Predicting Prostatic Obstruction and Bladder Outlet Dysfunction in Men with Lower Urinary Tract Symptoms and Small-to-Moderate Prostate Volume Using Noninvasive Diagnostic Tools
Abstract
1. Introduction
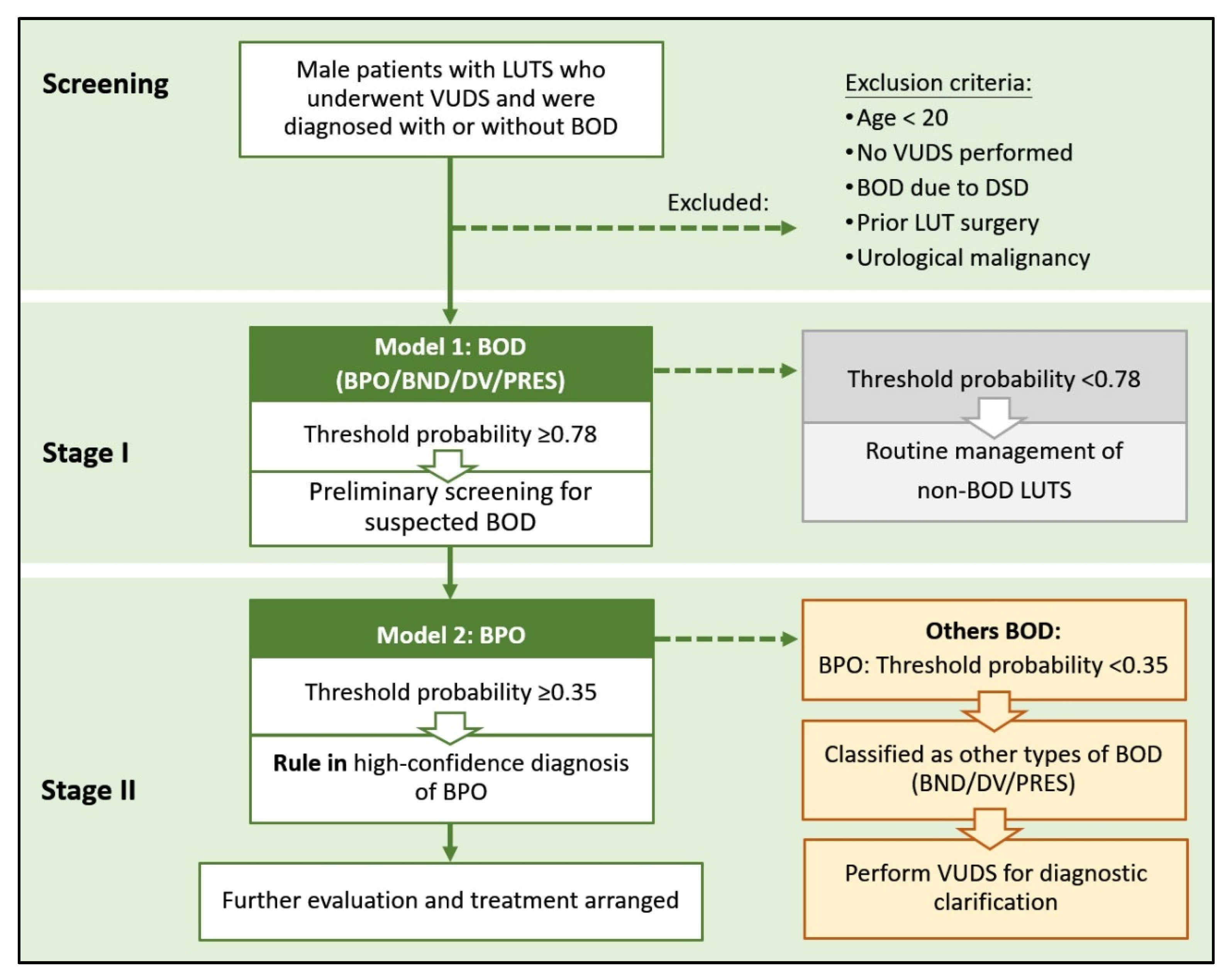
2. Materials and Methods
2.1. Characteristics of the Patients
2.2. Noninvasive Assessment Variables and VUDS
2.3. Definition of the Prediction Targets
2.4. Synthetic Oversampling for Imbalanced Data
2.5. Model Derivation and Internal Validation
2.6. Nomogram
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Predictors of BOD and Each BOD Subtype
3.3. Clinical Nomogram and Decision-Making Framework
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sekido, N.; Omae, K.; Haga, N.; Kubota, Y.; Saito, M.; Sakakibara, R.; Yoshida, M.; Mitsui, T.; Masumori, N.; Takahashi, S. Prevalence, impact on quality of life, and predictive factors of coexistence of overactive bladder and underactive bladder in men. Sci. Rep. 2025, 15, 21313. [Google Scholar] [CrossRef]
- Kuo, H.C. Videourodynamic analysis of pathophysiology of men with both storage and voiding lower urinary tract symptoms. Urology 2007, 70, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Wang, C.C.; Kuo, H.C. Videourodynamic findings of lower urinary tract dysfunctions in men with persistent storage lower urinary tract symptoms after medical treatment. PLoS ONE 2018, 13, e0190704. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Chen, S.F.; Kuo, H.C. Role of videourodynamic study in precision diagnosis and treatment for lower urinary tract dysfunction. Tzu Chi Med. J. 2019, 32, 121–130. [Google Scholar] [PubMed]
- Lee, C.L.; Kuo, H.C. Tailoring Medication for Lower Urinary Tract Symptoms in Men Based on International Prostate Symptom Score Voiding to Storage Ratio. Urology 2018, 120, 30–35. [Google Scholar] [CrossRef]
- Kuo, H.C. Clinical prostate score for diagnosis of bladder outlet obstruction by prostate measurements and uroflowmetry. Urology 1999, 54, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Gaur, S.; Pal, D.K. Role of videourodynamics in the identification of causes of lower urinary tract symptoms and low uroflow in young men. Urol. Ann. 2022, 14, 332–335. [Google Scholar] [CrossRef]
