Abstract
Background/Objectives: Ischemic heart disease remains a leading cause of morbidity and mortality worldwide, accompanied by a major decline in local myocardial pH. However, the mechanisms of pH regulation and the homeostasis of H+ neutralizing buffers, such as HCO3−, in cardiomyocytes remain incompletely understood. We identified a solute carrier, Slc26a6, in mouse and human hearts playing key roles in the regulation of cardiac pH, excitability, and contractility. Slc26a6 is an acid loader, so we hypothesized that ablation of Slc26a6 may protect the heart from ischemia/reperfusion (I/R) injury. Methods: The I/R model was generated using wild type (WT) and Slc26a6 knockout (Slc26a6−/−) mice. Multidisciplinary in vivo, in vitro, and ex vivo approaches were used, including echocardiography, electrophysiology, hemodynamic monitoring, fluorescence microscopy, histochemistry, and cellular Ca2+ transients, sarcoplasmic reticulum Ca2+ load, and sarcomere shortening were recorded. Results: Troponin I level was lower in Slc26a6−/− I/R mice. Slc26a6−/− mice showed better systolic and diastolic function, reduced collagen deposition, and reduced infarct size compared to that of WT mice. Cellular experiments in measurement of sarcomere shortening, Ca2+ transients, and sarcoplasmic reticulum Ca2+ load in cardiomyocytes from the infarct zone supported the in vivo findings, demonstrating better single cell function in Slc26a6−/− compared to WT mice. Ex vivo pHi measurement showed elevated pHi in Slc26a6−/− mouse heart. Conclusions: Ablation of Slc26a6 protects the heart from I/R injury, suggesting the importance of Cl−/HCO3− exchange in cardiac pH regulation and I/R injury. The elevated pHi in Slc26a6−/− mouse heart may counterbalance the effects of the myocardium acidosis resulting from ischemia.
1. Introduction
Ischemic heart disease represents the most common type of heart disease, that can lead to heart failure, lethal ventricular arrhythmias, and sudden cardiac death. Cardiac ischemia induces a major decline in local myocardial pH resulting in depressed myocardial contractility, disturbances in cellular Ca2+ signaling, and cardiac arrhythmia [1,2,3,4,5]. However, the mechanisms of pH regulation in cardiomyocytes remain incompletely understood. The critical knowledge gaps stem from the fact that the proton neutralizing buffers such as HCO3− have not been well studied. Several H+-equivalent transporters have been reported to contribute to cardiac pH regulation including Na+/H+ exchangers (NHEs), Na+-HCO3− cotransporters (NBCs), anion exchangers or Cl−/HCO3− exchangers (AEs or CBE), Cl−/OH− exchangers (CHEs), and monocarboxylate transporters (MCTs) [1,2]. Ischemia leads to local myocardial acidosis due to increased anaerobic metabolism coupled with decreased vascular acid removal, accompanied by a downregulation of NHE and possible NBC activity [1,6]. Therefore, the intracellular pH (pHi) is significantly reduced during ischemia. Reperfusion enhances the acid extrusion through MCTs, NHEs, and NBCs [1,7,8].
NHEs and NBCs are Na+-dependent H+ extruders in the heart. In contrast, H+-equivalent transporters such as AEs and CHEs are chloride-coupled anion transporters in the heart associated with Cl− and H+ loading into the cardiomyocytes. However, the molecular identity and physiological significance of these transporters in the heart are not well understood. Three AE family members AE1, AE2, and AE3 encoded by solute carrier SLC4A genes have been identified in the heart, with relative higher expression of AE3 [9,10,11,12]. AE1, AE2, and AE3 are electroneutral Cl−/HCO3− exchangers [13]. AE3 was reported to be involved in the myocardial pHi recovery from cellular alkalization [14], and ablation of AE3 showed phenotypical normal cardiac function and had no effect on the ischemia/reperfusion injury [15]. Further studies showed that loss of the AE3 in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure [16], and AE3 is required for cardiac protection by sasanquasaponin against ischemia/reperfusion (I/R) injury [17]. In addition, AE3 was recently proposed to be responsible for active transport-mediated disposal of CO2 in the heart [18]. In a zebrafish model, AE3 knockdown caused increased cardiac pHi, short QT syndrome, and reduced systolic duration [19].
Paradoxically, a recent study found that AE1, AE2, and AE3 are not the major Cl−/HCO3− exchangers in the heart; instead, a new solute carrier, Slc26a6, is the predominant Cl−/HCO3− and Cl−/OH− exchanger with nearly 100-fold higher expression level than AE1, AE2, and AE3 in the heart [20]. To this end, we decided to investigate and have cloned several isoforms of mouse and human cardiac Slc26a6. We demonstrated that Slc26a6 is highly expressed in atrial and ventricular myocytes. Importantly, we showed that Slc26a6 mediates electrogenic Cl−/HCO3− exchange activities in cardiomyocytes suggesting the potential role of Slc26a6 not only in the regulation of cardiac Cl− homeostasis, but also in cardiac excitability and pHi [21]. We tested the triple roles of Slc26a6 by taking advantage of the null deletion of Slc26a6 and demonstrated that the null deletion results in action potential shortening, elevated pHi, fragmented QRS complexes, slower heart rate, and reduced cardiac function compared to WT littermates [22]. Therefore, Slc26a6 may represent a novel and predominant Cl−/HCO3− exchanger in the heart with significant functional impact on cardiac function.
An early study found that intracellular Cl− activities increase in ventricular muscles during simulated ischemia [23]. Inhibition of Cl−/HCO3− exchange activities has been suggested to be a possible cardioprotective strategy against I/R injury [24]. In an I/R-induced ventricular fibrillation model, chloride substitution was suggested to be an effective antiarrhythmic approach [25,26]. Cl− substitution has been shown to produce protective effects against I/R injury, and an anion exchange inhibitor, 4-acetamido-4′-isothiocyanato-stilbene-2,2′-disulfonic acid (SITS) was shown to protect the heart from I/R injury [24,27]. A recent study found that blockade of transmembrane Cl− flux mitigates I/R-induced cardiac injury [28]. Slc26a6 represents the key Cl−/HCO3− exchanger in the heart critical for cardiac Cl− homeostasis and pHi regulation. Here, we hypothesize that ablation of Slc26a6 will result in a significant reduction of acid loading into cardiomyocytes, which may have protective effects on I/R injury. At the translational level, Slc26a6 may represent a novel therapeutic target for cardiac protection during I/R.
