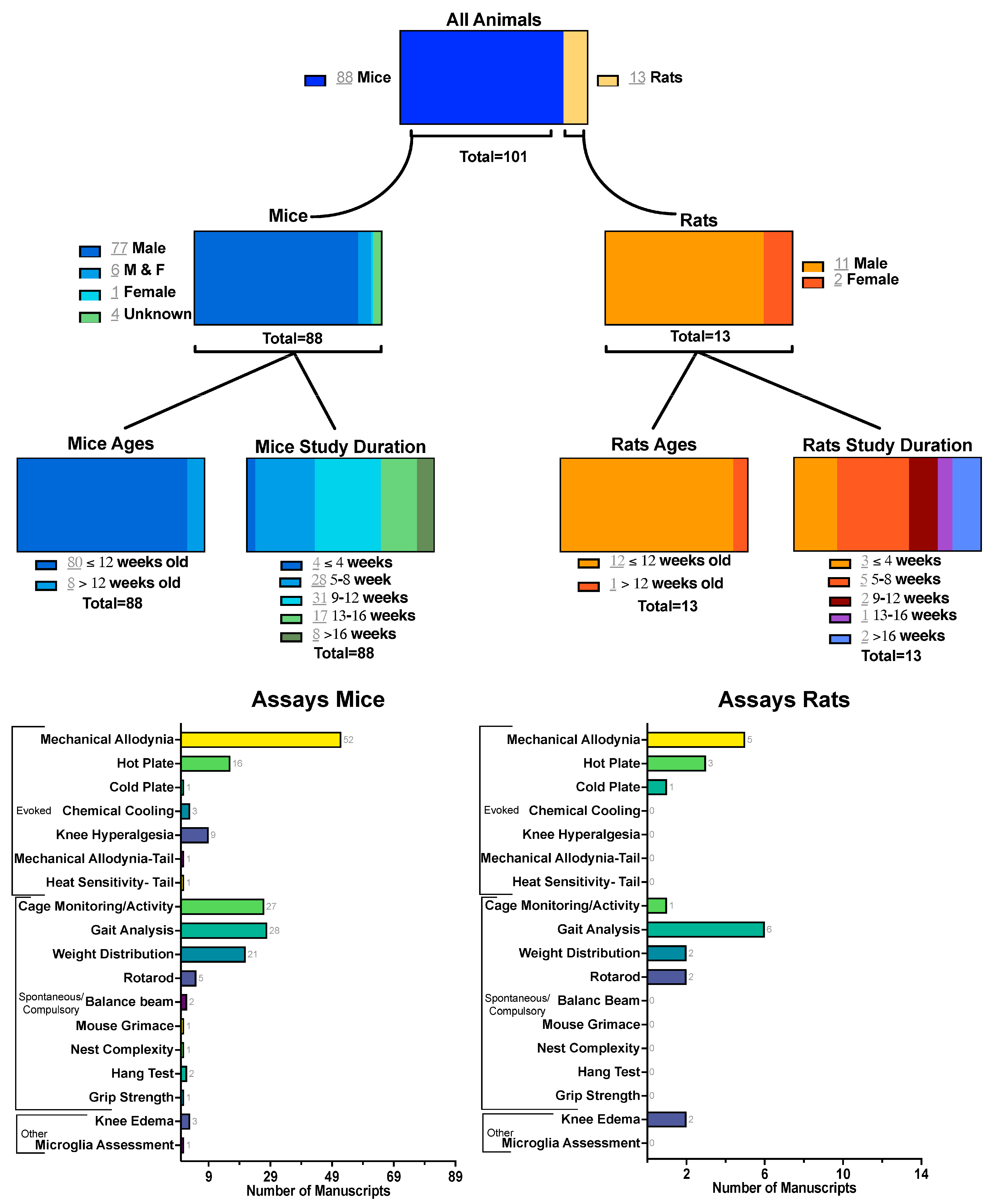
A Review of Rodent Behavior, Mobility, and Pain Modifications in Response to Destabilization of the Medial Meniscus Injury
Abstract
1. Introduction
2. Inclusion/Exclusion Criteria
3. Discussion–Mice
3.1. Evoked Measures
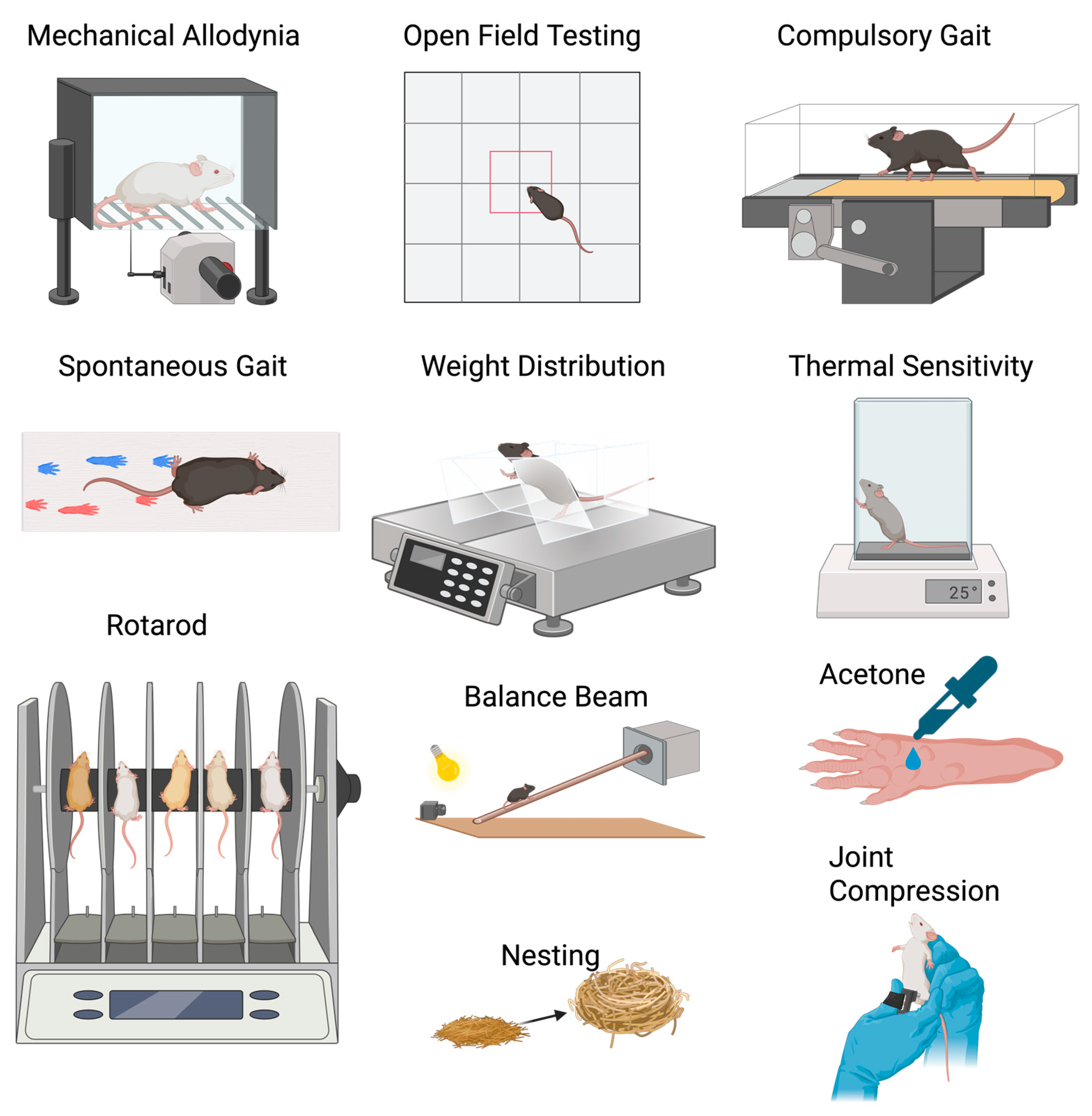
3.1.1. Mechanical Allodynia
- Foot-Brushing and Pin-Prick Tests
3.1.2. Thermal Sensitivity
- Hot Plate or Hot Allodynia
- Cold Plate or Cold Allodynia
- Evaporative Cooling/Chemical Sensitivity
3.1.3. Knee Hyperalgesia
3.1.4. Mechanical Allodynia-Tail
3.1.5. Heat Sensitivity-Tail
3.2. Spontaneous and Compulsory Measures
3.2.1. Cage Monitoring
3.2.2. Gait Analysis
3.2.3. Weight Distribution
3.2.4. Balance
- Rotarod test
- Balance beam
3.2.5. Hang Test
3.2.6. Grip Strength
3.2.7. Mouse Grimace Scale
3.2.8. Nest Complexity
3.3. Other
3.3.1. Knee Edema
3.3.2. Microglia Assessment
4. Discussion–Rats
4.1. Evoked Measures
4.1.1. Mechanical Allodynia
4.1.2. Thermal Sensitivity
- Hot plate or hot allodynia
- Cold Plate or Cold Allodynia
4.2. Spontaneous and Compulsory Measures
4.2.1. Cage Monitoring
4.2.2. Gait Analysis
4.2.3. Weight Distribution
4.2.4. Balance
- Rotarod
4.3. Other
Knee Edema
5. Consideration of Analgesics
- Wan et al. treated mice with baicalein or baicalein + ferroptosis inhibitor ferrosatin-1 (fer-1), and treatment with baicalein significantly helped animals maintain mobility in comparison to the DMM group at all measured weeks (2, 4, and 10), but treatment with baicalein and fer-1 significantly improved mobility from baicalein alone. Treated animals were closer to uninjured control mobility for both measures [75].
- Sun et al. treated mice with celecoxib. At 8 weeks PI, for the celecoxib-treated animals, there were significant increases in distance traveled, mean speed, and active time compared to the vehicle controls. The treated group was also equivalent to the sham [49].
- Tang et al. treated mice with 4-octyl itaconate. Starting at week 4, treatment with 4-OI ameliorated behavior/mobility changes seen in the DMM animals, equivalent to shams for both distance traveled and average speed [92].