- Khodabakhsh, A.; Arabi, H.; Zaidi, H. U-net based estimation of functional connectivity from time series multi-channel eeg from schizophrenia patients. In Proceedings of the 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Yokohama, Japan, 16–23 October 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Tajvidi Asr, R.; Rahimi, M.; Hossein Pourasad, M.; Zayer, S.; Momenzadeh, M.; Ghaderzadeh, M. Hematology and hematopathology insights powered by machine learning: Shaping the future of blood disorder management. Iran. J. Blood Cancer 2024, 16, 9–19. [Google Scholar] [CrossRef]
- Shin, H.; Ko, K.J.; Park, W.J.; Han, D.H.; Yeom, I.; Lee, K.S. Machine Learning Models for the Noninvasive Diagnosis of Bladder Outlet Obstruction and Detrusor Underactivity in Men with Lower Urinary Tract Symptoms. Int. Neurourol. J. 2024, 28 (Suppl. S2), S74–S81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Q.; Li, G.; Cui, K.; Mao, W.; Lin, D.; Yang, Z.; Chen, Z.; Hu, Y.; Zhang, X. Using machine learning to construct the diagnosis model of female bladder outlet obstruction based on urodynamic study data. Investig. Clin. Urol. 2024, 65, 559–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Başaranoğlu, M.; Taşdemir, İ.K.; Akbay, E.; Doruk, H.E. Artificial intelligence-based prediction of treatment failure and medication non-adherence in overactive bladder management. BMC Urol. 2025, 25, 209. [Google Scholar] [CrossRef]
- Rosier, P.F.W.M.; Gammie, A.; Valdevenito, J.P.; Speich, J.; Smith, P.; Sinha, S.; members of the ICS Working Group PFS23. ICS-SUFU standard: Theory, terms, and recommendations for pressure-flow studies performance, analysis, and reporting. Part 2: Analysis of PFS, reporting, and diagnosis. Neurourol. Urodyn. 2023, 42, 1603–1627. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Tian, J.H.; Lee, C.C.; Kuo, H.C. Building Dual AI Models and Nomograms Using Noninvasive Parameters for Aiding Male Bladder Outlet Obstruction Diagnosis and Minimizing the Need for Invasive Video-Urodynamic Studies: Development and Validation Study. J. Med. Internet Res. 2024, 26, e58599. [Google Scholar] [CrossRef]
- Lee, C.L.; Ong, H.L.; Kuo, H.C. Therapeutic efficacy of mirabegron 25 mg monotherapy in patients with nocturia-predominant hypersensitive bladder. Tzu Chi Med. J. 2019, 32, 30–35. [Google Scholar]
- Tkocz, M.; Prajsner, A. Comparison of long-term results of transurethral incision of the prostate with transurethral resection of the prostate, in patients with benign prostatic hypertrophy. Neurourol. Urodyn. 2002, 21, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Jiang, Y.H.; Lee, C.L.; Kuo, H.C. Precision medicine in the diagnosis and treatment of male lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Tzu Chi Med. J. 2019, 32, 5–13. [Google Scholar] [PubMed]
