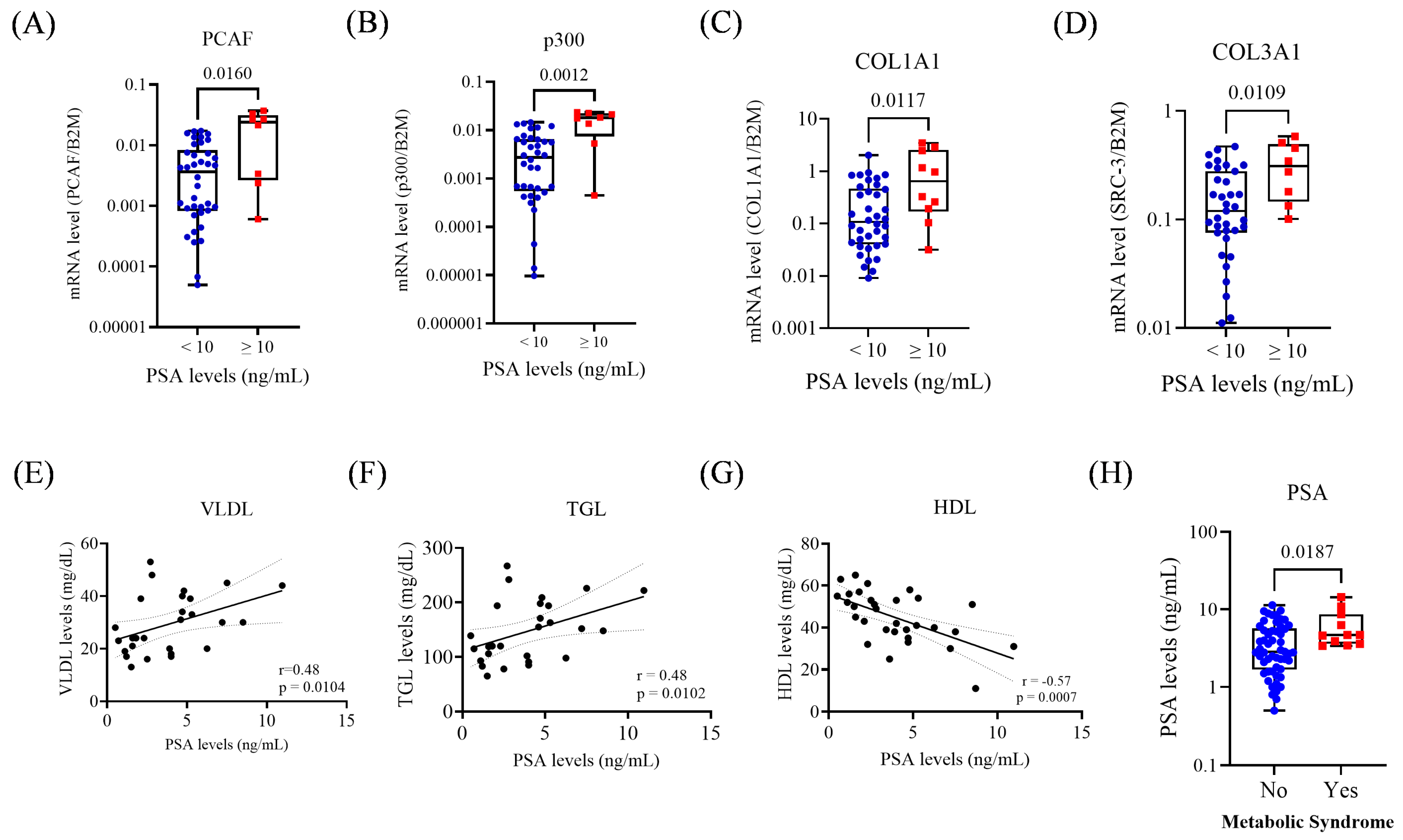
3.2. PSA Levels and Their Association with Gene Expression and Metabolic Parameters
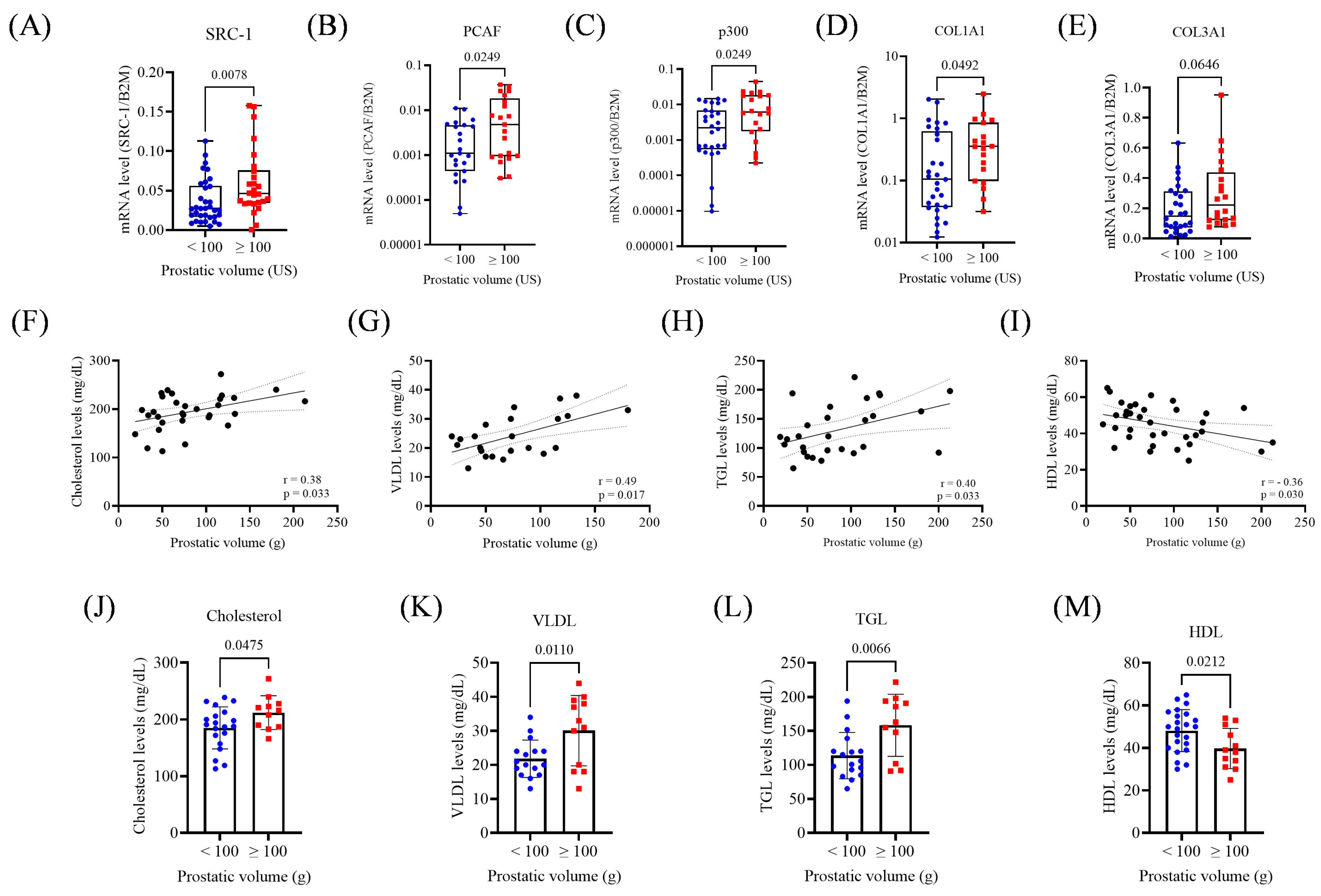
3.3. Prostate Volume: Associations with Gene Expression and Lipid Metabolism
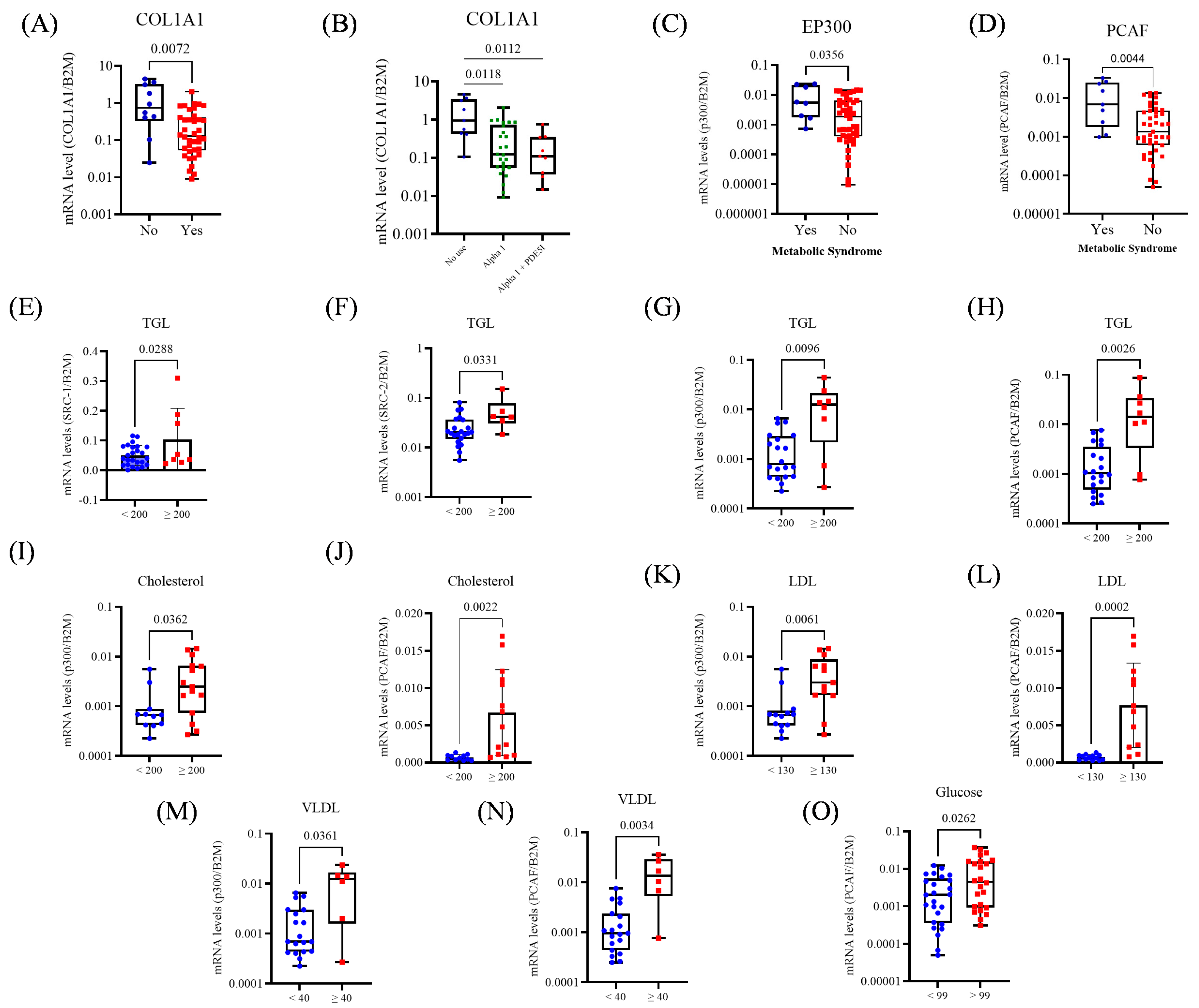
3.4. Correlation Between Collagen and AR Coregulators-Positive Association with the p160 Family
3.5. Gene Expression and Its Association with BPH Treatment and Metabolic Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Benign Prostatic Hyperplasia Collaborators. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2022, 3, e754–e776. [Google Scholar] [CrossRef]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- McConnell, J.D.; Roehrborn, C.G.; Bautista, O.M.; Andriole, G.L.; Dixon, C.M.; Kusek, J.W.; Lepor, H.; McVary, K.T.; Nyberg, L.M.J.; Clarke, H.S.; et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003, 349, 2387–2398. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Siami, P.; Barkin, J.; Damião, R.; Major-Walker, K.; Nandy, I.; Morrill, B.B.; Gagnier, R.P.; Montorsi, F. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur. Urol. 2010, 57, 123–131, Erratum in Eur. Urol. 2010, 58, 801. [Google Scholar] [CrossRef]
- Fogaing, C.; Alsulihem, A.; Campeau, L.; Corcos, J. Is Early Surgical Treatment for Benign Prostatic Hyperplasia Preferable to Prolonged Medical Therapy: Pros and Cons. Medicina 2021, 57, 368. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B. The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer. Mol. Cell. Biochem. 2003, 253, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Androgen Receptor Coactivators in Regulation of Growth and Differentiation in Prostate Cancer. J. Cell. Physiol. 2016, 231, 270–274. [Google Scholar] [CrossRef]