- Xu et al. treated mice with recombinant human midkine (rhMK) and saw that treatment increased rearing and movement to levels similar to controls. However, at week 8 PI, the rhMK-treated group decreased temporarily to the level of the DMM vehicle group [101].
- Liu et al. treated mice with betulinic acid (BA). At 8 weeks PI, the high-dose injection of BA significantly improved gait for base of support (BOS), right hind paw stride length, and right-left hind paw distance compared to the vehicle controls. Low dose of BA treatment saw improvements from the vehicle for BOS. Improved measures were similar to the shams [134].
- Wang et al. treated mice with Eucommia ulmoides Oliv. and Glycyrrhiza uralensis Fisch. (E.G.). At week 12 PI, the DMM animals had significantly altered gait compared to the shams. Treatment with E.G. ameliorated the effects of the DMM injury on the gait [14].
- Xu et al. treated mice with Glycyrrhiza uralensis Fisch. (GC). At week 12 PI, the DMM animals had significantly altered gait, and treatment with GC helped animals maintain a gait similar to the shams [113].
- Westhof et al. rats treated with Zilretta. They measured only the percent print length of the contralateral limb for gait and saw differences between the control and DMM groups only at week 1 PI, with all other weeks measured displaying no differences in gait for any of the three groups. The treatment with Zilretta mirrored the vehicle treatment for gait [147].
- Jin et al. treated mice with Prostaglandin E receptor 4 (EP4), grapiprant, HL-43, and celecoxib. They saw that the EP4-inhibited mice had a more equal distribution than untreated DMM animals at 8 weeks PI [24].
- Hwang et al. treated mice with capsazepine (CPZ) and saw at 8 and 10 PI that the male mice had a significant improvement in weight-bearing symmetry compared to untreated animals when given CPZ. Females did not exhibit different pain behaviors when treated [38].
- Westhof et al. saw that at 1 week PI, the DMM rats had significantly decreased percent incapacitance of the contralateral limb compared to controls, and that at 2 and 5 weeks PI, the Zilretta treatment significantly increased symmetry [147].
- Wan et al. saw that in WT mice, both treatments with baicalein and baicalein + fer-1 significantly reduced sensitivity in comparison to DMM for every tested week, and that in KO mice, only the baicalein + fer-1 significantly reduced sensitivity in comparison to DMM for every tested week [75].
- Jin et al. saw that the EP4 KO mice had decreased sensitivity in comparison to the vehicle controls at 8 weeks PI. All 3 drug treatments in the WT mice demonstrated significantly reduced sensitivity in comparison with the untreated control at week 6 PI [24].
- Sun et al. saw that at 8 weeks PI, celecoxib-treated mice had significantly reduced sensitivity compared to the vehicle controls [49].
- Miller et al. treated mice with morphine or clozapine-N-oxide (CNO). At 12 weeks, PI treatment with morphine significantly reduced sensitivity for WT mice compared to vehicle. Treatment with CNO significantly reduced sensitivity to von Frey at 8 weeks PI in Nav mice 1–2 h after treatment, but not 4 h. Treatment at 4, 8, and 16 weeks PI showed no benefit to CNO therapy [87].
- Tang et al. saw that the treatments with 4-OI ameliorated the effects of the DMM injury in mice [92].
- Xu et al. saw that treatments with rhMK reduced sensitivity compared to the DMM-injured mice starting at 2 weeks post-treatment (week 20 PI) in both the injured and contralateral limbs [101].
- Hwang et al. saw that the treatment with CPZ did not decrease sensitivity to the von Frey filaments for male or female mice [38].
- Jin et al. found that all 3 treatments led to decreased sensitivity to the thermal hyperalgesia tests at week 6 PI when compared to untreated mice [24].
- Liu et al. treated mice with U50,488H and found that the treatment reduced sensitivity to heat at 8 weeks PI in comparison to the untreated mice [110].
- Wang et al. found that at week 12 PI, DMM mice had significantly increased sensitivity to heat. Treatment with E.G. ameliorated this [14].
- Xu et al. saw that at week 12 PI, DMM mice had significantly increased sensitivity to heat compared to shams and that GC treatment significantly decreased the sensitivity seen in DMM animals [113].
- Gao et al. treated mice with Tetrandrine (Tet), celecoxib (CXB), or indomethacin (INDO) and found that mice treated with any of the treatments had significantly decreased sensitivity weeks 2–8 PI [109].
- Gao et al. saw that all three treatments significantly decreased sensitivity to acetone cooling compared to untreated mice at weeks 2–8 PI [109].
- Sun et al. found that at 8 weeks PI, celecoxib-treated mice had significantly reduced sensitivity compared to the vehicle controls [49].
- Miller et al. saw that treatment with morphine significantly reduced knee sensitivity 15 to 75 min after administration at week 4 in WT mice and that Nav mice had a significant reduction in sensitivity up to 4 h after CNO therapy compared to the controls at week 4 PI [87].
- Gao et al. saw that all three treatments significantly increased hang time from the vehicle control for the inverted wire mesh hang test at week 4 PI [109].
6. Conclusions
Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| PTOA | Pots-traumatic osteoarthritis |
| DMM | Destabilization of the medial meniscus |
| ACLT | Anterior cruciate ligament transection |
| PI | Post-injury |
| PWT | Paw withdrawal threshold |
| OFT | Open-field test |
| LABORAS | Laboratory Animal Behavior Observation Registration and Analysis System |
| WBA | Weight-bearing asymmetry |
| OVX | Ovariectomized |
Appendix A
| Activity Monitoring Type | Test Duration | Parameters Shown | DMM Compared Against | Baseline Measure | Timepoints Measures Shown | Distance Traveled/Track Length | Walking/Ambulation/Locomotion/Activity | Mean Speed/Velocity | Cimbing | Rearing | Grooming | Immobile/Inactive/Rest | Mobile Episodes | Time in Hut | Top of Hut Entries | Dig/Burrow | RDI | Sex, Age at Injury | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LABORAS (Assessment of Spontaneous movement) | 15 h | Assessed locomotive behaviors (distance traveled, climbing, and rearing) overnight | sham and/or naïve | no | weeks 4, 8, 16 | * 8, 16 ns 4 | * 8, * 16 ns 4 | * 8, * 16 ns 4 | males 10 weeks | [71] | |||||||||
| LABORAS (Assessment of Spontaneous movement) | 3 h | grooming, activity, climbing, immobility, measured viasingle cage vibration | sham | no | week 8 | * 8 | * 8 | ns 8 | * 8 | males 10–12 weeks | [118] | ||||||||
| LABORAS (assessment of spontaneous movement) | 15 h | travel distance, average walking speed, rearing frequency, and rearing duration | control and treatment | no | weeks 2, 6, 10 | * 6 ns 2, 10 | ns 2–10 | frequency * 6, ns 2, 10 duration * 6, ns 2, 10 | males 10 weeks | [74] | |||||||||
| LABORAS (Assessment of Spontaneous movement) | 14 h | grooming, locomotion, climbing, immobility via single cage vibration | baselines, treatments, and naïve | yes | weeks 0, 4, 8, 12, 16 | * 4–8 ns 12–16 | * 4 ns 8–16 | * 4–8 ns 12–16 | males 10–12 weeks | [123] | |||||||||
| LABORAS (Assessment of Spontaneous movement) | 14 h | climbing, locomotion, eating, drinking, immobile, and grooming | baselines, treatments, and naïve | yes | weeks 0, 4, 8, 12, 16 | * 8–16 ns 4 | * 4–8 ns 12–16 | ns | ns | * 8–16 ns 4 | males 10 weeks | [119] | |||||||
| LABORAS (Assessment of Spontaneous movement) | 15 h | distance of locomotion, average speed of locomotion, rearing frequency, and rearing duration via single cage vibration | baselines, shams, and treatments | yes | week 0, 2, 6, 10 | * 2–10 | * 2–10 | frequency * 2–10 duration * 2–10 | males 12 weeks | [41] | |||||||||
| LABORAS (assessment of spontaneous movement) | 15 h | average speed, distance traveled | control and treatments | no | weeks 2, 6, 10 | * 2–10 | * 2–10 | males 10 weeks | [75] | ||||||||||
| Open field analysis (Zhenghua Technology) | 3 min | relative activity, active time, distance, mean speed | shams, controls, and treatments | no | week 12 | * 12 | relative activity * 12 active time * 12 | * 12 | males 12 weeks | [78] | |||||||||
| Open field analysis (Zhenghua Technology) | 3 min | relative activity, active time, distance, mean speed | shams and treatments | no | week 12 | * 12 | relative activity * 12 active time * 12 | * 12 | males 12 weeks | [80] | |||||||||
| Open field analysis (Zhenghua Technology) | 3 min | relative activity, active time, distance, mean speed | shams and treatments | no | week 12 | * 12 | relative activity * 12 active time * 12 | * 12 | males 12 weeks | [15] | |||||||||
| Open field analysis (Activity Monitor: Med Associates) | 30 min | distance traveled, ambulatory time, ambulatory counts, ambulatory episodes, resting time, average velocity | shams and treatments | no | week 8 | ns 8 | ns 8 | ns 8 | ns 8 | males 12 weeks | [16] | ||||||||
| Open field testing (EthoVision XT 11) | 30 min | distance, average speed | shams and treatments | yes | weeks 0, 4, 8, 12, 16 | * 4–12 | * 4–12 | males 9 weeks | [92] | ||||||||||
| Open field testing (Smart Video Tracking Software (Panlab)) | 10 min | total distance traveled, mean velocity (excluding the immobility time), and immobility time. | naïve and shams | no | weeks 4, 6, 8, 10, 12 | ns 4–12 | * 10 ns 4–8, 12 | ns 4–12 | males 12 weeks | [48] | |||||||||
| Open field testing (SMART, Panlab SL, Spain) | 6 min | distance traveled, rearing count | treatments | yes | week 0, 4, 8, 12 | * 12 ns 4–8 | * 4, 12 ns 8 | males 10 weeks | [124] | ||||||||||