- Chang, T.L.; Chen, S.F.; Kuo, H.C. Surgical outcome of male patients with chronic central nervous system disorders and voiding dysfunction due to bladder outlet obstruction. Int. Urol. Nephrol. 2022, 54, 2511–2519. [Google Scholar] [CrossRef]
- Rosier, P.F.; Giarenis, I.; Valentini, F.A.; Wein, A.; Cardozo, L. Do patients with symptoms and signs of lower urinary tract dysfunction need a urodynamic diagnosis? ICI-RS 2013. Neurourol. Urodyn. 2014, 33, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Finazzi Agrò, E.; Rosato, E.; Pletto, S.; Sampogna, G. What is the Role of Invasive Urodynamics in the Assessment of Male Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia. Eur. Urol. Focus 2025, S2405-4569(25)00094-X. [Google Scholar] [CrossRef]
- Raffo, M.; Capogrosso, P.; Pozzi, E.; Belladelli, F.; Corsini, C.; Bertini, A.; Negri, F.; Candela, L.; Larcher, A.; Matloob, R.; et al. Clinical predictors of bladder outlet obstruction in men consulting for routine urological screening. Int. Urol. Nephrol. 2025, 57, 2513–2518. [Google Scholar] [CrossRef]
- Gharbieh, S.; Reeves, F.; Challacombe, B. The prostatic middle lobe: Clinical significance, presentation and management. Nat. Rev. Urol. 2023, 20, 645–653. [Google Scholar] [CrossRef]
- Ku, J.H.; Ko, D.W.; Cho, J.Y.; Oh, S.J. Correlation between prostatic urethral angle and bladder outlet obstruction index in patients with lower urinary tract symptoms. Urology 2010, 75, 1467–1471. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Ikeguchi, E.F.; Santarosa, R.P.; D’Alisera, P.M.; Hendricks, J.; Te, A.E.; Miller, M.I. Etiology of voiding dysfunction in men less than 50 years of age. Urology 1996, 47, 836–839. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, S.S.; Chen, Y.T.; Hsieh, J.H. Videourodynamics identifies the causes of young men with lower urinary tract symptoms and low uroflow. Eur. Urol. 2003, 43, 386–390. [Google Scholar] [CrossRef]
- Akkoç, Y.; Ersöz, M.; Yüceyar, N.; Tunç, H.; Köklü, K.; Yoldaş, T.K.; Dönmez, Ü.; Uzunköprü, C.; Alemdaroğlu, E.; Bilen, Ş.; et al. Overactive bladder symptoms in patients with multiple sclerosis: Frequency, severity, diagnosis and treatment. J. Spinal Cord Med. 2016, 39, 229–233. [Google Scholar] [CrossRef]
