A Novel Homozygous Mutation in PMFBP1 Associated with Acephalic Spermatozoa Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. Whole Exome Sequencing (WES) and Bioinformatic Analysis
2.3. Sanger Sequencing of PMFBP1
2.4. In Vitro Fertilization and Embryo Culture
2.5. Semen Analysis
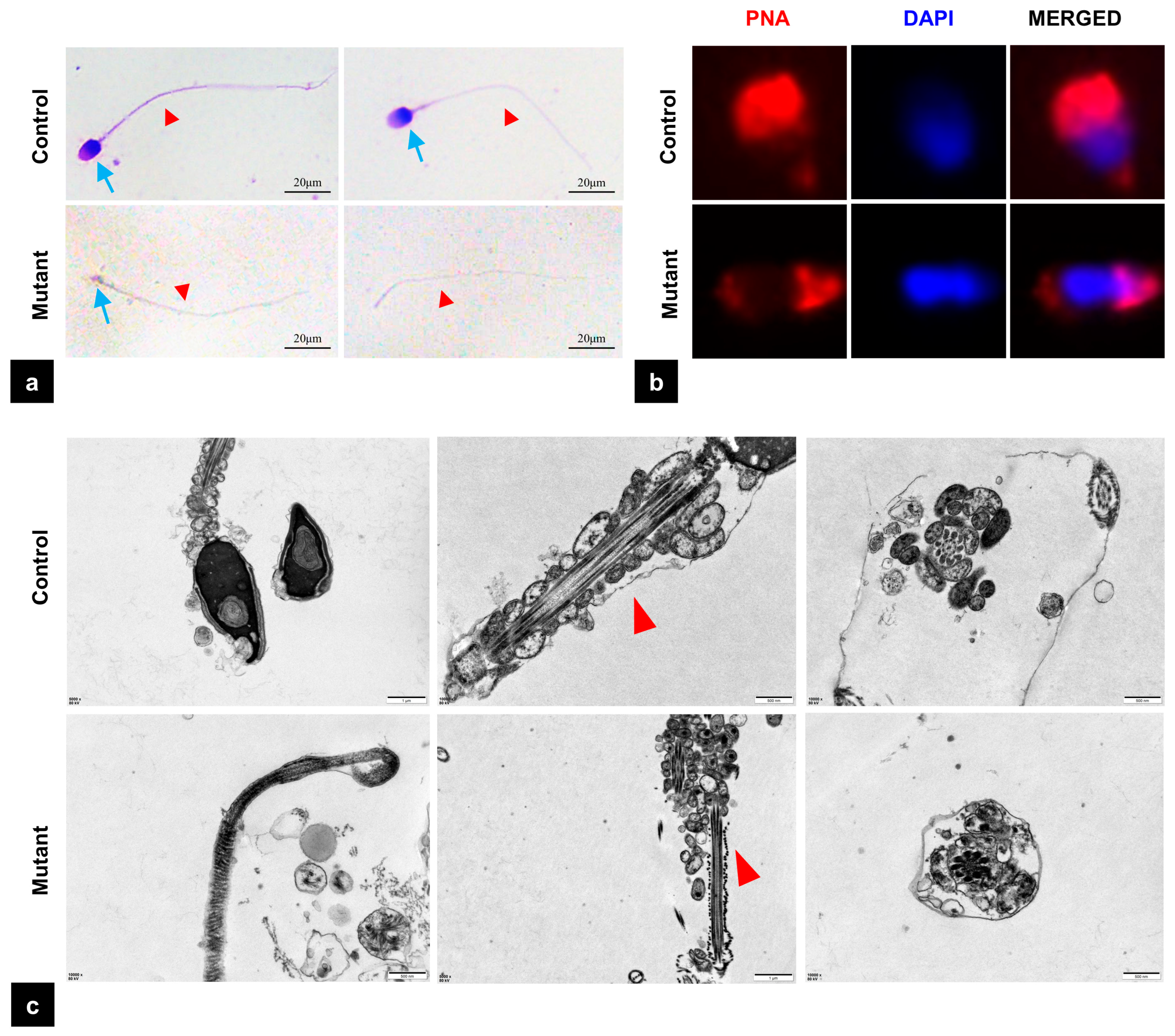
2.6. Peanut Agglutinin (PNA) Staining
2.7. Transmission Electron Microscopy Analysis
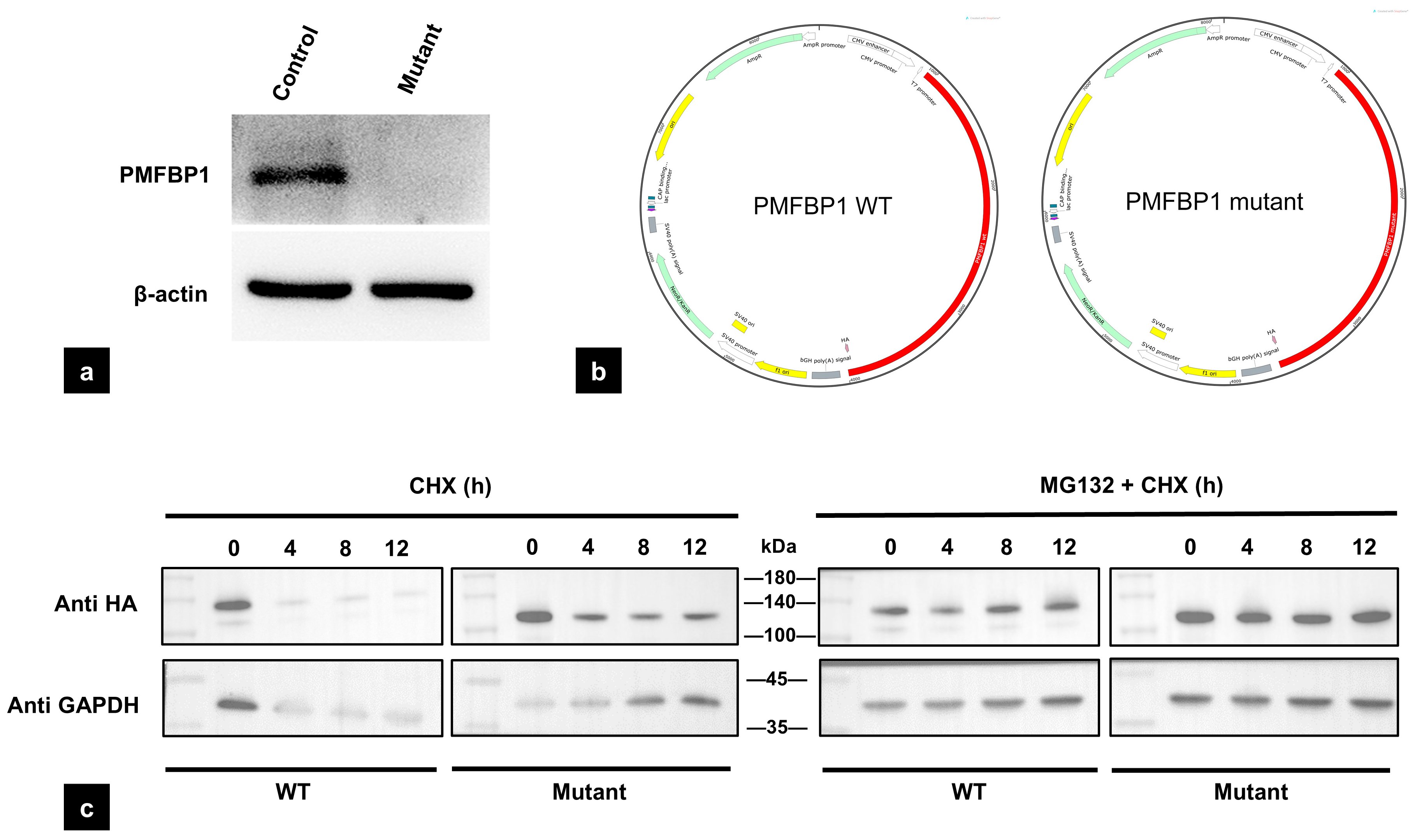
2.8. Western Blotting
2.9. Protein Stability Analysis
3. Results
3.1. IVF Outcomes
3.2. Identification of a Novel Mutation in PMFBP1
3.3. PMFBP1 Mutation Analysis
3.4. The Mutation of PMFBP1 Leads to Abnormal Sperm Morphology
3.5. The Mutation of PMFBP1 Leads to Altered Protein Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| AOA | Artificial oocyte activation |
| ART | Assisted reproductive technology |
| ASS | Acephalic spermatozoa syndrome |
| BCA | Bicinchoninic acid assay |
| BSA | Bovine serum albumin |
| CHX | Cycloheximide |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECL | Enhanced chemiluminescence |
| ExAC | Exome Aggregation Consortium |
| FBS | Fetal bovine serum |
| GATK | Genome Analysis Toolkit |
| GnomAD | Genome Aggregation Database |
| GRCh37 | Genome Reference Consortium Human Build 37 |
| HGVS | Human Genome Variation Society |
| HTCA | Head–tail coupling apparatus |
| ICSI | Intracytoplasmic sperm injection |
| PCR | Polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| PNA | Peanut agglutinin |
| PVDF | Polyvinylidene fluoride |
| RIPA | Radioimmunoprecipitation assay buffer |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| Smc | Structural maintenance of chromosomes |
| TBST | Tris-buffered saline with Tween |
| TEM | Transmission electron microscopy |
| TESE | Testicular sperm extraction |