- Pimenta, R.; Malulf, F.C.; Romão, P.; Caetano, G.V.B.; da Silva, K.S.; Ghazarian, V.; dos Santos, G.A.; Guimarães, V.; Silva, I.A.; de Camargo, J.A.; et al. Evaluation of AR, AR-V7, and p160 family as biomarkers for prostate cancer: Insights into the clinical significance and disease progression. J. Cancer Res. Clin. Oncol. 2024, 150, 70. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, R.; Camargo, J.A.; Candido, P.; Ghazarian, V.; Gonçalves, G.L.; Guimarães, V.R.; Romão, P.; Chiovatto, C.; Mioshi, C.M.; dos Santos, G.A.; et al. Cholesterol Triggers Nuclear Co-Association of Androgen Receptor, p160 Steroid Coactivators, and p300/CBP-Associated Factor Leading to Androgenic Axis Transactivation in Castration-Resistant Prostate Cancer. Cell. Physiol. Biochem. 2022, 56, 1–15. [Google Scholar]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Primers 2016, 2, 16031. [Google Scholar] [CrossRef]
- Daher, M.; Saqer, T.; Jabr, M.; Al-Mousa, S. Benign prostatic hyperplasia and metabolic syndrome; prevalence and association: A cross-sectional study in Syria. BMC Urol. 2023, 23, 187. [Google Scholar] [CrossRef]
- Hammarsten, J.; Högstedt, B.; Holthuis, N.; Mellström, D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998, 1, 157–162. [Google Scholar] [CrossRef]
- Pimenta, R.; Mioshi, C.M.; Gonçalves, G.L.; Candido, P.; Camargo, J.A.; Guimarães, V.R.; Chiovatto, C.; Ghazarian, V.; Romão, P.; da Silva, K.S.; et al. Intratumoral Restoration of miR-137 Plus Cholesterol Favors Homeostasis of the miR-137/Coactivator p160/AR Axis and Negatively Modulates Tumor Progression in Advanced Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 9633. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, B.; Agarwal, A.; Gupta, A. Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer. Front. Endocrinol. 2022, 13, 886594. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ding, L.; Bohrer, L.R.; Pan, Y.; Liu, P.; Zhang, J.; Sebo, T.J.; Karnes, R.J.; Tindall, D.J.; van Deursen, J.; et al. p300 acetyltransferase regulates androgen receptor degradation and PTEN-deficient prostate tumorigenesis. Cancer Res. 2014, 74, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, M.; Ou, Z.; He, W.; Chen, L.; Zhang, J.; He, Y.; Xu, R.; Jiang, S.; Qi, L.; et al. Long noncoding RNA DNM3OS promotes prostate stromal cells transformation via the miR-29a/29b/COL3A1 and miR-361/TGFβ1 axes. Aging 2019, 11, 9442–9460. [Google Scholar] [CrossRef]
- Hueber, P.A.; Ben-Zvi, T.; Liberman, D.; Bhojani, N.; Gautam, G.; Deklaj, T.; Katz, M.; Zorn, K.C. Mid term outcomes of initial 250 case experience with GreenLight 120W-HPS photoselective vaporization prostatectomy for benign prostatic hyperplasia: Comparison of prostate volumes < 60 cc, 60 cc–100 cc and >100 cc. Can. J. Urol. 2012, 19, 6450–6458. [Google Scholar]
- Litman, H.J.; McKinlay, J.B. The future magnitude of urological symptoms in the USA: Projections using the Boston Area Community Health survey. BJU Int. 2007, 100, 820–825, Erratum in BJU Int. 2007, 100, 97. [Google Scholar] [CrossRef]
- Bortnick, E.; Brown, C.; Simma-Chiang, V.; Kaplan, S.A. Modern best practice in the management of benign prostatic hyperplasia in older people. Ther. Adv. Urol. 2020, 12, 1756287220929486. [Google Scholar] [CrossRef]
- Marmiroli, R.; Antunes, A.A.; Reis, S.T.; Nakano, E.; Srougi, M. Standard surgical treatment for benign prostatic hyperplasia is safe for patients over 75 years: Analysis of 100 cases from a high-volume urologic center. Clinics 2012, 67, 1415–1418. [Google Scholar] [CrossRef]