| Open field testing infrared array sensors (Taimeng, Chengdu, China) | 12 h | mobile episodes, rearing | naïve, shams, and treatments | yes | weeks 14, 16, 18, 20, 22, 24, 28, 30 | * 14–30 | * 14–30 | males 8 weeks | [101] | ||||||||||
| Open field analysis( VersaMax activity monitors) | 20 min | Distance traveled, ambulatory movements, resting time | treatments | yes | weeks 0, 6, 12 | * 12 ns 6 | * 12 ns 6 | * 12 ns 6 | males 12 weeks | [61] | |||||||||
| multi-rodent tracking system (open field) | 1 h | total distance of movement | shams and treatments | no | weeks 8, 12 | * 8–12 | males 12 weeks | [81] | |||||||||||
| Voluntary Wheel Running | 24 h | active time, distance traveled, and mean speed | shams and treatments | no | week 8 | * 8 | * 8 | * 8 | males 12 weeks | [49] | |||||||||
| Observations of digging and burrowing behaviors Digital Ventilated Cages (DVCs) Weekly regulatory disruption index (RDI) | unknown weekly (lights on period) | digging duration, # burrows, RDI | baselines, shams, and treatments | yes | weeks -1 through 16 (weekly) | Digging * 16 ns 1–15 burrowing * 16 ns 1–15 | * 16 ns 1–15 | males 10–12 weeks | [120] | ||||||||||
| ANY-maze overhead cage monitoring | 10 min | Distance traveled, mean speed, time in hut, top of hut entries | Baselines and treatments | yes | weeks 0, 2, 3, 4 | * 4 ns 2–3 | * 4 ns 2–3 | * 4 ns 2–3 | * 4 ns 2–3 | males 12 weeks | [6] | ||||||||
| Open field analysis | 6 min | distance traveled and number of rears manually tracked | shams and treatments | no | week 8 | * 8 | * 8 | males 21–25 weeks | [21] | ||||||||||
| Open field analysis | 6 min | distance traveled and number of rears manually tracked | naïve and treatments | no | week 8 | * 8 | * 8 | males 22–26 weeks | [10] | ||||||||||
| Open field testing (Photobeam Activity System) | 30 min | ambulation, rearing | shams | yes | weeks 0, 2, 4, 8, 12, 16 | * 8–16 ns 2–4 | * 8–16 ns 2–4 | unknown 10 weeks | [112] | ||||||||||
| Open field analysis (Accuscan analysis software) | 1 h | distance traveled | treatments | no | weeks 6, 14, 24 | ns (6–24) | unknown 16 weeks | [111] | |||||||||||
| Locomotor activity, Overnight (6 h) Ambulation, (Opto-Varames Micro Animal Activity System) | 6 h | Total ambulation | baseline | yes | weeks 0, 2, 4, 6, 8 | ns 2–8 Males and females look different | F&M 8–12 weeks | [55] | |||||||||||
| Open field analysis (biobserve) | 15 min | track length, average velocity | shams and treatments | no | weeks 2, 8 | * 2 ns 8 | * 2 ns 8 | Females 8 weeks | [40] | ||||||||||
| RFID tracking of colony movement at jump holes | 16 h | cage movement | healthy and different injury types | yes | Weeks 1–12 | * 1 ns 2–12 | rats males 8–9 weeks | [125] |
| Test Style | Parameters Shown | DMM Compared Against | Baseline | Timepoints Shown | Stance | Swing | Step | Paw Area | Paw Angle | Duty | Stride/Stance/Step Length | Stride/Stance/Step Width/BOS | Velocity | Break | Propel | F/R or L/R | Sex and Age at Time of Injury | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| catwalk | paw area, contact area, and swing speed. | Sham and treated | no | weeks 4, 8 | * 4–8 | paw * 4–8 contact * 4–8 | males 10 weeks | [72] | ||||||||||
| catwalk | print area, max contact area | treated | no | week 8 | print * 8 max * 8 | males 8 weeks | [79] | |||||||||||
| catwalk | duty cycle (stance time/(stance time + swing time) * 100%) | baseline and treated | yes | day 0, 1 week 1, 2 | * d1,w1 ns w2 | males 10–12 weeks | [130] | |||||||||||
| catwalk | mean intensity, swing speed, swing time, step cycle | treated and contralateral | no | week 12 | time * 12 speed * 12 | * 12 | * 12 | males 8 weeks | [131] | |||||||||
| catwalk | % mean intensity, % print area, % swing speed, % duty cycle | naked eye vs. microscope DMM | no | weeks 8, 10, 12, 14, 16 | * 8 ns 10–12 | area * 8–16 mean intensity * 8–14, ns 16 | * 8–14 ns 16 | males 8 weeks | [67] | |||||||||
| catwalk | velocity, paw print area, and duty cycle | naïve and bilateral DMM | yes | weeks 0–16 every week | * 1, 4–16 ns 2–3 | * 1 ns 2–16 | * 1 ns 2–16 | males 10 weeks | [34] | |||||||||
| catwalk | RH/LH % print area, RH/LH % Stress, RH/LH % swing speed | Sham and treated | no | weeks 2, 4, 6, 8 | * 2–8 | * 2–8 | * 2–8 | males 8 weeks | [96] | |||||||||
| catwalk | normalized paw print area %, weight bearing | baseline and treated | yes | weeks 0–12 weekly | * 12 ns 11 weight bearing * 12 ns 11 | males 12 weeks | [36] | |||||||||||
| catwalk | stance phase, swing phase, single stance, duty cycle, swing speed. | Sham and treated | yes | weeks 0, 3, 8, 12, 16 | phase * 12–16 ns 3–8 single * 12–16 ns 3–8 | phase * 12–16 ns 3–8 speed * 12–16 ns 3–8 | * 12–16 ns 3–8 | males 9 weeks | [132] | |||||||||
| custom gait analysis-free movement down a walkway (MouseWalker) | anterior extreme position (AEP) in parallel, posterior extreme position (PEP) in parallel, stance instability, swing speed, step distance, footprint area imbalance (LH-RH), stance offset, stance duration, and duty factor imbalance. | naïve/baseline | yes | weeks 1, 2, 4, 8, 12 | offset * 4 ns 1–2, 8–12 duration * 4 ns 1–2, 8–12 | * 4–12 ns 1–2 | ns 1–12 | * 12 ns 1–8 | stance instability * 12 ns 1–8 | AEP * 1–12 PEP ns 1–12 LH-RH * 12 ns 1–8 | males 12 weeks | [60] | ||||||
| footprint analysis | relative stride length, relative step length, and relative front/rear print length | Sham and treated | no | week 12 | stride * 12 stance * 12 | * 12 | males 12 weeks | [78] | ||||||||||
| footprint analysis | relative stride length, relative step length, relative front/rear print length | Sham and treated | no | week 12 | stride * 8 step * 8 | * 8 | males 12 weeks | [80] | ||||||||||
| footprint analysis | front stride length, hindlimb stride length | Sham and treated | no | week 4 | front stride * 4 rear stride * 4 | males 12 weeks | [81] | |||||||||||
| footprint analysis | measured stride length, stride width, step distance, and % of right-to-left stride length | Sham and treated | no | weeks 4, 8 | distance * 8 ns 4 stride length * 4–8 | * 8 ns 4 | * 4–8 | males 12 weeks | [133] | |||||||||
| footprint analysis | relative stride length, relative step length, relative front/rear print length | Sham and treated | no | week 12 | stride * 12 step * 12 | * 12 | males 12 weeks | [15] | ||||||||||
| footprint analysis | base of support (BOS), right hind paw stride length, and right-left hind paw distance | Sham and treated | no | week 8 | * 8 | * 8 | * 8 | males 8 weeks | [134] | |||||||||
| footprint analysis | stride length, stance length, sway distance | Sham and treated | no | weeks 2, 4, 6, 8 | Stride * 2–8 stance * 2–8 | sway * 2–8 | males 12 weeks | [16] | ||||||||||
| footprint analysis | stride length, right-left hind paws distance, and base of support (BOS) | Sham and treated | no | week 8 | * 8 | * 8 | * 8 | males 12 weeks | [135] | |||||||||
| footprint analysis | stride length | Baseline and sham | yes | weeks 0.4, 1, 2, 4, 8, 12, 16 | * 4 ns 1–2, 8–16 | males 11 weeks | [51] | |||||||||||
| footprint analysis | r-l distance, rotation of paws, relative step length, toe spread (TS), intermediate toe spread (ITS, print length (PL). | naïve and sham | no | weeks 4, 6, 8, 10, 12 | PL ns 2–12 TS ns 2–12 ITS ns2–12 | ns 2–12 | ns 2–12 | ns 2–12 | males 12 weeks | [48] | ||||||||
| compulsory treadmill running (DigiGait)18 cm/s | stride length, paw area, and swing time | Sham and treated | no | week 12 | * 12 | * 12 | * 12 | males 10 weeks | [14] | |||||||||
| compulsory treadmill running (DigiGait) 18 cm/s | stride length, stance width, paw area, and swing time | Sham and treated | no | week 12 | * 12 | * 12 | * 12 | * 12 | males 10 weeks | [113] | ||||||||
| compulsory treadmill running (DigiGait) 17 cm/s | stride length, stance time, paw area, and swing time | Sham and treated | no | week 8 | * 8 | * 8 | * 8 | * 8 | males 10 weeks | [137] | ||||||||
| compulsory treadmill running DigiGait 35 cm/sec | % swing stride, % stance stride, % break stride, % propel stride | baseline and treated | yes | week 0, 2, 3, 4 | * 2, 4 ns 3 | * 2, 4 ns 3 | * 3 ns 2, 4 | * 4 ns 2–3 | males 12 weeks | [6] | ||||||||
| catwalk | mean intensity | All other paws | yes | weeks 0, 2, 4, 6 | ns 2–6 | females and males 8–12 weeks | [55] | |||||||||||
| catwalk | log paw intensity | sham | no | weeks 2, 5, 10 | * 10 ns 2–5 | unknown 10 weeks | [46] | |||||||||||
| compulsory treadmill running DigiGait 30 cm/sec | break time, paw area, paw angle, midline distance | treated | no | week 16 | F * 16 | F * 16 | F * 16 | F * 16 | F&M 16 weeks | [138] | ||||||||
| custom gait analysis (free walking gait) | velocity, stance time, stride length, step width, temporal symmetry, and spatial symmetry | sham | yes | week 0, 4, 6, 8, 10, 12 | * Temporal ns 4–12 | * Temporal, 8 ns 4–6, 10–12 | * Temporal, 6 ns 4, 8–12 | * Temporal ns 4–12 | temporal and spatial symmetry ns 4–12 | F&M 16 weeks | [136] |
References
- Glasson, S.; Blanchet, T.; Morris, E. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Ma, H.-L.; Blanchet, T.; Peluso, D.; Hopkins, B.; Morris, E.; Glasson, S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr. Cartil. 2007, 15, 695–700. [Google Scholar] [CrossRef]
- Glasson, S.; Blanchet, T.; Morris, E. Less Severe OA is Observed in IL-1 KO Mice and More Severe OA Is Observed in MMP-9 and MK2 KO Mice in a Surgical Model of OA. 2005. Available online: https://www.ors.org/transactions/51/0251.pdf (accessed on 12 December 2024).