2. Materials and Methods
All animal care and procedures were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Animal use was in accordance with the National Institutes of Health guidelines. We used 129S6/SvEv wild type (WT) and Slc26a6−/− mice previously generated and reported [29]. The validation of the absence of Slc26a6 in the heart of the global Slc26a6−/− mouse was reported in our previous publication supporting the deletion of Slc26a6 in the heart in this mouse model [22]. We used the same animal model in this study. For further validating the global Slc26a6−/− mouse model used in this study, we provided the genotyping data as shown in Supplemental Figure S1. All experiments described in the study were conducted in a blinded fashion. Specifically, the investigators who performed the physiological recordings, cell/tissue dissection, and staining had no knowledge of the genotypes of the animals. Chemicals used in this study were products of Sigma-Aldrich (St. Louis, MO, USA), unless specifically indicated.
2.1. I/R Mouse Model
Mouse I/R model was generated as we previously described [30]. Briefly, mice were injected intraperitoneally with ketamine 50–80 mg/kg and xylazine 5 mg/kg to achieve anesthesia during surgery. Mice were intubated and mechanically ventilated with isoflurane anesthetic (2%) and supplemental oxygen at a respiratory rate of 100–200 breaths/min and tidal volume of 0.13–0.2 mL. Surface ECG recording was recorded before and during the I/R surgery. An 8 mm cut was made 2 mm left of the sternal border in the 4th intercostal space. A retractor was used to gently widen the incision. The pericardium was clamped and fixed by the retractor. The left anterior descending (LAD) coronary artery was ligated 1–2 mm below the tip of the left auricle in its normal position, which induced approximately 40–50% ischemia of the LV. Occlusion was confirmed by the change of color of the anterior wall of the LV and ST elevation in ECG. The ligation was left for a period of 45 min after which the occlusion was removed. The retractor was then taken out, a temporary chest tube was inserted to re-establish the negative plural pressure until the spontaneous breathing recovered, and the chest cut was sutured. We performed the same operative procedures in sham mice except for LAD ligation. Additional postoperative treatment included supplemental buprenorphine twice daily as an analgesic for two days and a warm environment. Mice were monitored daily until the wound was healed.
2.2. Evaluation of Cardiac Function by Echocardiography
Echocardiograms using M-mode and two-dimensional (2D) measurements to assess systolic function were performed in conscious animals using Vevo 2100 (FUJIFILM VisualSonics, Toronto, ON, Canada), as we previously described [21,22]. Briefly, we performed at least two sperate scans to measure six cardiac cycles with papillary muscles as the position references. Left ventricle (LV) end diastolic and end systolic dimensions were measured for calculation of fractional shortening (FS) and ejection fraction (EF) defined as follows: FS% = (EDD − ESD)/EDD × 100, where EDD and ESD are LV end diastolic and end systolic dimension, respectively; EF% = (EDV − ESV)/EDV × 100, where EDV and ESV are LV end diastolic and end systolic volume, respectively.
2.3. Electrocardiographic Recordings
ECG recordings were performed using Bioamplifier (BMA 831, CWE, Inc., Ardmore, PA, USA), as we previously described [21,22]. Briefly, mice were placed on a temperature-controlled warming blanket at 37 °C. Four consecutive two-minute epochs of ECG data were recorded. Signals were low-pass filtered at 0.2 kHz and digitized using Digidata 1200 (Molecular Devices LLC., Sunnyvale, CA, USA). A total of 100 beats were analyzed from each animal in a blinded fashion.
2.4. Hemodynamic Monitoring
We performed hemodynamic surgery and measurement as we previously reported [22]. Briefly, Mice were anesthetized and maintained at 37 °C. We inserted the catheter with pressure and volume sensors into the LV through the carotid artery. The LV pressure and volume were recorded by a Millar Pressure–Volume System MPVS-300 (Millar, Inc., Houston, TX, USA), Power Lab, and Lab Chart 6.0 software (AD Instruments, Colorado Springs, CO, USA).
2.5. Cardiac Tissue Preparation and Cardiomyocyte Isolation
Cardiomyocyte isolation was performed as we reported [21,22]. We performed rapid heart excision, retrograde perfusion, and enzymatic dissociation.
2.6. Histological Analyses
Histological analyses of cardiac tissue were performed as we previously described [22]. Briefly, after washing out the blood, the mouse heart was embedded in paraffin. We then made precision cuts of heart slices (5 μm thick) along the short axis and stained the slices with Picrosirius red for collagen deposition assessment.
2.7. Measurement of Sarcomere Shortening and Ca2+ Transient (CaT)
The IonOptix sarcomere detection (IonOptix LLC., Westwood, MA, USA) and fast Fourier transform (FFT) method were used to measure sarcomere contraction as we previously reported [22]. The sarcomere contraction and CaT were measured simultaneously by the IonOptix cardiac contraction system. The sarcomere shortening was quantified by the percentage changes of sarcomere length during contraction relative to the diastolic length.
2.8. Ex Vivo pHi Measurement by Confocal Imaging
The mouse was injected intraperitoneally with ketamine 50–80 mg/kg and xylazine 5 mg/kg to achieve anesthesia, and the heart was rapidly excised and placed in the cold Ca2+-free and HEPES-buffered Tyrode’s solution containing (in mM): 145 NaCl, 4 KCl, 1 MgCl2, 10 glucose, 0.33 NaH2PO4, 10 HEPES, and pH 7.4 by NaOH. After trimming the connected tissues, the aorta was cannulated, and retrograde perfusion was performed to clear the blood followed by perfusion for 30 min using 10 mL Ca2+-free and HEPES-buffered Tyrode’s solution containing 10 µM SNARF-1 at room temperature (22–24 °C) by circulating the solution using an electrical pump. The heart was subsequently perfused and washed twice by 10 mL Ca2+-free Tyrode’s solution each time. The heart was then transferred to a chamber with a cover glass bottom (#1.5 cover glass), mounted on the confocal microscope (LSM 700, Carl Zeiss, Oberkochen, Germany) stage, and perfused by HCO3−-buffered Tyrode’s solution containing the following (in mM): 120 NaCl, 24 NaHCO3, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, 0.33 NaH2PO4, gassed by 5% CO2 and 95% O2. Confocal imaging was performed by focusing on the epicardium of the heart. For pHi measurement in the epicardial cells, the cells were excited by 555 nm laser and the emission images were acquired simultaneously at 580 and 640 nm using two band pass emission filters (585 ± 10 nm, and 630 ± 15 nm). The heart was perfused for 10 min by HCO3−-buffered Tyrode’s solution gassed by 5% CO2 and 95% O2, and the emission images were acquired for pHi quantification. The SNARF emission ratio (F580/F640) was converted to a pHi value using standard calibration as we reported before [22,31]. The calibration was performed by measuring the SNARF emission ratios (F580/F640) when cardiomyocytes were perfused by calibration solutions with five different pH values. The calibration solutions contained the following (in mM): 140 KCl, 1 MgCl2, 20 HEPES (or MES at pH 5.5), with pH 5.5, 6.5, 7.5, 8.5. Then, 10 µM nigericin (a K+/H+ antiporter ionophore) was added to the calibration solution before use.