- Ngai, H.Y.; Yuen, K.S.; Ng, C.M.; Cheng, C.H.; Chu, S.P. Metabolic syndrome and benign prostatic hyperplasia: An update. Asian J. Urol. 2017, 4, 164–173. [Google Scholar] [CrossRef]
- Zamani, A.; Rafiee, M.; Alikhani, M.Y.; Mohagheghi, S.; Pakrad, B.; Borzouei, S. Serum Interleukin-17, Carcinoembryonic Antigen, and Prostate-Specific Antigen in High Lipid Profile Individuals. J. Interferon. Cytokine Res. 2020, 40, 218–224. [Google Scholar] [CrossRef]
- Arab, D.; Ardestani Zadeh, A.; Mirmohammadkhani, M.; Beiglarzadeh, A. Prostate-specific antigen rising in Iranian men in correlation with body mass index, fasting blood sugar and blood lipid profile. J. Nephropathol. 2016, 5, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef]
- Heemers, H.V.; Tindall, D.J. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef]
- Wang, T.; Yao, W.; Shao, Y.; Zheng, R.; Huang, F. PCAF fine-tunes hepatic metabolic syndrome, inflammatory disease, and cancer. J. Cell. Mol. Med. 2018, 22, 5787–5800. [Google Scholar] [CrossRef] [PubMed]
- Youn, D.H.; Park, J.; Kim, H.L.; Jung, Y.; Kang, J.; Lim, S.; Song, G.; Kwak, H.J.; Um, J.-Y. Berberine Improves Benign Prostatic Hyperplasia via Suppression of 5 Alpha Reductase and Extracellular Signal-Regulated Kinase in Vivo and in Vitro. Front. Pharmacol. 2018, 9, 773. [Google Scholar] [CrossRef]
- Linja, M.J.; Porkka, K.P.; Kang, Z.; Savinainen, K.J.; Jänne, O.A.; Tammela, T.L.; Vessella, R.L.; Palvimo, J.J.; Visakorpi, T. Expression of androgen receptor coregulators in prostate cancer. Clin. Cancer Res. 2004, 10, 1032–1040. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors. 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Yuan, Y.; Geng, H.; Xia, S.J. Influence of immune inflammation on androgen receptor expression in benign prostatic hyperplasia tissue. Asian J. Androl. 2012, 14, 316–319. [Google Scholar] [CrossRef]