- Ma, H.L.; Blanchet, T.; Morris, E.A.; Glasson, S.S. Disease Progression in Surgically Induced Murine Osteoarthritis is Strain and Sex Dependent. Annu. Meet. Orthop. Res. Soc. 2005, 30, 1422. [Google Scholar]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.A.; Blanchet, T.; Ma, H.; Flannery, C.R.; Kanki, K.; Wang, E.; Peluso, D.; et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4–knockout mice. Arthritis Rheum. 2004, 50, 2547–2558. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Timkovich, A.E.; Sikes, K.J.; Chow, L.; Hendrickson, D.A.; Becker, J.R.; Webster, A.; Santangelo, K.S.; Dow, S. Erythrocyte removal from bone marrow aspirate concentrate improves efficacy as intra-articular cellular therapy in a rodent osteoarthritis model. Ann. Transl. Med. 2023, 11, 311. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal Models of Osteoarthritis: Challenges of Model Selection and Analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kazezian, Z.; Pitsillides, A.A.; Bull, A.M.J. A synoptic literature review of animal models for investigating the biomechanics of knee osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1408015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Alves-Simões, M. Rodent models of knee osteoarthritis for pain research. Osteoarthr. Cartil. 2022, 30, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Park, D.; Han, S. Animal Models in Osteoarthritis Research: Pain Behavioral Methods and Clinical Significance. Int. J. Pain 2023, 14, 39–47. [Google Scholar] [CrossRef]
- Neugebauer, V.; Han, J.S.; Adwanikar, H.; Fu, Y.; Ji, G. Techniques for assessing knee joint pain in arthritis. Mol. Pain 2007, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, J.; Sun, Q.; Ge, Q.; Qiu, M.; Zou, K.; Ying, J.; Yuan, W.; Chen, J.; Zeng, Q.; et al. Chondroprotective Mechanism of Eucommia ulmoides Oliv.-Glycyrrhiza uralensis Fisch. Couplet Medicines in Knee Osteoarthritis via Experimental Study and Network Pharmacology Analysis. Drug Des. Dev. Ther. 2023, 17, 633–646. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, K.; Wan, G.; Liao, W.; Jin, J.; Wang, P.; Sun, X.; Wang, W.; Jiang, Q. A ROS-responsive hydrogel encapsulated with matrix metalloproteinase-13 siRNA nanocarriers to attenuate osteoarthritis progression. J. Nanobiotechnol. 2025, 23, 18. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Chung, J.; Abdeen, A.; Thompson, A.; Bouboukas, A.; Pinamont, W.J.; Yoshioka, N.K.; Sepulveda, D.E.; Raup-Konsavage, W.M.; Graziane, N.M.; et al. Therapeutic Effects of Non-Euphorigenic Cannabis Extracts in Osteoarthritis. Cannabis Cannabinoid Res. 2023, 8, 1030–1044. [Google Scholar] [CrossRef]
- Deng, X.; Xu, H.; Pan, C.; Hao, X.; Liu, J.; Shang, X.; Chi, R.; Hou, W.; Xu, T. Moderate mechanical strain and exercise reduce inflammation and excessive autophagy in osteoarthritis by downregulating mitofusin 2. Life Sci. 2023, 332, 122020. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Liu, C.; Dawson, B.; Ketkar, S.; Patel, S.; Lee, B.; Grol, M. Leukocyte-dependent effects of platelet-rich plasma on cartilage loss and thermal hyperalgesia in a mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2020, 28, 1385–1393. [Google Scholar] [CrossRef]
- Izukashi, K.; Okumo, T.; Tatsuo, T.; Kachi, I.; Iida, Y.; Nishio, T.; Ikemoto, H.; Adachi, N.; Kanzaki, K.; Sunagawa, M. Early Intervention with Boiogito to Suppress Knee Osteoarthritis Progression: An Experimental Approach Using a Medial Meniscus Instability Rat Model. Cureus 2025, 17, e77311. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; A Hardin, J.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508, Erratum in Arthritis Res Ther. 2019, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.; Little, C.; McDougall, J. A commentary on modelling osteoarthritis pain in small animals. Osteoarthr. Cartil. 2013, 21, 1316–1326. [Google Scholar] [CrossRef]
- Blaker, C.L.; Clarke, E.C.; Little, C.B. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 424–439. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Q.; Chen, P.; Zhao, S.; Jiang, W.; Wang, F.; Li, P.; Zhang, Y.; Lu, W.; Zhong, T.P.; et al. A novel prostaglandin E receptor 4 (EP4) small molecule antagonist induces articular cartilage regeneration. Cell Discov. 2022, 8, 24. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- Wallace, C.W.; Hislop, B.; Hahn, A.K.; Erdogan, A.E.; Brahmachary, P.P.; June, R.K. Correlations between metabolites in the synovial fluid and serum: A mouse injury study. J. Orthop. Res. 2022, 40, 2792–2802. [Google Scholar] [CrossRef] [PubMed]
- Timkovich, A.E.; Sikes, K.J.; Andrie, K.M.; Afzali, M.F.; Sanford, J.; Fernandez, K.; Burnett, D.J.; Hurley, E.; Daniel, T.; Serkova, N.J.; et al. Full and Partial Mid-substance ACL Rupture Using Mechanical Tibial Displacement in Male and Female Mice. Ann. Biomed. Eng. 2023, 51, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; McCarthy, H.S.; Bou-Gharios, G.; van’t Hof, R.; Milner, P.I.; Mennan, C.; Roberts, S. Injected human umbilical cord-derived mesenchymal stromal cells do not appear to elicit an inflammatory response in a murine model of osteoarthritis. Osteoarthr. Cartil. Open 2020, 2, 100044. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Skelly, J.D.; Ayers, D.C.; Song, J. Age-dependent Changes in the Articular Cartilage and Subchondral Bone of C57BL/6 Mice after Surgical Destabilization of Medial Meniscus. Sci. Rep. 2017, 7, srep42294. [Google Scholar] [CrossRef]
- Geraghty, T.; Obeidat, A.M.; Ishihara, S.; Wood, M.J.; Li, J.; Lopes, E.B.P.; Scanzello, C.R.; Griffin, T.M.; Malfait, A.; Miller, R.E. Age-Associated Changes in Knee Osteoarthritis, Pain-Related Behaviors, and Dorsal Root Ganglia Immunophenotyping of Male and Female Mice. Arthritis Rheumatol. 2023, 75, 1770–1780. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A Review of Translational Animal Models for Knee Osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Gowler, P.R.W.; Mapp, P.I.; Burston, J.J.; Shahtaheri, M.; Walsh, D.A.; Chapman, V. Refining surgical models of osteoarthritis in mice and rats alters pain phenotype but not joint pathology. PLoS ONE 2020, 15, e0239663. [Google Scholar] [CrossRef] [PubMed]
- Fouasson-Chailloux, A.; Dauty, M.; Bodic, B.; Masson, M.; Maugars, Y.; Metayer, B.; Veziers, J.; Lesoeur, J.; Rannou, F.; Guicheux, J.; et al. Posttraumatic Osteoarthritis Damage in Mice: From Histological and Micro–Computed Tomodensitometric Changes to Gait Disturbance. Cartilage 2021, 13, 1478S–1489S. [Google Scholar] [CrossRef]
- von Loga, I.S.; Batchelor, V.; Driscoll, C.; Burleigh, A.; Chia, S.L.; Stott, B.; Miotla-Zarebska, J.; Riley, D.; Dell’aCcio, F.; Vincent, T.L. Does Pain at an Earlier Stage of Chondropathy Protect Female Mice Against Structural Progression After Surgically Induced Osteoarthritis? Arthritis Rheumatol. 2020, 72, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.E.; Wolter, A.; Muschter, D.; Grässel, S.; Lang, A. Impact of sensory neuropeptide deficiency on behavioral patterns and gait in a murine surgical osteoarthritis model. J. Orthop. Res. 2024, 42, 2673–2682. [Google Scholar] [CrossRef]
- Weaver, S.R.; Arnold, K.M.; Peralta-Herrera, E.; Oviedo, M.; Zars, E.L.; Bradley, E.W.; Westendorf, J.J. Postnatal deletion of Phlpp1 in chondrocytes delays post-traumatic osteoarthritis in male mice. Osteoarthr. Cartil. Open 2024, 7, 100525. [Google Scholar] [CrossRef]
- Hwang, H.; Park, I.; Hong, J.; Kim, J.; Kim, H. Comparison of joint degeneration and pain in male and female mice in DMM model of osteoarthritis. Osteoarthr. Cartil. 2021, 29, 728–738. [Google Scholar] [CrossRef]
- Gil Alabarse, P.; Chen, L.; Oliveira, P.; Qin, H.; Liu-Bryan, R. Targeting CD38 to Suppress Osteoarthritis Development and Associated Pain After Joint Injury in Mice. Arthritis Rheumatol. 2023, 75, 364–374. [Google Scholar] [CrossRef]
- Corciulo, C.; Scheffler, J.M.; Humeniuk, P.; Pons, A.D.C.; Stubelius, A.; Von Mentzer, U.; Drevinge, C.; Barrett, A.; Wüstenhagen, S.; Poutanen, M.; et al. Physiological levels of estradiol limit murine osteoarthritis progression. J. Endocrinol. 2022, 255, 39–51. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Chen, D.; Liu-Bryan, R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthr. Cartil. 2022, 30, 160–171. [Google Scholar] [CrossRef]