- Araki, I.; Kitahara, M.; Oida, T.; Kuno, S. Voiding dysfunction and Parkinson’s disease: Urodynamic abnormalities and urinary symptoms. J. Urol. 2000, 164, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Ransmayr, G.N.; Holliger, S.; Schletterer, K.; Heidler, H.; Deibl, M.; Poewe, W.; Madersbacher, H.; Kiss, G. Lower urinary tract symptoms in dementia with Lewy bodies, Parkinson disease, and Alzheimer disease. Neurology 2008, 70, 299–303. [Google Scholar] [CrossRef]
- Charalampous, I.; Tsikopoulos, I.; Mitkani, C.; Samarinas, M.; Yuan, Y.; Vouros, I.; Tsafrakidis, P.; Anastasios, A.; Gkotsi, A.; Sakalis, V. Does Surgical Treatment for Benign Prostate Enlargement (BPE)-Related Bladder Outlet Obstruction (BOO) Benefit Patients with Central Nervous System Diseases? A Systematic Review. J. Clin. Med. 2024, 13, 5846. [Google Scholar] [CrossRef]
- Ke, Q.S.; Jiang, Y.H.; Kuo, H.C. Role of Bladder Neck and Urethral Sphincter Dysfunction in Men with Persistent Bothersome Lower Urinary Tract Symptoms after α-1 Blocker Treatment. Low Urin. Tract Symptoms 2015, 7, 143–148. [Google Scholar] [CrossRef]
- Lee, C.L.; Jhang, J.F.; Ho, H.C.; Jiang, Y.H.; Hsu, Y.H.; Kuo, H.C. Therapeutic outcome of active management in male patients with detrusor underactivity based on clinical diagnosis and videourodynamic classification. Sci. Rep. 2022, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E. The many faces of impaired bladder emptying. Curr. Opin. Urol. 2014, 24, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.P. Aging and the underactive detrusor: A failure of activity or activation? Neurourol. Urodyn. 2010, 29, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.S.; Ou, Y.C.; Cheng, Y.S.; Wu, K.Y.; Wang, C.T.; Huang, Y.C.; Kao, Y.L. Surgical Outcomes and Predictive Factors in Patients with Detrusor Underactivity Undergoing Bladder Outlet Obstruction Surgery. Int. Neurourol. J. 2024, 28, 59–66. [Google Scholar] [CrossRef] [PubMed]



| Total | Training Set | Test Set | p-Value | ||
|---|---|---|---|---|---|
| 307 (100%) | 215 (70%) | 92 (30%) | |||
| VUDS Diagnosis, n (%) | |||||
| Non-BOD | 55 (17.9) | 38 (17.7) | 17 (18.5) | 0.866 | |
| BOD (BND/BPO/DV/PRES) | 252 (82.1) | 177 (82.3) | 75 (81.5) | ||
| BND | Yes | 120 (47.6) | 84 (47.5) | 36 (48.0) | 0.937 |
| No | 132 (52.4) | 93 (52.5) | 39 (52.0) | ||
| BPO | Yes | 87 (34.5) | 61 (34.5) | 26 (34.7) | 0.975 |
| No | 165 (65.5) | 116 (65.5) | 49 (65.3) | ||
| DV | Yes | 24 (9.5) | 17 (9.6) | 7 (9.3) | 0.947 |