- Lu, T.; Lin, W.J.; Izumi, K.; Wang, X.; Xu, D.; Fang, L.Y.; Li, L.; Jiang, Q.; Jin, J.; Chang, C. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol. Endocrinol. 2012, 26, 1707–1715. [Google Scholar] [CrossRef]
- Schneider, A.J.; Serrell, E.C.; Grimes, M.; Wang, S.; Bushman, W. Histologic inflammation and collagen content are not positively correlated in human BPH. Prostate 2023, 83, 1529–1536. [Google Scholar] [CrossRef]
- Delella, F.K.; de Almeida, F.L.A.; Nunes, H.C.; Rinaldi, J.C.; Felisbino, S.L. Fibrillar collagen genes are not coordinately upregulated with TGF β1 expression in finasteride-treated prostate. Cell Biol. Int. 2017, 41, 1214–1222. [Google Scholar] [CrossRef]
- Delella, F.K.; Lacorte, L.M.; Almeida, F.L.; Pai, M.D.; Felisbino, S.L. Fibrosis-related gene expression in the prostate is modulated by doxazosin treatment. Life Sci. 2012, 91, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.J.; Jacobson, D.J.; Girman, C.J.; Roberts, R.O.; Rhodes, T.; Guess, H.A.; Lieber, M.M. Natural history of prostatism: Risk factors for acute urinary retention. J. Urol. 1997, 158, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; McConnell, J.D.; Lieber, M.; Kaplan, S.; Geller, J.; Malek, G.H.; Castellanos, R.; Coffield, S.; Saltzman, B.; Resnick, M.; et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999, 53, 473–480. [Google Scholar] [CrossRef]
- Nickel, J.C. Benign prostatic hyperplasia: Does prostate size matter? Rev. Urol. 2003, 5, S12–S17. [Google Scholar]
- Carithers, L.J.; Ardlie, K.; Barcus, M.; Branton, P.A.; Britton, A.; Buia, S.A.; Compton, C.C.; DeLuca, D.S.; Peter-Demchok, J.; Gelfand, E.T.; et al. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv. Biobank. 2015, 13, 311–319. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Control (N = 5) | BPH (N = 76) | p-Value |