- Arant, K.; Katz, J.; Neogi, T. Quantitative sensory testing: Identifying pain characteristics in patients with osteoarthritis. Osteoarthr. Cartil. 2022, 30, 17–31. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.-M. Osteoarthritis pain: What are we learning from animal models? Best Pr. Res. Clin. Rheumatol. 2017, 31, 676–687. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, S17–S23. [Google Scholar] [CrossRef]
- Haubruck, P.; Heller, R.; Blaker, C.L.; Clarke, E.C.; Smith, S.M.; Burkhardt, D.; Liu, Y.; Stoner, S.; Zaki, S.; Shu, C.C.; et al. Streamlining quantitative joint-wide medial femoro-tibial histopathological scoring of mouse post-traumatic knee osteoarthritis models. Osteoarthr. Cartil. 2023, 31, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Huang, L.; Welch, I.; Norley, C.; Holdsworth, D.W.; Beier, F.; Cai, D. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci. Rep. 2018, 8, 2855. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Hong, B.-K.; Pham, T.H.N.; Kim, W.-U.; Kim, H.A. Interferon-gamma signaling promotes cartilage regeneration after injury. Sci. Rep. 2024, 14, 8046. [Google Scholar] [CrossRef]
- Alves, C.J.; Couto, M.; Sousa, D.M.; Magalhães, A.; Neto, E.; Leitão, L.; Conceição, F.; Monteiro, A.C.; Ribeiro-Da-Silva, M.; Lamghari, M. Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model. Sci. Rep. 2020, 10, 15271. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Ding, Y.; Xie, W.; Li, H.; Li, S.; Li, Y.; Cai, M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells 2022, 11, 2760. [Google Scholar] [CrossRef]
- Willcockson, H.; Ozkan, H.; Arbeeva, L.; Mucahit, E.; Musawwir, L.; Longobardi, L. Early ablation of Ccr2 in aggrecan-expressing cells following knee injury ameliorates joint damage and pain during post-traumatic osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1616–1630. [Google Scholar] [CrossRef]
- Zaki, S.; Smith, M.; Little, C. Pathology-pain relationships in different osteoarthritis animal model phenotypes: It matters what you measure, when you measure, and how you got there. Osteoarthr. Cartil. 2021, 29, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Olex, A.L.; McNulty, M.A.; Carlson, C.S.; Callahan, M.F.; Ferguson, C.M.; Chou, J.; Leng, X.; Fetrow, J.S. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012, 64, 705–717. [Google Scholar] [CrossRef]
- Kwok, J.; Onuma, H.; Olmer, M.; Lotz, M.; Grogan, S.; D’LIma, D. Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthr. Cartil. 2016, 24, 709–718. [Google Scholar] [CrossRef]
- Willcockson, H.; Ozkan, H.; Valdés-Fernández, J.; Arbeeva, L.; Mucahit, E.; Musawwir, L.; Hooper, L.B.; Granero-Moltó, F.; Prósper, F.; Longobardi, L. CC-Chemokine Receptor-2 Expression in Osteoblasts Contributes to Cartilage and Bone Damage during Post-Traumatic Osteoarthritis. Biomolecules 2023, 13, 891. [Google Scholar] [CrossRef]
- Malfait, A.; Ritchie, J.; Gil, A.; Austin, J.-S.; Hartke, J.; Qin, W.; Tortorella, M.D.; Mogil, J. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthr. Cartil. 2010, 18, 572–580. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Li, X.; Liao, T.; Ma, Z.; Xing, R.; Wang, P.; Mao, J. Characteristics of sensory innervation in synovium of rats within different knee osteoarthritis models and the correlation between synovial fibrosis and hyperalgesia. J. Adv. Res. 2022, 35, 141–151. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Zhou, Z.; Yang, J.; Li, A.; Wu, Y.; Peng, Z.; Li, X.; Liu, Z.; Leng, X.; et al. Integrated Network Pharmacology and Experimental Validation Approach to Investigate the Mechanisms of Radix Rehmanniae Praeparata—Angelica Sinensis—Radix Achyranthis Bidentatae in Treating Knee Osteoarthritis. Drug Des. Dev. Ther. 2024, 18, 1583–1602. [Google Scholar] [CrossRef] [PubMed]
- Chuluunbat, O.; Ikemoto, H.; Okumo, T.; Adachi, N.; Hisamitsu, T.; Sunagawa, M. Electroacupuncture Inhibits Cartilage Degeneration in a Rat Knee Osteoarthritis (KOA) Model by Suppressing ADAMTS5 Expression. Cureus 2024, 16, e73736. [Google Scholar] [CrossRef]
- Flannery, C.; Seaman, S.; Buddin, K.; Nasert, M.; Semler, E.; Kelley, K.; Long, M.; Favret, J.; Pavesio, A.; Loeser, R. A novel placental tissue biologic, PTP-001, inhibits inflammatory and catabolic responses in vitro and prevents pain and cartilage degeneration in a rat model of osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Croen, B.J.; Carballo, C.B.; Green, S.J.; Sevick, J.L.; Piacentini, A.N.; Chen, T.; Rodeo, S.A. Markers of Pain in 3 Different Murine Models of Posttraumatic Osteoarthritis: A Comparison Using Gait Analysis, Imaging, Histology, and Gene Expression. Am. J. Sports Med. 2023, 51, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Feigenson, M.; Begun, D.L.; Shull, L.C.; Culley, K.L.; Otero, M.; Goldring, M.B.; Ta, L.E.; Kakar, S.; Bradley, E.W.; et al. Phlpp inhibitors block pain and cartilage degradation associated with osteoarthritis. J. Orthop. Res. 2018, 36, 1487–1497. [Google Scholar] [CrossRef]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- Molstad, D.H.H.; Bradley, E.W. Pain and Activity Measurements. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021; pp. 291–299. [Google Scholar] [CrossRef]
- Creamer, P. Osteoarthritis pain and its treatment. Curr. Opin. Rheumatol. 2000, 12, 450–455. [Google Scholar] [CrossRef]
- Piel, M.J.; Kroin, J.S.; van Wijnen, A.J.; Kc, R.; Im, H.-J. Pain assessment in animal models of osteoarthritis. Gene 2014, 537, 184–188. [Google Scholar] [CrossRef]
- NIH HEAL Initiative. NIH. 2025. Available online: https://heal.nih.gov/research/training-next-generation-researchers (accessed on 20 May 2025).
- Hu, W.; Lin, J.; Wei, J.; Yang, Y.; Fu, K.; Zhu, T.; Zhu, H.; Zheng, X. Modelling osteoarthritis in mice via surgical destabilization of the medial meniscus with or without a stereomicroscope. Bone Jt. Res. 2022, 11, 518–527. [Google Scholar] [CrossRef]
- Parksl, E.L.; Gehal, P.Y.; Balikil, M.N.; Katzl, J.; Schnitzerl, T.J.; Apkarianl, A.V. Brain activity for chronic knee osteoarthritis: Dissociating evoked pain from spontaneous pain. Eur. J. Pain 2011, 15, 843.e1–843.e14. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; King, T.; Morgan, M.M. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci. Biobehav. Rev. 2019, 100, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.; Chanalaris, A.; Knights, C.; Ismail, H.; Sacitharan, P.K.; Gentry, C.; Bevan, S.; Vincent, T.L. Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol. 2016, 68, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Tran, P.B.; Das, R.; Ghoreishi-Haack, N.; Ren, D.; Miller, R.J.; Malfait, A.-M. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 20602–20607. [Google Scholar] [CrossRef]
- Sun, Q.; Zhen, G.; Li, T.P.; Guo, Q.; Li, Y.; Su, W.; Xue, P.; Wang, X.; Wan, M.; Guan, Y.; et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. eLife 2021, 10, e66532. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.; Liu, W.-X.; Lu, K.; Pan, H.; Wang, T.; Oh, C.-D.; Yi, D.; Huang, J.; Zhao, L.; et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020, 79, 635–645. [Google Scholar] [CrossRef]
- Wan, Y.; Shen, K.; Yu, H.; Fan, W. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free. Radic. Biol. Med. 2023, 196, 108–120. [Google Scholar] [CrossRef]
- Chen, H.-K.; Li, Y.-Z.; Ge, A.-N.; Zhu, Y.-B.; Wu, S.-J.; Bai, X.; Bai, H.-H.; Liu, Y.-N. Cbl-b modulated TrkA ubiquitination and function in the dorsal root ganglion of mice. Eur. J. Pharmacol. 2022, 921, 174876. [Google Scholar] [CrossRef]
- Ma, J.-C.; Luo, T.; Feng, B.; Huang, Z.; Zhang, Y.; Huang, H.; Yang, X.; Wen, J.; Bai, X.; Cui, Z.-K. Exploring the translational potential of PLGA nanoparticles for intra-articular rapamycin delivery in osteoarthritis therapy. J. Nanobiotechnol. 2023, 21, 361. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lv, Z.; Wang, P.; Xie, Y.; Sun, W.; Guo, H.; Jin, X.; Liu, Y.; Jiang, R.; Fei, Y.; et al. Near Infrared Responsive Gold Nanorods Attenuate Osteoarthritis Progression by Targeting TRPV1. Adv. Sci. 2024, 11, e2307683. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Fan, M.; Kuang, B.; Zhang, F.; Xian, M.; Deng, L.; Chen, C.; Pan, Y.; Chen, J.; Feng, N.; et al. 10-hydroxy-2-decenoic acid prevents osteoarthritis by targeting aspartyl β hydroxylase and inhibiting chondrocyte senescence in male mice preclinically. Nat. Commun. 2024, 15, 7712. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, P.; Li, W.; Xie, Y.; Sun, W.; Jin, X.; Jiang, R.; Fei, Y.; Liu, Y.; Shi, T.; et al. Bifunctional TRPV1 Targeted Magnetothermal Switch to Attenuate Osteoarthritis Progression. Research 2024, 7, 0316. [Google Scholar] [CrossRef]