2.9. Data Analysis and Statistics
The sample size of 5 was estimated based on the detection of at least 15% differences with alpha = 0.05 for a two-tailed test with the power > 0.95, with the standard deviation of the differences assumed to be 5% (SigmaStat, Grafiti LLC, Palo Alto, CA, USA). All data were included. Normal distribution was tested for choosing the appropriate statistical methods. For three or more groups, one-way ANOVA combined with Tukey’s post hoc analyses was used except where specified in the figure legends. For comparisons between two groups with equal sample numbers, two-sample t-test was used; for comparisons between unequal sample numbers in two groups, Welch’s t-test was used. If the data did not follow a normal distribution, non-parametric paired Wilcoxon signed-rank test was used. Statistical significance was defined as p < 0.05. Multiple software tools were used for statistical analyses including Origin Pro 2021 (OriginLab, Northampton MA, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± S.E.M.
3. Results
3.1. I/R Mouse Model and Ischemia Monitoring During I/R Surgery
To test the effects of I/R on cardiac function and structural remodeling, we generated I/R models in both WT and Slc26a6−/− mice by performing left anterior descending coronary artery (LAD) ligation surgery in the heart. Figure 1 shows the experimental design to test the short-term and long-term effects of I/R injury. The reperfusion time was set to be 24 h and four weeks in short-term and long-term experiments, respectively.
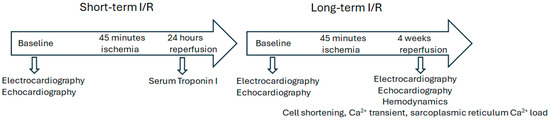
Figure 1.
I/R experimental design. The mouse I/R model and experimental design are shown. Both WT and Slc26a6−/− male and female mice were used. Sham mice were generated by performing similar surgical incision but without left anterior descending coronary artery (LAD) ligation.
For short-term reperfusion, we tested the serum troponin I level to evaluate myocardiac injuries; for long-term reperfusion, we investigated the effect on structural remodeling, cardiac function changes, hemodynamics alterations, and single cell function. We performed ECG recordings during I/R surgery to monitor the ischemia progress and the correlated ECG alterations with the surgery effects to be sure of the success of the ligation. Figure 2A shows the ECG traces recorded during I/R surgery. We found significant ST segment elevation in both WT and Slc26a6−/− I/R mice during LAD ligation surgery, supporting the success in making the I/R models in both WT and Slc26a6−/− mice. The ST elevation was more significant in WT mice during the I/R surgery as shown in Figure 2B.
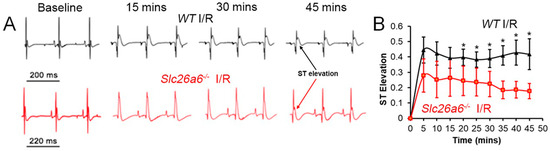
Figure 2.
ECG monitoring during I/R surgery. (A) Representative traces of surface ECG during the LAD ligation surgery in WT and Slc26a6−/− mice. (B) Comparisons of ST segment elevation during LAD ligation. Biological replicates or mouse numbers: WT (12), Slc26a6−/− (12). (* p < 0.05 by two-sample t-test).
3.2. Less Structural Remodeling in Slc26a6−/− I/R Mice
To test the potential effects of I/R in WT and Slc26a6−/− mice, we compared the morphology of the heart between sham and I/R in WT and Slc26a6−/− mice. We found significant hypertrophy post I/R in both WT and Slc26a6 −/− mice as shown in Figure 3A,B, supported by the increased heart weight/body weight ratios in both groups. To quantify the fibrosis, we performed Picrosirius red staining on heart slices to assess collagen deposition in infracted areas as shown in Figure 3C. The collagen deposition was further quantified as shown in Figure 3D. The percentage of collagen deposition is significantly higher in the I/R heart slice compared to the sham group. Interestingly, the deposition percentage is significantly smaller in the Slc26a6−/− I/R heart compared to the WT I/R heart, suggesting reduced injuries and structural remodeling in Slc26a6−/− mice. Consistently, the troponin I level in the plasma of Slc26a6−/− mice is significantly lower than that of WT mice as shown in Figure 3E.
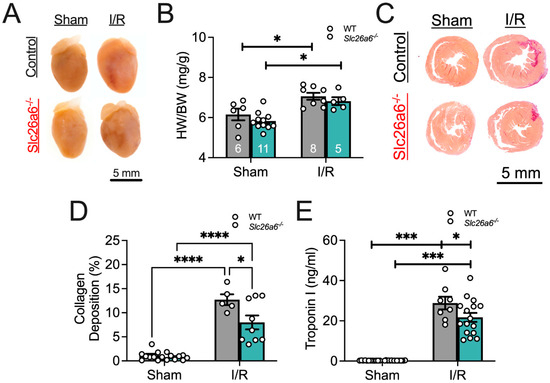
Figure 3.
Comparisons of cardiac I/R injury and structural remodeling. (A) Heart images before and after I/R. (B) Comparisons of the ratios of heart weight to body weight (HW/BW) in WT and Slc26a6−/− mice from long-term I/R model. Biological replicates or mouse numbers: WT sham (6); Slc26a6−/− sham (11); WT I/R (8); Slc26a6−/− I/R (5). (* p < 0.05 by one-way ANOVA combined with Tukey–Kramer post hoc analyses). The data points in bar figures show the animal number for different experiments. Grey color bar: WT; cyan blue color bar: Slc26a6−/−. (C) Fibrosis analysis using Picrosirius red staining to assess the collagen deposition. (D) Summary data of cardiac collagen deposition in WT and Slc26a6−/− mouse from long-term I/R model. Biological replicates or mouse numbers: WT sham (9); Slc26a6−/− sham (8); WT I/R (5); Slc26a6−/− I/R (9). (* p < 0.05, **** p < 0.0001 by one-way ANOVA combined with Tukey–Kramer post hoc analyses). Grey color bar: WT; cyan blue color bar: Slc26a6−/−. (E) Comparisons of serum troponin I levels in WT and Slc26a6−/− mice from short-term I/R model. Biological replicates or mouse numbers: WT sham (9); Slc26a6−/− sham (8); WT I/R (8); Slc26a6−/− I/R (16). (* p < 0.05, *** p < 0.001 by one-way ANOVA combined with Tukey–Kramer post hoc analyses). Grey color bar: WT; cyan blue color bar: Slc26a6−/−.