| No | 228 (90.5) | 160 (90.4) | 68 (90.7) | ||
| PRES | Yes | 21 (8.3) | 15 (8.5) | 6 (8.0) | |
| No | 231 (91.7) | 162 (91.5) | 69 (92.0) | 0.901 | |
| Characteristics, Mean ± SD | |||||
| Age (years) | 67.8 ± 9.7 | 67.8 ± 9.8 | 67.9 ± 9.5 | 0.927 | |
| Qmax (mL/s) | 10.2 ± 6.5 | 10.6 ± 7.0 | 9.4 ± 5.1 | 0.105 | |
| VoL (mL) | 196.8 ± 124.1 | 200.1 ± 124.7 | 189.2 ± 123.0 | 0.482 | |
| PVR (mL) | 44.1 ± 83.9 | 48.7 ± 96.3 | 33.5 ± 41.2 | 0.146 | |
| TPV (mL) | 38.1 ± 19.5 | 39.2 ± 20.9 | 35.4 ± 15.7 | 0.119 | |
| TZI | 0.40 ± 0.16 | 0.40 ± 0.16 | 0.42 ± 0.14 | 0.393 | |
| IPP (cm) | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.3 ± 0.5 | 0.390 | |
| PUA (degree) | 26.3 ± 18.7 | 25.9 ± 18.8 | 27.4 ± 18.3 | 0.517 | |
| Non-BOD (n = 55) | BND (n = 120) | BPO (n = 87) | DV (n = 24) | PRES (n = 21) | p-Value # | |
|---|---|---|---|---|---|---|
| Age (years) | 66.0 ± 11.0 | 67.5 ± 9.2 | 70.4 ± 8.0 | 68.8 ± 10.3 | 62.9 ± 12.1 | 0.007 |
| Qmax (mL/s) | 13.0 ± 8.1 | 9.7 ± 6.1 | 8.8 ± 4.3 | 11.1 ± 7.9 | 11.4 ± 7.9 | 0.002 |
| VoL (mL) | 200.8 ± 121.3 | 209.8 ± 122.3 | 167.1 ± 110.4 | 185.8 ± 122.0 | 247.4 ± 172.5 | 0.039 |
| PVR (mL) | 44.0 ± 115.4 | 36.9 ± 44.0 | 63.2 ± 113.7 | 28.5 ± 29.4 | 24.5 ± 31.5 | 0.117 |
| TPV (mL) | 31.4 ± 13.8 | 32.0 ± 11.5 | 54.0 ± 24.2 | 30.9 ± 8.8 | 32.4 ± 19.4 | <0.001 |
| TZI | 0.36 ± 0.15 | 0.37 ± 0.14 | 0.50 ± 0.13 | 0.37 ± 0.13 | 0.31 ± 0.18 | <0.001 |
| IPP (cm) | 0.10 ± 0.26 | 0.16 ± 0.40 | 0.74 ± 0.76 | 0.10 ± 0.29 | 0.17 ± 0.66 | <0.001 |
| PUA (degree) | 17.4 ± 17.2 | 25.9 ± 17.8 | 36.2 ± 15.5 | 16.5 ± 15.9 | 22.6 ± 23.2 | <0.001 |
| OR | 95% CI | p-Value | Hosmer-Lemeshow p-Value | |
|---|---|---|---|---|
| Model 1: BOD (BND/BPO/DV/PRES) | ||||
| Qmax (mL/s) | 0.925 | 0.872–0.982 | 0.010 | 0.407 |
| Voided volume (mL) | 1.004 | 1.000–1.008 | 0.045 | |
| IPP (cm) | 2.356 | 0.807–6.873 | 0.117 | |
| PUA (degree) | 1.018 | 0.995–1.042 | 0.118 | |
| Model 2: BND | ||||
| TPV (mL) | 0.964 | 0.939–0.990 | 0.006 | 0.484 |
| IPP (cm) | 0.475 | 0.205–1.100 | 0.082 | |
| Model 3: BPO | ||||
| PVR (mL) | 1.006 | 0.999–1.014 | 0.081 | 0.788 |
| TPV (mL) | 1.064 | 1.033–1.096 | <0.001 | |
| IPP (cm) | 2.254 | 0.960–5.289 | 0.062 | |
| Model 4: DV (after SMOTE, ratio 1:4) | ||||
| Qmax (mL/s) | 1.105 | 1.015–1.209 | 0.025 | 0.381 |
| Voided volume (mL) | 0.990 | 0.983–0.995 | 0.001 | |
| TPV (mL) | 0.941 | 0.897–0.978 | 0.006 | |
| TZI | 20.051 | 0.689–704.728 | 0.087 | |
| PUA (degree) | 0.958 | 0.930–0.984 | 0.002 | |