- Qian, Y.; Rao, S.; Tan, Y.; Wang, Z.; Yin, H.; Wan, T.; He, Z.; Wang, X.; Hong, C.; Zeng, H.; et al. Intermittent Fasting Targets Osteocyte Neuropeptide Y to Relieve Osteoarthritis. Adv. Sci. 2024, 11, e2400196. [Google Scholar] [CrossRef]
- Gao, J.; Pei, H.; Lv, F.; Niu, X.; You, Y.; He, L.; Hu, S.; Shah, K.M.; Liu, M.; Chen, Y.; et al. JD-312—A novel small molecule that facilitates cartilage repair and alleviates osteoarthritis progression. J. Orthop. Transl. 2024, 44, 60–71. [Google Scholar] [CrossRef]
- Geng, N.; Xian, M.; Deng, L.; Kuang, B.; Pan, Y.; Liu, K.; Ye, Y.; Fan, M.; Bai, Z.; Guo, F. Targeting the senescence-related genes MAPK12 and FOS to alleviate osteoarthritis. J. Orthop. Transl. 2024, 47, 50–62. [Google Scholar] [CrossRef]
- Shin, Y.; Cho, D.; Kim, S.K.; Chun, J.-S. STING mediates experimental osteoarthritis and mechanical allodynia in mouse. Arthritis Res. Ther. 2023, 25, 90. [Google Scholar] [CrossRef]
- Lu, R.; Qu, Y.; Wang, Z.; He, Z.; Xu, S.; Cheng, P.; Lv, Z.; You, H.; Guo, F.; Chen, A.; et al. TBK1 pharmacological inhibition mitigates osteoarthritis through attenuating inflammation and cellular senescence in chondrocytes. J. Orthop. Transl. 2024, 47, 207–222. [Google Scholar] [CrossRef]
- Miller, R.E.; Belmadani, A.; Ishihara, S.; Tran, P.B.; Ren, D.; Miller, R.J.; Malfait, A. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through toll-like receptor 4. Arthritis Rheumatol. 2015, 67, 2933–2943. [Google Scholar] [CrossRef]
- Miller, R.E.; Ishihara, S.; Bhattacharyya, B.; Delaney, A.; Menichella, D.M.; Miller, R.J.; Malfait, A. Chemogenetic Inhibition of Pain Neurons in a Mouse Model of Osteoarthritis. Arthritis Rheumatol. 2017, 69, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xu, Y.; Jiang, H.; Zeng, W.; Wang, Y.; Zhu, L.; Lin, C.; Lou, C.; Shen, H.; Ye, H.; et al. CDK8 mediated inflammatory microenvironment aggravates osteoarthritis progression. J. Adv. Res. 2025, 77, 585–603. [Google Scholar] [CrossRef]
- Miller, R.; Tran, P.; Ishihara, S.; Larkin, J.; Malfait, A. Therapeutic effects of an anti-ADAMTS-5 antibody on joint damage and mechanical allodynia in a murine model of osteoarthritis. Osteoarthr. Cartil. 2016, 24, 299–306. [Google Scholar] [CrossRef]
- Tran, P.; Miller, R.; Ishihara, S.; Miller, R.; Malfait, A. Spinal microglial activation in a murine surgical model of knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 718–726. [Google Scholar] [CrossRef]
- Teng, H.; Chen, S.; Fan, K.; Wang, Q.; Xu, B.; Chen, D.; Zhao, F.; Wang, T. Dexamethasone Liposomes Alleviate Osteoarthritis in miR-204/-211-Deficient Mice by Repolarizing Synovial Macrophages to M2 Phenotypes. Mol. Pharm. 2023, 20, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Z.; Chen, W.; Xu, B.-Y.; He, G.; Fan, X.-C.; Tang, K.-L. 4-Octyl itaconate inhibits synovitis in the mouse model of post-traumatic osteoarthritis and alleviates pain. Chin. J. Traumatol. 2024, 28, 50–61. [Google Scholar] [CrossRef]
- Dyson, G.; Barrett, M.; Schlupp, L.; Prinz, E.; Hannebut, N.; Szymczak, A.; Brawner, C.M.; Jeffries, M.A. Ketogenic Diet-Associated Worsening of Osteoarthritis Histologic Secerity, Increased Pain Sensitivity and Gut Microbiome Dysbiosis in Mice. ACR Open Rheumatol. 2025, 7, e11794. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, Y.H.; Han, S. Increased oxidative phosphorylation through pyruvate dehydrogenase kinase 2 deficiency ameliorates cartilage degradation in mice with surgically induced osteoarthritis. Exp. Mol. Med. 2025, 57, 390–401. [Google Scholar] [CrossRef]
- Yang, X.; Guo, H.; Ye, W.; Yang, L.; He, C. Pulsed Electromagnetic Field Attenuates Osteoarthritis Progression in a Murine Destabilization-Induced Model through Inhibition of TNF-α and IL-6 Signaling. Cartilage 2021, 13, 1665S–1675S. [Google Scholar] [CrossRef]
- Shi, X.; Chen, H.; Yang, H.; Xue, S.; Li, Y.; Fang, X.; Ding, C.; Zhu, Z. Aptamer-Modified Tetrahedral Framework Nucleic Acid Synergized with TGF-β3 to Promote Cartilage Protection in Osteoarthritis by Enhancing Chondrogenic Differentiation of MSCs. ACS Appl. Mater. Interfaces 2024, 16, 50484–50496. [Google Scholar] [CrossRef]
- Shu, C.C.; Zaki, S.; Ravi, V.; Schiavinato, A.; Smith, M.M.; Little, C.B. The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: Differential effect of stem cell and hyaluronan treatment. Arthritis Res. Ther. 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Gowler, P.R.W.; Turnbull, J.; Shahtaheri, M.; Walsh, D.A.; Barrett, D.A.; Chapman, V. Interplay between cellular changes in the knee joint, circulating lipids and pain behaviours in a slowly progressing murine model of osteoarthritis. Eur. J. Pain 2022, 26, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, X.; Terkeltaub, R.; Lin, H.; Zhang, Y.; Zhou, B.; He, K.; Li, K.; Liu, Z.; Wei, J.; et al. Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Pham, T.H.N.; Kim, W.-U.; Kim, H.A. A causative role for periarticular skeletal muscle weakness in the progression of joint damage and pain in OA. Sci. Rep. 2023, 13, 21349. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, S.; Wu, M.; Zhao, Y.; Han, W.; Yu, Y. The Therapeutic Effect of rhMK on Osteoarthritis in Mice, Induced by Destabilization of the Medial Meniscus. Biol. Pharm. Bull. 2014, 37, 1803–1810. [Google Scholar] [CrossRef]
- Schmidt, R.; Schmelz, M.; Ringkamp, M.; Handwerker, H.O.; Torebjörk, H.E. Innervation Territories of Mechanically Activated C Nociceptor Units in Human Skin. J. Neurophysiol. 1997, 78, 2641–2648. [Google Scholar] [CrossRef]
- de Sousa, M.V.P.; Ferraresi, C.; de Magalhães, A.C.; Yoshimura, E.M.; Hamblin, M.R. Building, testing and validating a set of home-made von Frey filaments: A precise, accurate and cost effective alternative for nociception assessment. J. Neurosci. Methods 2014, 232, 1–5. [Google Scholar] [CrossRef]
- Obeidat, A.M.; Wood, M.J.; Adamczyk, N.S.; Ishihara, S.; Li, J.; Wang, L.; Ren, D.; Bennett, D.A.; Miller, R.J.; Malfait, A.-M.; et al. Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis. Nat. Commun. 2023, 14, 2479. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Xu, B.-X.; Fan, K.-J.; Fan, Y.-S.; Teng, H.; Wang, T.-Y. Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief. Ann. Transl. Med. 2021, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, Z.; Niu, X.; Li, R.; Wang, F.; You, Y.; Gao, J.; Zhao, L.; Shah, K.M.; Fan, J.; et al. GPRC5B protects osteoarthritis by regulation of autophagy signaling. Acta Pharm. Sin. B 2023, 13, 2976–2989. [Google Scholar] [CrossRef]
- Gao, P.; Rao, Z.-W.; Li, M.; Sun, X.-Y.; Gao, Q.-Y.; Shang, T.-Z.; Chen, C.; Zhang, C.-L. Erratum to: Tetrandrine Represses Inflammation and Attenuates Osteoarthritis by Selective Inhibition of COX-2. Curr. Med Sci. 2023, 43, 845–846. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.; Zhuo, Z.; Zhang, X.; Wu, L.; Guo, Z.; Wen, F.; An, L.; Yuan, H.; Zhang, Y.; et al. Activation of kappa opioid receptor suppresses post-traumatic osteoarthritis via sequestering STAT3 on the plasma membrane. Cell Commun. Signal. 2024, 22, 335. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Jain, D.; McNeill, J.N.; Little, D.; A Anderson, J.; Huebner, J.L.; Kraus, V.B.; Rodriguiz, R.M.; Wetsel, W.C.; Guilak, F. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann. Rheum. Dis. 2015, 74, 2076–2083. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, S.; Zhao, L.; Chang, X.; Han, L.; Huang, J.; Chen, D. Acute synovitis after trauma precedes and is associated with osteoarthritis onset and progression. Int. J. Biol. Sci. 2020, 16, 970–980. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Q.; Qiu, M.; Wu, Y.; Cheng, L.; Jiang, N.; Zhang, R.; Chen, J.; Yuan, W.; Jin, H.; et al. Exploring the pharmacological mechanism of Glycyrrhiza uralensis against KOA through integrating network pharmacology and experimental assessment. J. Cell. Mol. Med. 2024, 28, e18319. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Obeidat, A.M.; Wokosin, D.L.; Ren, D.; Miller, R.J.; Malfait, A.-M.; Miller, R.E. The role of intra-articular neuronal CCR2 receptors in knee joint pain associated with experimental osteoarthritis in mice. Arthritis Res. Ther. 2021, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.Y.; Kloefkorn, H.E.; Allen, K.D. Gait Analysis Methods for Rodent Models of Osteoarthritis. Curr. Pain Headache Rep. 2014, 18, 456. [Google Scholar] [CrossRef]