| WES | Whole exome sequencing |
| WHO | World Health Organization |
| WT | Wild type |
References
- Shi, Z.; Yu, M.; Guo, T.; Sui, Y.; Tian, Z.; Ni, X.; Chen, X.; Jiang, M.; Jiang, J.; Lu, Y.; et al. MicroRNAs in spermatogenesis dysfunction and male infertility: Clinical phenotypes, mechanisms and potential diagnostic biomarkers. Front. Endocrinol. 2024, 15, 1293368. [Google Scholar] [CrossRef] [PubMed]
- Atmoko, W.; Savira, M.; Shah, R.; Chung, E.; Agarwal, A. Isolated teratozoospermia: Revisiting its relevance in male infertility: A narrative review. Transl. Androl. Urol. 2024, 13, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, M.-F.; Zheng, N.; Cao, Y.-X.; Zhu, F.-X. Genetic pathogenesis of acephalic spermatozoa syndrome: Past, present, and future. Asian J. Androl. 2022, 24, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chemes, H.; Puigdomenech, E.; Carizza, C.; Olmedo, S.B.; Zanchetti, F.; Hermes, R. Acephalic spermatozoa and abnormal development of the head–neck attachment: A human syndrome of genetic origin. Hum. Reprod. 1999, 14, 1811–1818. [Google Scholar] [CrossRef]
- Ying, L.-J.; Yu, L.; Yang, T.; Wu, Y.-B.; Xu, J.-Y.; Jia, Y.-L.; Zheng, Y.; Li, F. Semen parameters are seriously affected in acephalic spermatozoa syndrome. Basic Clin. Androl. 2022, 32, 20. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Hamzeiy, H.; Baghbanzadeh, A.; Pashazadeh, F.; Sakhinia, E. Genetic basis of acephalic spermatozoa syndrome, and intracytoplasmic sperm injection outcomes in infertile men: A systematic scoping review. J. Assist. Reprod. Genet. 2021, 38, 573–586. [Google Scholar] [CrossRef]
- Cazin, C.; Boumerdassi, Y.; Martinez, G.; Ben Mustapha, S.F.; Whitfield, M.; Coutton, C.; Thierry-Mieg, N.; Di Pizio, P.; Rives, N.; Arnoult, C.; et al. Identification and Characterization of the Most Common Genetic Variant Responsible for Acephalic Spermatozoa Syndrome in Men Originating from North Africa. Int. J. Mol. Sci. 2021, 22, 2187. [Google Scholar] [CrossRef]