| Model 5: PRES (after SMOTE, ratio 1:4) | ||||
| Age (years) | 0.920 | 0.876–0.960 | <0.001 | 0.394 |
| Voided volume (mL) | 1.003 | 1.000–1.006 | 0.041 | |
| PVR (mL) | 0.988 | 0.974–0.998 | 0.048 | |
| TZI | 0.032 | 0.001–0.620 | 0.002 | |
| Stage I Model | Stage II Model | ||||
|---|---|---|---|---|---|
| BOD | BND | BPO | DV | PRES | |
| Training set | |||||
| AUC | 0.71 | 0.70 | 0.85 | 0.84 | 0.83 |
| Threshold of training set | 0.78 | 0.40 | 0.35 | 0.17 | 0.23 |
| Accuracy | 0.73 | 0.68 | 0.82 | 0.72 | 0.81 |
| Sensitivity | 0.77 | 0.88 | 0.75 | 0.95 | 0.81 |
| Specificity | 0.55 | 0.49 | 0.86 | 0.66 | 0.81 |
| PPV | 0.89 | 0.61 | 0.74 | 0.41 | 0.52 |
| NPV | 0.34 | 0.82 | 0.87 | 0.98 | 0.94 |
| AUC | 0.70 ± 0.10 | 0.70 ± 0.07 | 0.84 ± 0.07 | 0.80 ± 0.06 | 0.81 ± 0.08 |
| Accuracy | 0.74 ± 0.06 | 0.66 ± 0.06 | 0.81 ± 0.06 | 0.68 ± 0.07 | 0.78 ± 0.06 |
| Test set | |||||
| AUC | 0.70 | 0.68 | 0.79 | 0.61 | 0.73 |
| Accuracy | 0.74 | 0.60 | 0.73 | 0.63 | 0.80 |
| Sensitivity | 0.80 | 0.83 | 0.58 | 0.71 | 0.33 |
| Specificity | 0.47 | 0.38 | 0.82 | 0.62 | 0.84 |
| PPV | 0.87 | 0.56 | 0.63 | 0.16 | 0.15 |
| NPV | 0.35 | 0.71 | 0.78 | 0.95 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.-H.; Hsieh, T.-C.; Kuo, H.-C. Predicting Prostatic Obstruction and Bladder Outlet Dysfunction in Men with Lower Urinary Tract Symptoms and Small-to-Moderate Prostate Volume Using Noninvasive Diagnostic Tools. Biomedicines 2025, 13, 2894. https://doi.org/10.3390/biomedicines13122894
Tian J-H, Hsieh T-C, Kuo H-C. Predicting Prostatic Obstruction and Bladder Outlet Dysfunction in Men with Lower Urinary Tract Symptoms and Small-to-Moderate Prostate Volume Using Noninvasive Diagnostic Tools. Biomedicines. 2025; 13(12):2894. https://doi.org/10.3390/biomedicines13122894
Chicago/Turabian StyleTian, Jing-Hui, Tsung-Cheng Hsieh, and Hann-Chorng Kuo. 2025. "Predicting Prostatic Obstruction and Bladder Outlet Dysfunction in Men with Lower Urinary Tract Symptoms and Small-to-Moderate Prostate Volume Using Noninvasive Diagnostic Tools" Biomedicines 13, no. 12: 2894. https://doi.org/10.3390/biomedicines13122894
APA StyleTian, J.-H., Hsieh, T.-C., & Kuo, H.-C. (2025). Predicting Prostatic Obstruction and Bladder Outlet Dysfunction in Men with Lower Urinary Tract Symptoms and Small-to-Moderate Prostate Volume Using Noninvasive Diagnostic Tools. Biomedicines, 13(12), 2894. https://doi.org/10.3390/biomedicines13122894