- Vrinten, D.H.; Hamers, F.F. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. PAIN® 2003, 102, 203–209. [Google Scholar] [CrossRef]
- Turner, P.V.; Pang, D.S.; Lofgren, J.L. A Review of Pain Assessment Methods in Laboratory Rodents. Comp. Med. 2019, 69, 451–467. [Google Scholar] [CrossRef]
- Inglis, J.J.; McNamee, K.E.; Chia, S.; Essex, D.; Feldmann, M.; Williams, R.O.; Hunt, S.P.; Vincent, T. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008, 58, 3110–3119. [Google Scholar] [CrossRef]
- Sambamurthy, N.; Zhou, C.; Nguyen, V.; Smalley, R.; Hankenson, K.D.; Dodge, G.R.; Scanzello, C.R. Deficiency of the pattern-recognition receptor CD14 protects against joint pathology and functional decline in a murine model of osteoarthritis. PLoS ONE 2018, 13, e0206217. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Hotham, W.E.; Pattison, L.A.; Ma, Q.; Henson, F.M.; Smith, E.S.J. Role of Human Mesenchymal Stem Cells and Derived Extracellular Vesicles in Reducing Sensory Neuron Hyperexcitability and Pain Behaviors in Murine Osteoarthritis. Arthritis Rheumatol. 2023, 75, 352–363. [Google Scholar] [CrossRef]
- Yu, S.P.; Ferreira, M.L.; Duong, V.; Caroupapoullé, J.; Arden, N.K.; Bennell, K.L.; Hunter, D.J. Responsiveness of an activity tracker as a measurement tool in a knee osteoarthritis clinical trial (ACTIVe-OA study). Ann. Phys. Rehabil. Med. 2022, 65, 101619. [Google Scholar] [CrossRef] [PubMed]
- Östlind, E.; Eek, F.; Stigmar, K.; Sant’anna, A.; Hansson, E.E. Promoting work ability with a wearable activity tracker in working age individuals with hip and/or knee osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 112. [Google Scholar] [CrossRef]
- Sambamurthy, N.; Nguyen, V.; Smalley, R.; Xiao, R.; Hankenson, K.; Gan, J.; Miller, R.E.; Malfait, A.; Dodge, G.R.; Scanzello, C.R. Chemokine receptor-7 (CCR7) deficiency leads to delayed development of joint damage and functional deficits in a murine model of osteoarthritis. J. Orthop. Res. 2018, 36, 864–875. [Google Scholar] [CrossRef]
- Nekomoto, A.; Nakasa, T.; Ikuta, Y.; Ding, C.; Miyaki, S.; Adachi, N. Feasibility of administration of calcitonin gene-related peptide receptor antagonist on attenuation of pain and progression in osteoarthritis. Sci. Rep. 2023, 13, 15354. [Google Scholar] [CrossRef] [PubMed]
- Brenneis, C.; Menges, S.; Westhof, A.; Lindemann, S.; Thudium, C.; Kleinschmidt-Doerr, K. Colony housing promotes structural and functional changes during surgically induced osteoarthritis in rats. Osteoarthr. Cartil. Open 2020, 2, 100100. [Google Scholar] [CrossRef] [PubMed]
- Van Zeeland, E.M.; Kassel, B.; Montoya, T.; Saviola, A.J.; Burton, L.H.; Easley, J.T.; Nelson, B.B.; Santangelo, K.S.; Sikes, K.J. Structural, functional, and proteomic based sex differences in murine post-traumatic osteoarthritis development following mechanical anterior cruciate ligament injury. Osteoarthr. Cartil. 2025, 33, 992–1006. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Effects of Early Motion on Healing of Musculoskeletal Tissues. Hand Clin. 1996, 12, 13–24. [Google Scholar] [CrossRef]
- Greising, S.M.; Corona, B.T.; Call, J.A. Musculoskeletal Regeneration, Rehabilitation, and Plasticity Following Traumatic Injury. Int. J. Sports Med. 2020, 41, 495–504. [Google Scholar] [CrossRef]
- Timkovich, A.E.; Holling, G.A.; Afzali, M.F.; Kisiday, J.; Santangelo, K.S. TLR4 antagonism provides short-term but not long-term clinical benefit in a full-depth cartilage defect mouse model. Connect. Tissue Res. 2023, 65, 26–40. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.; Ouyang, J.; Xie, Y.; Chen, L.; Tan, Q.; Du, X.; Su, N.; Ni, Z.; Chen, L. SQSTM1-dependent autophagic degradation of PKM2 inhibits the production of mature IL1B/IL-1β and contributes to LIPUS-mediated anti-inflammatory effect. Autophagy 2020, 16, 1262–1278. [Google Scholar] [CrossRef]
- Feng, N.; Ye, Y.; Pan, Y.; Kuang, B.; Du, Y.; Geng, N.; Chen, C.; Liu, K.; Liang, L.; Xian, M.; et al. The circUbqln1, regulated by XBP1s, interplays with 14–3–3ζ to inhibit collagen synthesis and promote osteoarthritis by controlling PRODH activity and proline metabolism. J. Adv. Res. 2024, 66, 267–284. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Sasho, T.; Saito, M.; Yamaguchi, S.; Akagi, R.; Mukoyama, S.; Akatsu, Y.; Katsuragi, J.; Fukawa, T.; Endo, J.; et al. Preventive effects of hyaluronan from deterioration of gait parameters in surgically induced mice osteoarthritic knee model. Osteoarthr. Cartil. 2014, 22, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Ning, K.; Yang, B.; Dou, X.; Liu, S.; Wang, D.; Xu, H. Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice. Int. J. Mol. Sci. 2023, 24, 535. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Liang, T.; Zhou, Y.; Chen, G.; He, J.; Ji, C.; Liu, P.; Zhang, C.; Lin, J.; et al. Betulinic Acid Attenuates Osteoarthritis via Limiting NLRP3 Inflammasome Activation to Decrease Interleukin-1β Maturation and Secretion. Mediat. Inflamm. 2023, 2023, 3706421. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, J.; Chen, Q.; Wang, Z.; Yang, Z. Milk fat globule-epidermal growth factor 8 exerts anti-osteoarthritis effects by inhibiting apoptosis and inducing autophagy in mouse chondrocytes. Biochem. Biophys. Res. Commun. 2024, 741, 151022. [Google Scholar] [CrossRef]
- Chan, K.M.; Thurlow, N.A.; Maden, M.; Allen, K.D. African Spiny Mice (Acomys) Exhibit Mild Osteoarthritis Following Meniscal Injury. Cartilage 2023, 14, 94–105. [Google Scholar] [CrossRef]
- Ling, H.; Zeng, Q.; Ge, Q.; Chen, J.; Yuan, W.; Xu, R.; Shi, Z.; Xia, H.; Hu, S.; Jin, H.; et al. Osteoking Decelerates Cartilage Degeneration in DMM-Induced Osteoarthritic Mice Model Through TGF-β/smad-dependent Manner. Front. Pharmacol. 2021, 12, 678810. [Google Scholar] [CrossRef]
- Ge, X.; Ritter, S.Y.; Tsang, K.; Shi, R.; Takei, K.; O Aliprantis, A. Sex-specific protection of osteoarthritis by deleting cartilage acid protein 1. PLoS ONE 2016, 11, e0159157. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Gulati, V.; Southgate, D.F.; McGregor, A.H. Measuring body weight distribution during sit-to-stand in patients with early knee osteoarthritis. Gait Posture 2013, 38, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.L.; Stevens-Lapsley, J.E. Weight-bearing asymmetry in relation to measures of impairment and functional mobility for people with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2010, 91, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Kobayashi, T. Limited roles of Piezo mechanosensing channels in articular cartilage development and osteoarthritis progression. Osteoarthr. Cartil. 2023, 31, 775–779. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Zeng, B.; Yang, X.; Su, M. MicroRNA-183attenuates osteoarthritic pain by inhibiting theTGFα-mediatedCCL2/CCR2signalling axis. Bone Jt. Res. 2021, 10, 548–557. [Google Scholar] [CrossRef]
- Zarebska, J.M.; Chanalaris, A.; Driscoll, C.; Burleigh, A.; Miller, R.; Malfait, A.; Stott, B.; Vincent, T. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr. Cartil. 2017, 25, 406–412. [Google Scholar] [CrossRef]
- Lee, K.M.-C.; Lupancu, T.; Keenan, S.N.; Bing, G.; Achuthan, A.A.; Biondo, M.; Lieu, K.G.; Watt, M.J.; Maraskovsky, E.; Kingwell, B.A.; et al. Therapeutic blockade of CCL17 in obesity-exacerbated osteoarthritic pain and disease. PLoS ONE 2025, 20, e0317399. [Google Scholar] [CrossRef]
- McNamee, K.E.; Burleigh, A.; Gompels, L.L.; Feldmann, M.; Allen, S.J.; Williams, R.O.; Dawbarn, D.; Vincent, T.L.; Inglis, J.J. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. PAIN® 2010, 149, 386–392. [Google Scholar] [CrossRef]
- Huesa, C.; Ortiz, A.C.; Dunning, L.; McGavin, L.; Bennett, L.; McIntosh, K.; Crilly, A.; Kurowska-Stolarska, M.; Plevin, R.; Hof, R.J.v.; et al. Proteinase-activated receptor 2 modulates OA-related pain, cartilage and bone pathology. Ann. Rheum. Dis. 2016, 75, 1989–1997. [Google Scholar] [CrossRef]