- Elkhatib, R.A.; Paci, M.; Longepied, G.; Saias-Magnan, J.; Courbière, B.; Guichaoua, M.-R.; Lévy, N.; Metzler-Guillemain, C.; Mitchell, M.J. Homozygous deletion of SUN5 in three men with decapitated spermatozoa. Hum. Mol. Genet. 2017, 26, 3167–3171. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, J.; Zhu, F.; Yang, X.; Cui, Y.; Liu, J. Patients with acephalic spermatozoa syndrome linked to SUN5 mutations have a favorable pregnancy outcome from ICSI. Hum. Reprod. 2018, 33, 372–377. [Google Scholar] [CrossRef]
- Li, L.; Sha, Y.; Wang, X.; Li, P.; Wang, J.; Kee, K.; Wang, B. Whole-exome sequencing identified a homozygous BRDT mutation in a patient with acephalic spermatozoa. Oncotarget 2017, 8, 19914–19922. [Google Scholar] [CrossRef]
- Liu, G.; Wang, N.; Zhang, H.; Yin, S.; Dai, H.; Lin, G.; Li, W. Novel mutations in PMFBP1, TSGA10 and SUN5: Expanding the spectrum of mutations that may cause acephalic spermatozoa. Clin. Genet. 2020, 97, 938–939. [Google Scholar] [CrossRef]
- Lu, M.; Kong, S.; Xiang, M.; Wang, Y.; Zhang, J.; Duan, Z.; Zha, X.; Wang, F.; Cao, Y.; Zhu, F. A novel homozygous missense mutation of PMFBP1 causes acephalic spermatozoa syndrome. J. Assist. Reprod. Genet. 2021, 38, 949–955. [Google Scholar] [CrossRef]
- Sha, Y.; Wang, X.; Xu, X.; Ding, L.; Liu, W.; Li, P.; Su, Z.; Chen, J.; Mei, L.; Zheng, L.; et al. Biallelic mutations in PMFBP1 cause acephalic spermatozoa. Clin. Genet. 2019, 95, 277–286. [Google Scholar] [CrossRef]
- Sha, Y.; Sha, Y.; Ji, Z.; Mei, L.; Ding, L.; Zhang, Q.; Qiu, P.; Lin, S.; Wang, X.; Li, P.; et al. TSGA10 is a novel candidate gene associated with acephalic spermatozoa. Clin. Genet. 2018, 93, 776–783. [Google Scholar] [CrossRef]