3.3. Slc26a6 Knockout Reserved Cardiac Function in I/R Mice
The structural remodeling after I/R may cause alterations in cardiac function. To quantify cardiac function, we used echocardiography to assess the systolic and diastolic function in sham and I/R mice, focusing on the comparisons between WT and Slc26a6−/− mice. The representative images of echocardiography for systolic function in sham and I/R mice are shown in Figure 4A. We did not find significant changes in heart rate after I/R (Figure 4B), but the diastolic and systolic left ventricle volumes were significantly increased after I/R in both WT and Slc26a6−/− mice (Figure 4D,E). The ejection fraction (EF) and fractional shortening (FS) were also significantly reduced (Figure 4F,G). Interestingly, the systolic left ventricle volume was smaller and EF and FS are larger in Slc26a6−/− I/R mice compared to WT I/R mice, supporting the notion that the impairment of cardiac function in Slc26a6−/− I/R mice is less than that of WT I/R mice and demonstrating the protective roles of Slc26a6 deletion in I/R injury. To further assess the cardiac function, we measured the diastolic cardiac function as well. Figure 4H shows the representative images of echocardiography for testing diastolic function in sham and I/R mice. Analyses of the diastolic function showed reduced E/A ratio in WT I/R mice instead of Slc26a6−/− I/R mice (Figure 4I), and the E/A ratio in Slc26a6−/− I/R mice was significantly higher than that of WT I/R mice. In addition, the isovolumic relaxation time (IVRT) was prolonged in WT I/R mice but did not change in Slc26a6−/− I/R mice (Figure 4J), further supporting the protective roles of Slc26a6 deletion in I/R injury.
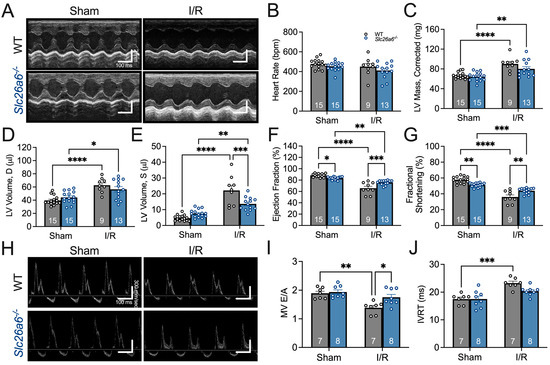
Figure 4.
Comparisons of cardiac function between WT and Slc26a6−/− I/R mice. (A) Representative images of M-mode echocardiogram before and after I/R surgery in WT and Slca6a6−/− mice. (B) Comparisons of heart rates. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (C) Comparisons of left ventricle (LV) mass. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (** p < 0.01, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). (D) Comparisons of diastolic LV volumes. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (* p < 0.05, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). The LV end-diastolic dimensions (in mm) are as follows: WT sham, 3.17 ± 0.08; WT I/R, 3.59 ± 0.14; Slc26a6−/− sham, 3.31 ± 0.08; Slc26a6−/− I/R, 3.61 ± 0.13. (E) Comparisons of systolic LV volume. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (** p < 0.01, *** p < 0.001, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). The LV end-systolic dimensions are as follows: WT sham, 1.35 ± 0.08; WT I/R, 2.36 ± 0.13; Slc26a6−/− sham, 1.57 ± 0.06; Slc26a6−/− I/R, 2.04 ± 0.08. (F) Comparisons of ejection fractions. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). (G) Comparisons of fractional shortening. Biological replicates or mouse numbers: WT sham (15); Slc26a6−/− sham (15); WT I/R (9); Slc26a6−/− I/R (13). (** p < 0.01, *** p < 0.001, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). (H) Echocardiogram images showing cardiac diastolic function. (I) Comparisons of E/A rations. Biological replicates or mouse numbers: WT sham (7); Slc26a6−/− sham (8); WT I/R (7); Slc26a6−/− I/R (8). (* p < 0.05, ** p < 0.01 by one-way ANOVA combined with Tukey’s post hoc analyses). (J) Comparisons of isovolumic relaxation time (IVRT). Biological replicates or mouse numbers: WT sham (7); Slc26a6−/− sham (8); WT I/R (7); Slc26a6−/− I/R (8). (*** p < 0.001 by one-way ANOVA combined with Tukey’s post hoc analyses). The data points in bar figures show the animal number for different experiments.
3.4. Improved Hemodynamics in Slc26a6−/− I/R Mice
To further characterize the cardiac function alterations, we performed hemodynamic recordings to directly assess the left ventricle mechanical properties in sham, WT I/R, and Slc26a6−/− I/R mice in the long-term reperfusion model. Figure 5A–C show the original recording traces of left ventricle pressure, volume, and the developed pressure (dP/dt), respectively. The summarized data are shown on the right panels. It was clearly shown that the end-systolic pressure is significantly reduced in WT I/R mice but not in Slc26a6−/− I/R mice. The end-systolic pressure in Slc26a6−/− I/R mice is higher than that in WT I/R mice, even the end-systolic pressure in Slc26a6−/− mice is lower than that of WT mice. In addition, the dP/dt in WT I/R mice is significantly smaller compared to the sham group. However, the dP/dt in Slc26a6−/− I/R mice did not have significant changes compared to that of sham mice supporting the protective roles of ablation of Slc26a6 in left ventricle function in Slc26a6−/− I/R mice.
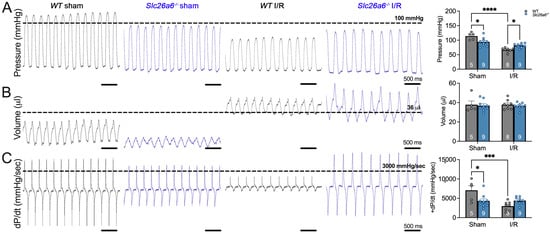
Figure 5.
Hemodynamic monitoring of the left ventricle function in WT and Slc26a6−/− I/R mice. (A) Representative traces of left ventricular pressures (left). The comparison of the systolic left ventricular pressures is shown on the right. Biological replicates or mouse numbers: WT sham (5); Slc26a6−/− sham (9); WT I/R (8); Slc26a6−/− I/R (9). (* p < 0.05, **** p < 0.0001 by one-way ANOVA combined with Tukey’s post hoc analyses). (B) Representative traces of left ventricular volumes (left). The comparison of the diastolic left ventricular volumes is shown on the right. Biological replicates or mouse numbers: WT sham (5); Slc26a6−/− Sham (9); WT I/R (8); Slc26a6−/− I/R (9). (C) Representative traces of left ventricular pressure development (derivative of pressure with respect to time, dP/dt). The comparison of the pressure development is shown on the right. Biological replicates or mouse numbers: WT sham (5); Slc26a6−/− sham (9); WT I/R (8); Slc26a6−/− I/R (9). (* p < 0.05, *** p < 0.001 by one-way ANOVA combined with Tukey’s post hoc analyses). The data points in bar figures show the animal number for different experiments.