|---|---|---|---|
| Age, n (%) | 5 | 76 | |
| ≤65 | 4 (80) | 27 (34.6) | 0.070 $ |
| >65 | 1 (20) | 49 (67.9) | |
| PSA, n (%) | 5 | 61 | |
| <10 | 5 (100) | 50 (64.1) | 0.58 $ |
| >10 | 0 | 11 (14.1) | |
| Prostatic Volume, n (%) | 66 | ||
| <100 | 40 (51.2) | ||
| >100 | 27 (34.6) | ||
| Use of Medications, n (%) | 66 | ||
| Yes | 53 (80.3) | ||
| No | 13 (19.7) | ||
| Use of Medications, n (%) | 53 | ||
| Alpha Blocker | 38 (71.6) | ||
| 5-alpha-reductase Inhibitor | 1 (1.8) | ||
| Anticholinergic | 1 (1.8) | ||
| Alpha Blocker + 5-alpha-reductase Inhibitor | 12 (22.6) | ||
| Alpha Blocker + Anticholinergic | 2 (3.7) | ||
| Type of Surgery | 71 | ||
| Transurethral Resection of the Prostate | 43 (60.5) | ||
| Transvesical Prostatectomy | 14 (19.7) | ||
| Millin’s Prostatectomy | 14 (19.7) | ||
| Comorbidity | 69 | ||
| SAH | 44 (64) | ||
| Diabetes Mellitus | 13 (19) | ||
| MetS | 12 (17) | ||
| Glucose (mg/dL) | 69 | ||
| <99 | 50 (72) | ||
| ≥99 | 19 (28) | ||
| Total Cholesterol (mg/dL) | 38 | ||
| <200 | 20 (53) | ||
| ≥200 | 18 (47) | ||
| HDL (mg/dL) | 38 | ||
| <100 | 14 (37) | ||
| ≥100 | 24 (63) | ||
| TGL (mg/dL) | 37 | ||
| <200 | 20 (54) | ||
| ≥200 | 17 (46) | ||
| VLDL (mg/dL) | 34 | ||
| <100 | 28 (82) | ||
| ≥100 | 6 (18) | ||
| LDL (mg/dL) | 38 | ||
| <130 | 24 (63) | ||
| ≥130 | 14 (37) |
| Genes | R | 95% CI | p-Value |
|---|---|---|---|
| COL1A1 vs. SRC-1 | 0.436 | 0.221–0.611 | <0.0005 |
| COL1A1 vs. SRC-2 | 0.437 | 0.217–0.615 | <0.0005 |
| COL1A1 vs. SRC-3 | 0.501 | 0.300–0.659 | <0.0005 |
| COL3A1 vs. SRC-1 | 0.553 | 0.367–0.696 | <0.0005 |
| COL3A1 vs. SRC-2 | 0.495 | 0.291–0.656 | <0.0005 |