- Sha, Y.-W.; Xu, X.; Ji, Z.-Y.; Lin, S.-B.; Wang, X.; Qiu, P.-P.; Zhou, Y.; Mei, L.-B.; Su, Z.-Y.; Li, L.; et al. Genetic contribution of SUN5 mutations to acephalic spermatozoa in Fujian China. Gene 2018, 647, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhu, F.; Wang, L.; Ouyang, Y.-C.; Dong, M.-Z.; Liu, C.; Zhao, H.; Cui, X.; Ma, D.; Zhang, Z.; et al. Essential role for SUN5 in anchoring sperm head to the tail. eLife 2017, 6, e28199. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Dai, S.; Shen, G.; Yang, Y.; Shen, Y. Identification of nonfunctional SPATA20 causing acephalic spermatozoa syndrome in humans. Clin. Genet. 2023, 103, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wei, X.; Sha, Y.; Li, N.; Yan, X.; Cheng, L.; Qiao, D.; Zhou, W.; Wu, R.; Liu, Q.; et al. Loss-of-function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol. Genet. Genom. Med. 2020, 8, e1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, W.-J.; Chen, G.-Y.; Dong, L.-H.; Tang, Y.; Zhang, H.; Li, Q.-Q.; Mei, X.-Y.; Wang, Z.-H.; Lan, F.-H. Pathogenesis of acephalic spermatozoa syndrome caused by SUN5 variant. Mol. Hum. Reprod. 2021, 27, gaab028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, F.; Yang, X.; Zhang, J.; Wu, H.; Zhang, Z.; Zhang, Z.; He, X.; Zhou, P.; Wei, Z.; et al. Biallelic SUN5 Mutations Cause Autosomal-Recessive Acephalic Spermatozoa Syndrome. Am. J. Hum. Genet. 2016, 99, 942–949. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, C.; Wang, F.; Yang, X.; Zhang, J.; Wu, H.; Zhang, Z.; He, X.; Zhang, Z.; Zhou, P.; et al. Mutations in PMFBP1 Cause Acephalic Spermatozoa Syndrome. Am. J. Hum. Genet. 2018, 103, 188–199. [Google Scholar] [CrossRef]