- Westhof, A.; Kleinschmidt-Doerr, K.; Michaelis, M.; Brenneis, C. Dynamic weight-bearing test during jumping: A sensitive outcome measure of chronic osteoarthritis pain in rats. Heliyon 2021, 7, e07906. [Google Scholar] [CrossRef]
- Fahlström, A.; Yu, Q.; Ulfhake, B. Behavioral changes in aging female C57BL/6 mice. Neurobiol. Aging 2011, 32, 1868–1880. [Google Scholar] [CrossRef]
- Jirkof, P.; Durst, M.; Klopfleisch, R.; Palme, R.; Thöne-Reineke, C.; Buttgereit, F.; Schmidt-Bleek, K.; Lang, A. Administration of Tramadol or Buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci. Rep. 2019, 9, 10749. [Google Scholar] [CrossRef]
- E Hess, S.; Rohr, S.; Dufour, B.D.; Gaskill, B.N.; A Pajor, E.; Garner, J.P. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 25–31. [Google Scholar]
- Taves, S.; Berta, T.; Chen, G.; Ji, R.-R. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast. 2013, 2013, 753656. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A.; Luisetto, R.; Pavan, M.; Guarise, C.; Beninatto, R.; Giraudo, C.; Galuppini, F.; Lazzarin, V.; Guzzardo, V.; Pennelli, G.; et al. Effect of intra-articular injection of a hyaluronic acid-alendronate conjugate on post-traumatic osteoarthritis induced by destabilization of the medial meniscus in rats. Sci. Rep. 2023, 13, 20692. [Google Scholar] [CrossRef]
- Hu, W.; Yao, X.; Li, Y.; Li, J.; Zhang, J.; Zou, Z.; Kang, F.; Dong, S. Injectable hydrogel with selenium nanoparticles delivery for sustained glutathione peroxidase activation and enhanced osteoarthritis therapeutics. Mater. Today Bio 2023, 23, 100864. [Google Scholar] [CrossRef] [PubMed]
- Altahla, R.; Tao, X. Thioredoxin-Interacting Protein’s Role in NLRP3 Activation and Osteoarthritis Pathogenesis by Pyroptosis Pathway: In Vivo Study. Metabolites 2024, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Bai, L.; Zhou, J.; Qian, Y.; Tang, Y.; Han, Q.; Zhang, X.; Zhang, M.; Yang, X.; Cui, W.; et al. Nanofat functionalized injectable super-lubricating microfluidic microspheres for treatment of osteoarthritis. Biomaterials 2022, 285, 121545. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde-García, C.; Burguera, E.F.; Vela-Anero, Á.; Hermida-Gómez, T.; Filgueira-Fernández, P.; Fernández-Rodríguez, J.A.; Meijide-Faílde, R.; Blanco, F.J. Intraarticular Administration Effect of Hydrogen Sulfide on an In Vivo Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 7421. [Google Scholar] [CrossRef] [PubMed]

| Test | Males ≤ 12 Weeks | Females ≤ 12 Weeks | Males > 12 Weeks | Females > 12 Weeks |
|---|---|---|---|---|
| Mechanical Allodynia | Day 1–Week 30 | Weeks 2–12 and 16 | Weeks 2–20 | |
| Hot Plate | Weeks 2–16 | Week 6 | ||
| Cold Plate | Week 12 | |||
| Chemical Cooling | Weeks 2–8 | |||
| Knee Hyperalgesia | Weeks 2–16 | |||
| Mechanical Allodynia-Tail | Week 8 | |||
| Heat Sensitivity-Tail | ||||
| Cage Monitoring | Weeks 2–30 | Week 2 | Week 8 | |
| Spontaneous Gait | Weeks 1–16 | Weeks 6–8 | Weeks 6–8 | |
| Compulsory Gait | Weeks 2–12 | Week 16 | Week 16 | |
| Weight Distribution | Day 1–Week 20 | Weeks 2–20 | Weeks 4–20 | |
| Rotarod | Weeks 8–20 | Week 20 | ||
| Balance Beam | Weeks 4–12 | |||
| Grimace and Nesting | Week 11 | |||
| Hang Test | Day 3–Week 9 | |||
| Grip Strength | Week 8 | |||
| Knee Edema | Week 12 | |||
| Microglia Assessment | Weeks 8–16 |
| Test Type | Temperature | Test Duration | Males ≤ 12 Weeks | Females ≤ 12 Weeks | Males > 12 Weeks | Ref |
|---|---|---|---|---|---|---|
| Radiant heat | 20% active intensity | <30 s | * 6 ns 2, 4, 8 weeks | * 6 ns 2, 4, 8 weeks | [55] | |
| Hotplate | 52 ± 1 °C | <20 s | ns week 8 | [111] | ||
| Radiant heat | 40 W | 20 s cut-off | * week 8 | [108] | ||
| Infrared | 25 infrared intensity | <20 s | * week 6 | [24] | ||
| Hotplate | NA | <25 s | * 6, 8 weeks | [83] | ||
| Hotplate | 55 °C | 45 s cut-off | * 5, 9, 13 weeks | [19] | ||
| Hotplate | 55 °C | 45 s cut-off | * 4, 6, 8, 10, 12 ns week 2 | [107] | ||
| Hotplate | 55 °C | 45 s cut-off | * week 8 | [110] | ||
| Hotplate | NA | <30 s | * 2, 3, 4, 5, 6, 7, 8 weeks | [94] | ||
| Hotplate | 55 °C | 30 s cut-off | * 8, 12, 16 ns 2, 4 weeks | [112] | ||
| Hotplate | 52 °C | 60 s cut-off | * 4, 8, 12, 16 ns 1, 2 weeks | [51] | ||
| Hotplate | 50 ± 0.1 °C | <30 s | * week 12 | [14] | ||
| Hotplate | 50 °C | <30 s | * week 12 | [113] | ||
| Infrared | 25 infrared intensity | <20 s | * week 8 | [82] | ||
| Infrared | 25 infrared intensity | <20 s | * week 8 | [76] | ||
| Hotplate | 54 ± 0.1 °C | 30 s cut-off | * 2, 4, 6, 8 weeks | [109] |
| Males ≤ 12 Weeks | Females ≤ 12 Weeks | Males > 12 Weeks | Ref. |
|---|---|---|---|
| * 11, 12 weeks ns day 1–3, weeks 2–10 | [70] | ||
| * 1–3 days, 16 weeks | [145] | ||
| * 8, 10–12 weeks ns days 1–3, weeks 1–7, 9 | * 9–12 weeks ns days 1–3, weeks 1–8 | [35] | |
| * 12, 14, 16 ns days 3, 7, 10 ns weeks 2, 4, 6, 8, 10 | [118] | ||
| * week 8 (between treatments, no controls shown) | [24] | ||
| * 4, 8, 12, 16, 20 weeks | [50] | ||
| * 4, 6, 8, 10 ns week 2 | [39] | ||
| * 8, 16, 20 ns 4, 12 weeks | [54] | ||
| * 13–16 ns 1–12 weeks | [33] | ||
| * 8, 10, 12, 14, 16 | [67] | ||
| * 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 weeks | *2, 4, 6, 8, 10, 12, 16 ns 14, 18, 20 weeks | [38] | |
| Stats NA (see Supplemental Table S1) | [146] | ||
| * 6–8 ns 2–5 weeks | [94] | ||
| * week 10–12 (between treatments, no stats to baseline) | [144] | ||
| * day 3, weeks 1, 2, 4, 8, 12, 16 | [51] | ||
| * 14–16 ns 1–13 weeks | [98] | ||
| * 2, 5–8 ns 1, 3–4 weeks | [99] | ||
| * 11–20 ns day 1, 3, weeks 1–10 | [143] | ||
| * week 8 | [142] | ||
| ns | [141] |
| Test | Males ≤ 12 Weeks | Females ≤ 12 Weeks | Females > 12 Weeks |
|---|---|---|---|
| Mechanical Allodynia | Weeks 1–12 | ||
| Hot Plate | Week 2 | ||
| Cold Plate | Week 2 | ||
| Cage Monitoring | Week 1 | ||
| Spontaneous Gait | Weeks 8–19 | Week 1 | |
| Compulsory Gait | Weeks 1–6 | ||
| Weight Distribution | Weeks 6–16 | Weeks 1–14 | |
| Rotarod | Week 2 | Weeks 1–7 | |
| Knee Edema | Week 2 | Weeks 1–13 |
| Test Style | Parameters Shown | DMM Compared Against | BASELINE | Timepoints Shown | Stance | Paw Area | Stride/Stance/Step Length | Stride/Stance/Step Width/BOS | Velocity | Sex and Age at Time of Injury | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catwalk | % print length of contralateral limb | healthy | yes | weeks 0, 2, 4, 6, 8, 10, 19 | * 19 ns 2–12 | males 8–9 weeks | [125] | ||||
| Footprint analysis | stride length, print area | sham and treated | no | week 8 | * 8 | * 8 | males 8 weeks | [153] | |||
| Footprint analysis | base of support (BOS), stride length, and print area | sham and treated | no | week 8 | * 8 | * 8 | * 8 | males 12 weeks | [155] | ||
| Compulsory treadmill running | maximum running speed | sham and treated | yes | weeks 0, 2, 4, 6 | * 6 2–4 | males 10 weeks | [20] | ||||
| Compulsory treadmill running (DigiGait) 25 cm/s | stride length, paw area, paw weight, pose duration | sham and treated | no | weeks 1, 2, 3, 4 | pose duration * 1–4 | area * 1–4 weight * 1–4 | * 1–4 | males 8 weeks | [57] | ||
| Catwalk | % print length of contralateral limb | naïve and treated | yes | weeks −1, and 1–14 every week | * 1 ns 2–14 | Females 13–14 weeks | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloser, H.; Henao-Tamayo, M.; Santangelo, K.S. A Review of Rodent Behavior, Mobility, and Pain Modifications in Response to Destabilization of the Medial Meniscus Injury. Biomedicines 2025, 13, 2886. https://doi.org/10.3390/biomedicines13122886
Kloser H, Henao-Tamayo M, Santangelo KS. A Review of Rodent Behavior, Mobility, and Pain Modifications in Response to Destabilization of the Medial Meniscus Injury. Biomedicines. 2025; 13(12):2886. https://doi.org/10.3390/biomedicines13122886
Chicago/Turabian StyleKloser, Heidi, Marcela Henao-Tamayo, and Kelly S. Santangelo. 2025. "A Review of Rodent Behavior, Mobility, and Pain Modifications in Response to Destabilization of the Medial Meniscus Injury" Biomedicines 13, no. 12: 2886. https://doi.org/10.3390/biomedicines13122886
APA StyleKloser, H., Henao-Tamayo, M., & Santangelo, K. S. (2025). A Review of Rodent Behavior, Mobility, and Pain Modifications in Response to Destabilization of the Medial Meniscus Injury. Biomedicines, 13(12), 2886. https://doi.org/10.3390/biomedicines13122886