| COL3A1 vs. SRC-3 | 0.570 | 0.390–0.708 | <0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluf, F.C.; da Silva, K.S.; Caetano, G.V.B.; Brito, P.H.S.; Candido, P.; dos Santos, G.A.; Guimarães, V.; Silva, I.A.; Antunes, A.A.; Leite, K.; et al. Molecular Profile and Clinical Associations of Androgen Receptor Coactivators and Structural Genes in Benign Prostatic Hyperplasia and Metabolic Syndrome. Biomedicines 2025, 13, 2896. https://doi.org/10.3390/biomedicines13122896
Maluf FC, da Silva KS, Caetano GVB, Brito PHS, Candido P, dos Santos GA, Guimarães V, Silva IA, Antunes AA, Leite K, et al. Molecular Profile and Clinical Associations of Androgen Receptor Coactivators and Structural Genes in Benign Prostatic Hyperplasia and Metabolic Syndrome. Biomedicines. 2025; 13(12):2896. https://doi.org/10.3390/biomedicines13122896
Chicago/Turabian StyleMaluf, Feres Camargo, Karina Serafim da Silva, Giovana Vilas Boas Caetano, Pedro Henrique Souza Brito, Patricia Candido, Gabriel A. dos Santos, Vanessa Guimarães, Iran Amorim Silva, Alberto Azoubel Antunes, Katia Leite, and et al. 2025. "Molecular Profile and Clinical Associations of Androgen Receptor Coactivators and Structural Genes in Benign Prostatic Hyperplasia and Metabolic Syndrome" Biomedicines 13, no. 12: 2896. https://doi.org/10.3390/biomedicines13122896
APA StyleMaluf, F. C., da Silva, K. S., Caetano, G. V. B., Brito, P. H. S., Candido, P., dos Santos, G. A., Guimarães, V., Silva, I. A., Antunes, A. A., Leite, K., Srougi, M., Nahas, W., Pimenta, R., & Reis, S. (2025). Molecular Profile and Clinical Associations of Androgen Receptor Coactivators and Structural Genes in Benign Prostatic Hyperplasia and Metabolic Syndrome. Biomedicines, 13(12), 2896. https://doi.org/10.3390/biomedicines13122896