3.5. Improved Sarcomere Contractility in Slc26a6−/− Cardiomyocytes from I/R Mice
The in vivo functional studies support the protective roles of Slc26a6−/− deletion in I/R injury. To further understand the cellular mechanisms under this finding, we isolated the cardiomyocytes from the remote and infarct zones of the left ventricle after long-term I/R challenges. We measured the sarcomere shortening in the cardiomyocytes from sham and I/R mice as shown in Figure 6A,B. The sarcomere shortening was quantified and compared in Figure 6C between WT and Slc26a6−/− cardiomyocytes from sham, I/R remote zone, and I/R infarct zone, respectively. We found that the sarcomere shortening is significantly smaller in the cardiomyocytes from the infarct zone, with a trend of reduction in the cardiomyocytes from the remote zone but without statistical significance. In addition, the sarcomere shortening is less reduced in the cardiomyocytes from the infarct zone of Slc26a6−/− I/R mice compared to that of WT I/R mice, further supporting the protective roles of Slc26a6−/− deletion in I/R injury at single cellular level. To reveal the mechanisms, we measured the calcium transient (CaT) in cardiomyocytes from sham and I/R mice as shown in Figure 6D,E. The quantifications and comparisons of CaT between WT and Slc26a6−/− cardiomyocytes from sham, I/R remote zone, and I/R infarct zone are shown in Figure 6F. We found significant reduction of CaT in cardiomyocytes from remote and infarct zones in WT I/R mice, but not in Slc26a6−/− I/R mice. Indeed, the CaT amplitudes in cardiomyocytes from Slc26a6−/− I/R mice are significantly higher than that of WT I/R mice, consistent with the less reduced sarcomere shortening in Slc26a6−/− I/R mice, as shown in Figure 6C. To demonstrate the contractility and cell shortening kinetics of the single cardiomyocyte, representative sarcomere contraction time-course traces at different conditions are shown in Supplemental Figure S2.
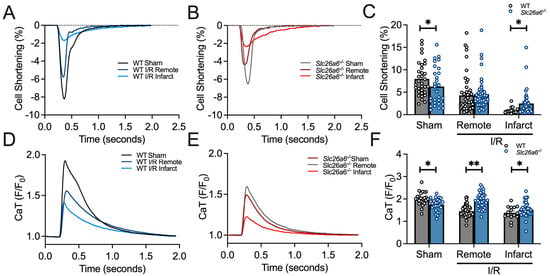
Figure 6.
Measurement and quantification of sarcomere contraction in WT and Slc26a6−/− I/R cardiomyocytes. (A,B) Representative traces of sarcomere shortening quantified by single cardiomyocyte recordings from WT, Slca6a6−/− I/R and sham mice. (C) Summary data of sarcomere shortening in cardiomyocytes isolated from remote and infarct zones of the heart from WT, Slc26a6−/− I/R, and sham mice. Biological replicates including cell numbers and mouse numbers: WT sham (34 cells from 12 mice); Slc26a6−/− sham (29 cells from 12 mice); WT I/R remote zone (42 cells from 12 mice); Slc26a6−/− I/R remote zone (45 cells from 12 mice); WT I/R infarct zone (16 cells from 12 mice); Slc26a6−/− I/R infarct zone (30 cells from 12 mice). Comparisons were performed between WT and Slc26a6−/− cardiomyocytes (* p < 0.05 by Welch’s t-test). (D,E) Representative traces of CaT quantified by single cardiomyocyte recordings from WT, Slc26a6−/− I/R and sham mice. (F) Summary data of CaT peak amplitude in cardiomyocytes isolated from remote and infarct zones of the heart from WT, Slc26a6−/− I/R and sham mice. Biological replicates including cell numbers and mouse numbers: WT sham (26 cells from 12 mice); Slc26a6−/− sham (32 cells from 12 mice); WT I/R remote zone (30 cells from 12 mice); Slc26a6−/− I/R remote zone (40 cells from 12 mice); WT I/R infarct zone (16 cells from 12 mice); Slc26a6−/− I/R infarct zone (30 cells from 12 mice). Comparisons were performed between WT and Slc26a6−/− cardiomyocytes (* p < 0.05, ** p < 0.01 by Welch’s t-test).
3.6. Elevated pHi in Slc26a6−/− Mouse Hearts
Slc26a6 is an acid loader in cardiomyocytes, and our previous study found that pHi is elevated in cardiomyocytes isolated from Slc26a6−/− mice [22]. We reasoned that the elevated pHi may play a role in the protection of I/R injury in Slc26a6−/− mice. To address this hypothesis, we developed an ex vivo confocal imaging technique to directly measure the epicardial pHi in a perfused heart. Mouse hearts were perfused by HCO3−-buffered Tyrode’s solution, gassed by 5% CO2 and 95% O2. We used a ratiometric pH dye, SNARF-1, as pHi indicator which was loaded into the heart by retrograde perfusion. During the measurement, the heart was perfused continuously. We found that the epicardial pHi of the heart can be well quantified by confocal imaging shown in Figure 7A, and the pHi in cardiomyocytes from Slc26a6−/− mouse heart is significantly higher than that from WT mice (Figure 7B), consistent with our cellular measurement reported before [22].
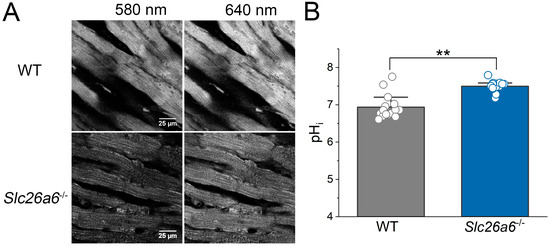
Figure 7.
Elevated pHi in Slc26a6−/− mouse hearts revealed by ex vivo pHi measurement using confocal imaging. (A) Representative confocal images of epicardial cells loaded with SNARF-1 from WT and Slc26a6−/− hearts. (B) Quantifications of epicardial pHi of WT and Slc26a6−/− mice. Biological replicates including cell numbers and mouse numbers: WT (15 cells from 6 mice); Slc26a6−/− (15 cells from 3 mice). (** p < 0.01 by two-sample t-test).