- Nie, H.; Tang, Y.; Qin, W. Beyond Acephalic Spermatozoa: The Complexity of Intracytoplasmic Sperm Injection Outcomes. BioMed Res. Int. 2020, 2020, 6279795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, G.; Xing, X.; Zhang, H.; Zhu, W.; Lin, G.; Lu, G.; Li, W. Patients with acephalic spermatozoa syndrome linked to novel TSGA10/PMFBP1 variants have favorable pregnancy outcomes from intracytoplasmic sperm injection. Clin. Genet. 2021, 100, 334–339. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.; Zhu, Z.; Zhi, E.; Lu, K.; Wang, X.; Liu, F.; Li, Z.; Xia, W. Detection of heterozygous mutation in hook microtubule-tethering protein 1 in three patients with decapitated and decaudated spermatozoa syndrome. J. Med Genet. 2018, 55, 150–157. [Google Scholar] [CrossRef]
- Gambera, L.; Falcone, P.; Mencaglia, L.; Collodel, G.; Serafini, F.; De Leo, V.; Piomboni, P. Intracytoplasmic sperm injection and pregnancy with decapitated sperm. Fertil. Steril. 2010, 93, 1347.e7–1347.e12. [Google Scholar] [CrossRef]
- Porcu, G.; Mercier, G.; Boyer, P.; Achard, V.; Banet, J.; Vasserot, M.; Melone, C.; Saias-Magnan, J.; D’ercole, C.; Chau, C.; et al. Pregnancies after ICSI using sperm with abnormal head-tail junction from two brothers: Case report. Hum. Reprod. 2003, 18, 562–567. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; Wang, Y.; Liu, C.; Sun, J.; Zhang, Z.; Guan, L.; Xiao, K.; Zhu, Z.; Luo, J. Association between mental health and male fertility: Depression, rather than anxiety, is linked to decreased semen quality. Front. Endocrinol. 2024, 15, 1478848. [Google Scholar] [CrossRef]
- Ohuchi, J.; Arai, T.; Kon, Y.; Asano, A.; Yamauchi, H.; Watanabe, T. Characterization of a novel gene, sperm-tail-associated protein (Stap), in mouse post-meiotic testicular germ cells. Mol. Reprod. Dev. 2001, 59, 350–358. [Google Scholar] [CrossRef]