4. Discussion
Slc26a6 is the predominant Cl−/HCO3− and Cl−/OH− exchanger in the heart with significant roles in the regulation of cardiac action potentials, pHi, and cardiac function [21,22]. Slc26a6 serves as an acid loader in cardiomyocytes, responsible for transporting Cl− into the cells and HCO3− out of the cells, therefore, affecting the intracellular Cl− homeostasis and pHi. Ischemia is characterized by a large fall of pHi and extracellular pH (pHo) due to the lack of vascular perfusion with a significant increase in anaerobic metabolism and local partial pressure of CO2 [1,32,33,34]. Reperfusion normalizes the pHo followed by a recovery of pHi by activating NHE and NBC, resulting in increased intracellular Na+ and subsequent Ca2+ overload through the action of NCX.
Ablation of Slc26a6 significantly elevated the pHi, and may play critical roles during I/R. Indeed, we found that Slc26a6−/− I/R mice demonstrated reduced structural remodeling, less impaired cardiac function, improved sarcomere shortening, and increased CaT in the in vivo and in vitro studies, compared with the WT I/R mice. Our ex vivo confocal imaging experiments showed an elevated pHi in Slc26a6−/− mouse hearts supporting the important roles of pHi regulation in the protection of I/R injury. Therefore, targeting pHi regulatory mechanisms and the H+-equivalent transport proteins in cardiomyocytes may represent an effective strategy in preventing cardiac I/R injury.
4.1. I/R Injury
I/R injury is an ubiquitous pathological condition occurring in multiple organs. It is also a concern in organ and tissue transplantation procedures. In the heart, acute ischemia caused by coronary artery occlusions represents one of the leading causes of morbidity and mortality, and is the most common cause of chronic heart failure (HF) worldwide [35]. Malignant ventricular arrhythmia after acute myocardial infarction is the major cause of sudden cardiac death [36]. Myocardial necrosis and the adverse remodeling of myocardium are the results of I/R injury leading to endothelial dysfunction, microvascular injury, abnormal Ca2+ handling in myocardium, and altered myocardial metabolism and endogenous protective mechanisms [37,38,39]. Inflammation, oxidative stress, Ca2+ overloading, and mitochondrial dysfunction are the important mechanisms underlying I/R injury [35,36,37,40]. Despite the identification of multiple mechanisms and therapeutic strategies, the accumulated knowledge to date is not sufficient for the effective treatment of I/R injury. There are still critical knowledge gaps in the mechanistic understanding of myocardial I/R injury. The abnormal Ca2+ handling and endothelial and mitochondrial dysfunction are highly associated with ion transport including Na+, Ca2+, and H+ [41]. However, the mechanistic roles of HCO3− and Cl− are largely unknown. HCO3− and Cl− transport through ion channels and transporters is highly associated with cellular Ca2+ signaling and H+ homeostasis in the heart; we thus aimed to test the mechanistic roles of Slc26a6, a predominant cardiac Cl−/HCO3− and Cl−/OH− exchanger, in I/R injury. The significance is in that Cl−/HCO3− and Cl−/OH− exchange is tightly coupled with cardiac pH regulation, which is a key regulatory component in ischemia [2,42].
4.2. Abnormal pH Regulation in I/R
Myocardial ischemia is accompanied by a major decline in myocardial pH, resulting in depressed myocardial contractility, distorted cellular Ca2+ signaling, and cardiac arrhythmia [1,2,3,4,5]. Local acidosis is a hallmark of ischemia, resulting from increased anaerobic metabolism, lack of vascular perfusion, and an increase in local partial pressure of CO2. The significance of pH regulation lies in that the homeostasis and transport of several important ions including Na+, Ca2+, K+, Cl−, and HCO3− are highly dependent on pHi and pHo [1,6,43,44,45,46,47,48,49,50,51]. In addition, pHi and pHo regulate the function of contractile proteins and enzymatic activities of critical signaling pathways that are disrupted in I/R injury [32,35,36,38,40,52,53,54,55,56,57,58,59,60]. Therefore, dysregulation of pHi and pHo plays an important role in I/R injury. However, the molecular mechanisms of pH regulation in I/R are still not fully understood, and the specific molecular targets responsible for I/R injury have not been well studied and clearly identified. NHE and NBCs are Na+-dependent acid extruders responsible for pHi and intracellular Na+ regulations, affecting the intracellular Ca2+ homeostasis. The functional role of NHE has been extensively studied in regards to I/R injury, and the inhibition of NHE in I/R animal models has shown protective effects against injury through reductions of intracellular Ca2+ overload [6,61,62,63,64,65,66,67,68,69,70]. However, clinical trials using NHE1 inhibitors as cardioprotective agents during I/R proved to be disappointing and without significant benefits, and they even presented certain risks of increased incidence of stroke when higher doses of some agents were used [1,61,64,71]. The role of NBCs has also been studied. Inhibition of NBCe was reported to reduce I/R injury [72,73,74,75,76], and ablation of NBCe1 caused reduced apoptosis following I/R injury [77]. Due to the complicated regulatory mechanisms and lack of specific inhibitors of NBC, the mechanistic roles of NBC in I/R injury need to be further studied. As we and others reported previously, Slc26a6 is the predominant Cl−/HCO3− exchanger in the heart with significant roles in the regulation of cardiac excitability and pHi [20,21,78]. In this study, we took advantage of the Slc26a6−/− mouse model to investigate the specific roles of Slc26a6 in I/R injury. We found less injury in Slc26a6−/− mice, supporting the importance of Slc26a6 in I/R injury protection. By establishing a new confocal microscopy technique to measure epicardial pHi in an ex vivo heart model, we identified elevated pHi in Slc26a6−/− mouse heart. The elevated pHi in Slc26a6−/− mouse heart may counter-balance the acidification caused by ischemia to provide potential protection in I/R injury.
4.3. Role of Chloride Transporters in I/R Injury
In cardiomyocytes, one critical group of H+-equivalent transporters are chloride-coupled anion transporters, including AEs and CHEs. The AE family has three members, AE1, AE2, and AE3, with relative higher expression of AE3 in the heart [9,10,11,12]. AEs are electroneutral Cl−/HCO3− exchangers [13]. AE3 was reported to mediate pHi recovery from cellular alkalization [14], but ablation of AE3 did not affect cardiac function with no significant demonstrable roles in I/R injury [15]. AE3 knockdown in zebrafish increased cardiac pHi, shortened QT, and reduced systolic duration [19]. However, AE1, AE2, and AE3 are not the major Cl−/HCO3− exchangers in the heart; instead, Slc26a6 is the predominant Cl−/HCO3− and Cl−/OH− exchanger [20]. Our previous studies demonstrated that Slc26a6 is highly expressed in atrial and ventricular myocytes and mediates electrogenic Cl−/HCO3− exchange [21], while ablation of Slc26a6 causes action potential shortening, elevated pHi, fragmented QRS complexes, slower heart rate, and reduced cardiac function [22]. Slc26a6 represents a predominant Cl−/HCO3− exchanger in the heart with important roles in the regulation of cardiac excitability, pHi, and function. However, the mechanistic roles of Slc26a6 in cardiac I/R injury are unknown.
It was reported that intracellular Cl− activities increased in ventricular muscles during simulated ischemia [23], suggesting the important roles of enhanced chloride transport into cardiomyocytes in ischemia. Interestingly, inhibition of Cl−/HCO3− exchange was proposed to be a novel cardioprotective strategy against I/R injury [24]. Reduced intracellular Cl− activities by Cl− substitution produced protective effects against I/R injury, and inhibition of Cl− transport by 4-acetamido-4′-isothiocyanato-stilbene-2,2′-disulfonic acid (SITS) showed protective roles in I/R injury [24,27]. Moreover, blockade of transmembrane Cl− flux mitigates I/R injury via the inhibition of calpain activity [28].
Slc26a6 represents the predominant Cl−/HCO3− and Cl−/OH− exchanger in the heart responsible for cellular Cl− homeostasis and pHi regulation. Slc26a6 is an acid and chloride loader, facilitating inward chloride and H+ transport. Ischemia causes significant myocardium acidification with a large fall of pHi and pHo, and the reperfusion removes the acid and activates NHE and NBC for pHi recovery.
In the current study, we directly demonstrated that ablation of Slc26a6 reduced acid loading and increased pHi in cardiomyocytes [22] and in ex vivo hearts (Figure 7B), while the fall of cardiac pHi in Slc26a6−/− mice during ischemia may be ameliorated compared to WT mice. As a result, the recovery of pHi during reperfusion induces NHE activation to a lesser extent, and the disturbance to intracellular Na+ and Ca2+ is relatively smaller. Our data supported the important roles of elevated pHi in Slc26a6−/− mice, and this may represent one of the mechanisms for the reduced I/R injury in Slc26a6−/− mice. In addition, shortened action potentials and reduced CaT in Slc26a6−/− cardiomyocytes may reduce Ca2+ influx and intracellular Ca2+ overload during reperfusion, which may protect the heart from injury as well.
Moreover, the reduced chloride loading in Slc26a6−/− I/R mice may represent another potential mechanism. Increased intracellular Cl− activities in ischemia have been reported [23], and reduced intracellular Cl− activities by inhibition of Cl− transport or Cl− substitution both have protective effects on I/R injury [24,27,28]. The underlying mechanisms may be related to the coupled Cl−/HCO3− exchange, intracellular Ca2+ overload, attenuation of oxidative stress, inhibition of NF-kappaB activation [27], anion exchange stimulation through protein kinase C activation [24], and the inhibition of calpain activity [28]. Further mechanistic study is necessary for the in-depth understanding of the roles of Cl− in the regulation of cellular function in I/R.
5. Conclusions
Slc26a6 plays critical roles in the regulation of cardiac pHi, excitability, and contractility. Due to its essential role in acid loading in cardiomyocytes, it is a potential target for correcting the abnormal pHi in pathological conditions including I/R with dysregulations of pHo and pHi. Our study demonstrated that ablation of Slc26a6 affords cardioprotective effects from I/R injury, highlighting the importance of Cl− and pH regulation in I/R. Targeting Cl−/HCO3− exchange mediated by Slc26a6 and other transporters may represent a new therapeutic strategy in the management of I/R injury.
6. Future Studies
Our study revealed that the elevated pHi in Slc26a6−/− mouse heart may be a potential mechanism in mitigating I/R injury. However, more studies are needed to directly test the impact of pH modulation, including using the ex vivo I/R model for pHi measurement, Langendorff perfusion strategy, and force and pressure measurement. Importantly, the downstream molecular mechanisms are still not well defined, and more efforts are needed to test the expression and function of other H+ equivalent transporters and ion channels, as well as pH-sensitive processes and mitochondrial function in the heart. Moreover, platelets play a crucial role in the occurrence and development of myocardial ischemia/reperfusion injury [79,80]. Some SLC gene family members have been identified in platelets [81], but whether Slc26a6 is expressed in platelets has not been addressed. Slc26a6 mediated Cl−/HCO3− exchange may also be involved in the regulation of platelet function. Future studies will be performed to test whether Slc26a6 is expressed in platelets and how it regulates platelet function in I/R injury.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13122874/s1: Figure S1: Photomicrograph showing genotype analyses using RT-PCR for wild-type (WT), and Slc26a6−/− mice. Each line represents the result from one mouse. The expected sizes for the 2 bands are 400 bp and 250 bp for the WT and mutant alleles, respectively. Three sets of primers were used with 2 forward (F) primers for WT and mutant (KO) alleles and one reverse (R) primer for both WT and mutant alleles; Figure S2: The original time-course of sarcomere shortening recorded in WT and Slc26a6−/− cardiomyocytes isolated from sham control, I/R remote and I/R infarct zone. The duration between two shortening peaks is 2 seconds.
Author Contributions
X.-D.Z. conceived and designed the study, performed the experiments, data analyses and data interpretation, and wrote the manuscript; P.N.T., L.R., D.A.D., P.T., V.T. and R.Q.N. performed the experiments and data analyses; N.Z. managed and maintained the experimental mouse colonies, and helped on experiments; N.C. helped on experimental design and data interpretation; N.C., P.T., L.R. and N.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health (NIH) R56 HL138392, NIH R01 HL158961 and the American Heart Association (AHA) 23SFRNPCS1061606 (to X.-D.Z.); NIH R01 HL085727, HL085844, HL137228, HL149431, R35 HL166575, VA Merit Review Grant I01 BX000576 and I01 CX001490, AHA 23SFRNCCS1052478, 23SFRNPCS1060482 (to N.C.); NIH F32 Postdoctoral Fellowship HL149288, Harold S. Geneen Charitable Trust Awards Program for Coronary Heart Disease, and AHA CDA 24CDA1276831 (to P.N.T.); NIH F31 Predoctoral Fellowship HL168956 (to D.A.D.) and NIH F31 Predoctoral Fellowship HL170698 (to P.T.).
Institutional Review Board Statement
The animal study protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Protocol #22455 with an approval date of 21 September 2023; Protocol #24053 with an approval date of 22 August 2024; Protocol #24045 with an approval date of 3 September 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the current study have not been deposited in a public repository because they have only been used for the publication of this manuscript, but are available from the corresponding author on request.