- Wu, B.; Gao, H.; Liu, C.; Li, W. The coupling apparatus of the sperm head and tail†. Biol. Reprod. 2020, 102, 988–998. [Google Scholar] [CrossRef]
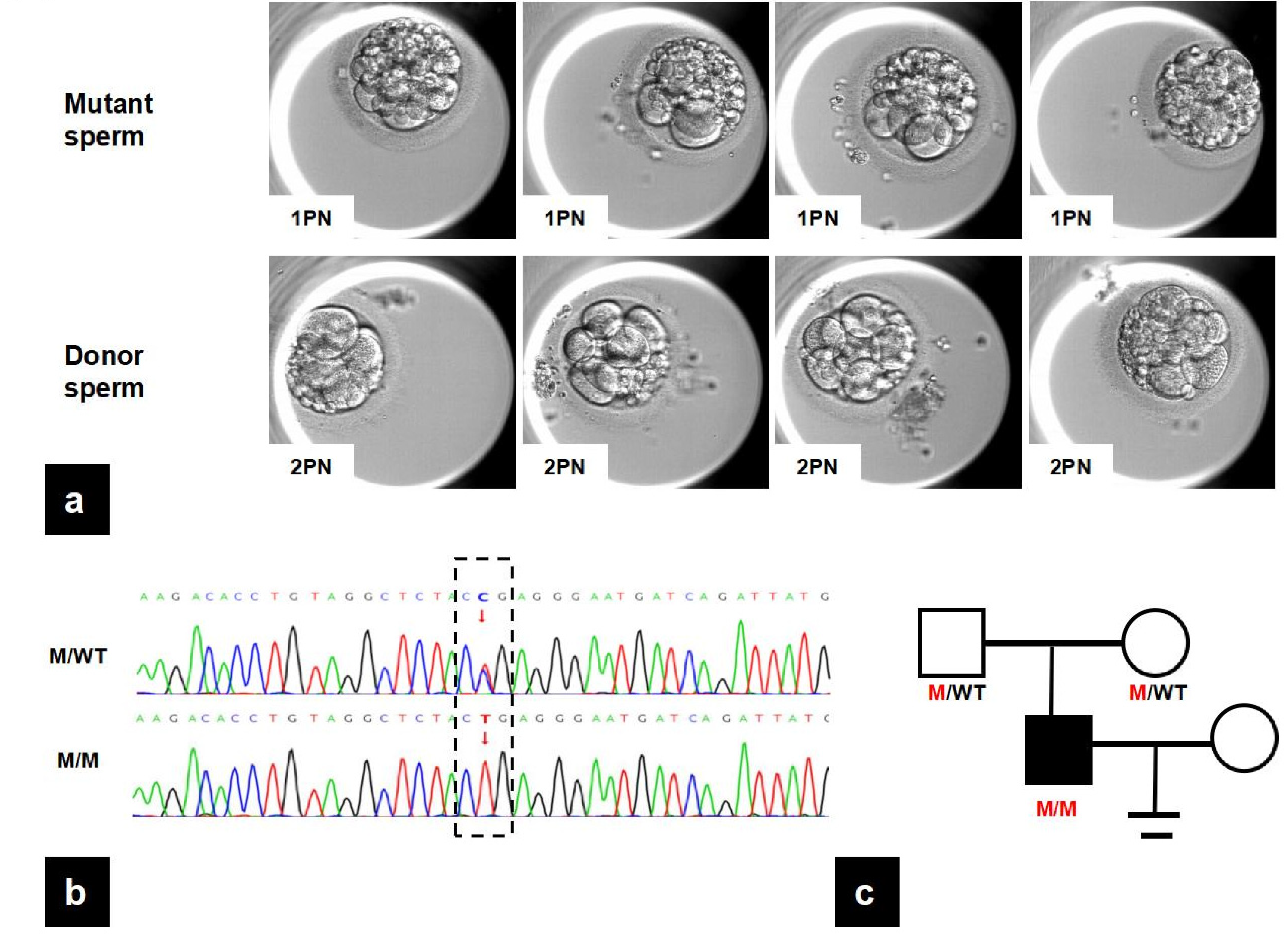

| Mutant Sperm | Donor Sperm | |
|---|---|---|
| In vitro fertilization cycle | ||
| oocytes retrieved | 6 | 7 |
| 2PN fertilization rate | 0 | 6 |
| Utilized embryos | 0 | 4 |
| Semen parameters | ||
| Concentration (×106/mL) | 9.6 | 63 |
| Progressive motility | 1 | 37 |
| Total motility | 9 | 50 |
| Normal morphology rate | 0 | 6 |
| Gene | Position | RefSeq ID | AA Alteration | Mutation Type | Status | 1000Ga | Gnom ADa | ExACa | Mutation Tasterbb | CADD_ph Red |
|---|---|---|---|---|---|---|---|---|---|---|
| PMFBP1 | Chr16:7157497 | NM_031293.3 | c.2641C>T p. Arg881Ter | Nonsense | Homo | NA | 7.955 × 10−6 | 8.25 × 10−6 | D | 36.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yin, X.; Yin, G.; Wang, J.; Chen, Y.; Zhang, Y.; Li, J.; Luo, J. A Novel Homozygous Mutation in PMFBP1 Associated with Acephalic Spermatozoa Defects. Biomedicines 2025, 13, 2882. https://doi.org/10.3390/biomedicines13122882
Liu C, Yin X, Yin G, Wang J, Chen Y, Zhang Y, Li J, Luo J. A Novel Homozygous Mutation in PMFBP1 Associated with Acephalic Spermatozoa Defects. Biomedicines. 2025; 13(12):2882. https://doi.org/10.3390/biomedicines13122882
Chicago/Turabian StyleLiu, Cong, Xinyue Yin, Gege Yin, Jinying Wang, Yirong Chen, Yi Zhang, Jie Li, and Jin Luo. 2025. "A Novel Homozygous Mutation in PMFBP1 Associated with Acephalic Spermatozoa Defects" Biomedicines 13, no. 12: 2882. https://doi.org/10.3390/biomedicines13122882
APA StyleLiu, C., Yin, X., Yin, G., Wang, J., Chen, Y., Zhang, Y., Li, J., & Luo, J. (2025). A Novel Homozygous Mutation in PMFBP1 Associated with Acephalic Spermatozoa Defects. Biomedicines, 13(12), 2882. https://doi.org/10.3390/biomedicines13122882