Acknowledgments
We thank Peter S. Aronson (Yale University) for his kind gift of Slc26a6 knockout mice.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| I/R | Ischemia/reperfusion |
| WT | Wild type |
| CaT | Calcium transient |
| AE | Anion exchanger |
| NHE | Na+/H+ exchanger |
| NBC | Na+-HCO3− cotransporter |
| CHE | Cl−/OH− exchanger |
| MCT | Monocarboxylate transporter |
| CBE | Cl−/HCO3− exchangers |
| SLC | Solute carrier |
| ECG | Electrocardiogram |
| LAD | Left anterior descending |
| LV | Left ventricle |
| EDD | End diastolic dimension |
| ESD | End systolic dimension |
| FS | Fractional shortening |
| EF | Ejection Fraction |
| FFT | Fast Fourier transform |
| SR | Sarcoplasmic reticulum |
| HF | Heart failure |
References
- Vaughan-Jones, R.D.; Spitzer, K.W.; Swietach, P. Intracellular pH regulation in heart. J. Mol. Cell Cardiol. 2009, 46, 318–331. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, Y.; Vairamani, K.; Shull, G.E. Critical role of bicarbonate and bicarbonate transporters in cardiac function. World J. Biol. Chem. 2014, 5, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, A.; Di Mattia, R.A.; Aiello, E.A. Intracellular pH Regulation in Ventricular Myocytes: Implications for Cardiac Health and Disease. Circ. Res. 2025, 136, 1636–1656. [Google Scholar] [CrossRef]
- Milliken, A.S.; Ciesla, J.H.; Nadtochiy, S.M.; Brookes, P.S. Distinct effects of intracellular vs. extracellular acidic pH on the cardiac metabolome during ischemia and reperfusion. J. Mol. Cell Cardiol. 2023, 174, 101–114. [Google Scholar] [CrossRef]
- Gonzalez Arbelaez, L.F.; Ciocci Pardo, A.; Burgos, J.I.; Vila Petroff, M.G.; Godoy Coto, J.; Ennis, I.L.; Mosca, S.M.; Fantinelli, J.C. New advances in the protective mechanisms of acidic pH after ischemia: Participation of NO. Arch. Biochem. Biophys. 2024, 758, 110059. [Google Scholar] [CrossRef]
- Allen, D.G.; Xiao, X.H. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc. Res. 2003, 57, 934–941. [Google Scholar] [CrossRef]
- Vandenberg, J.I.; Metcalfe, J.C.; Grace, A.A. Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ. Res. 1993, 72, 993–1003. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wang, X.; Poole, R.C.; Jackson, V.N.; Price, N.T. Lactate transport in heart in relation to myocardial ischemia. Am. J. Cardiol. 1997, 80, 17A–25A. [Google Scholar] [CrossRef]
- Kopito, R.R.; Lee, B.S.; Simmons, D.M.; Lindsey, A.E.; Morgans, C.W.; Schneider, K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell 1989, 59, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kudrycki, K.E.; Newman, P.R.; Shull, G.E. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl−/HCO3− exchanger. J. Biol. Chem. 1990, 265, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Linn, S.C.; Kudrycki, K.E.; Shull, G.E. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/HCO3− exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J. Biol. Chem. 1992, 267, 7927–7935. [Google Scholar] [PubMed]
- Yannoukakos, D.; Stuart-Tilley, A.; Fernandez, H.A.; Fey, P.; Duyk, G.; Alper, S.L. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ. Res. 1994, 75, 603–614. [Google Scholar] [CrossRef]
- Alper, S.L. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 2009, 212 Pt 11, 1672–1683. [Google Scholar] [CrossRef]
- Chiappe de Cingolani, G.E.; Ennis, I.L.; Morgan, P.E.; Alvarez, B.V.; Casey, J.R.; Camilion de Hurtado, M.C. Involvement of AE3 isoform of Na+-independent Cl−/HCO3− exchanger in myocardial pH(i) recovery from intracellular alkalization. Life Sci. 2006, 78, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Bodi, I.; Meyer, J.W.; Wang, Y.; Ashraf, M.; Engle, S.J.; Doetschman, T.; Sisco, K.; Nieman, M.L.; Miller, M.L.; et al. Impaired cardiac contractility in mice lacking both the AE3 Cl−/HCO3− exchanger and the NKCC1 Na+-K+-2Cl− cotransporter: Effects on Ca2+ handling and protein phosphatases. J. Biol. Chem. 2008, 283, 31303–31314. [Google Scholar] [CrossRef]
- Al Moamen, N.J.; Prasad, V.; Bodi, I.; Miller, M.L.; Neiman, M.L.; Lasko, V.M.; Alper, S.L.; Wieczorek, D.F.; Lorenz, J.N.; Shull, G.E. Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure. J. Mol. Cell Cardiol. 2011, 50, 137–146. [Google Scholar] [CrossRef]
- Chen, H.P.; He, M.; Mei, Z.J.; Huang, Q.R.; Peng, W.; Huang, M. Anion exchanger 3 is required for sasanquasaponin to inhibit ischemia/reperfusion-induced elevation of intracellular Cl− concentration and to elicit cardioprotection. J. Cell Biochem. 2011, 112, 2803–2812. [Google Scholar] [CrossRef]
- Vairamani, K.; Wang, H.S.; Medvedovic, M.; Lorenz, J.N.; Shull, G.E. RNA SEQ Analysis Indicates that the AE3 Cl−/HCO3− Exchanger Contributes to Active Transport-Mediated CO2 Disposal in Heart. Sci. Rep. 2017, 7, 7264. [Google Scholar] [CrossRef]
- Thorsen, K.; Dam, V.S.; Kjaer-Sorensen, K.; Pedersen, L.N.; Skeberdis, V.A.; Jurevicius, J.; Treinys, R.; Petersen, I.; Nielsen, M.S.; Oxvig, C.; et al. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat. Commun. 2017, 8, 1696. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Kieller, D.M.; Quon, A.L.; Markovich, D.; Casey, J.R. Slc26a6: A cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J. Physiol. 2004, 561 Pt 3, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Myers, R.; Sihn, C.R.; Rafizadeh, S.; Zhang, X.D. Slc26a6 functions as an electrogenic Cl/HCO3 exchanger in cardiac myocytes. Cardiovasc. Res. 2013, 100, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sirish, P.; Ledford, H.A.; Timofeyev, V.; Thai, P.N.; Ren, L.; Kim, H.J.; Park, S.; Lee, J.H.; Dai, G.; Moshref, M.; et al. Action Potential Shortening and Impairment of Cardiac Function by Ablation of Slc26a6. Circ. Arrhythm. Electrophysiol. 2017, 10, e005267. [Google Scholar] [CrossRef]
- Lai, Z.F.; Nishi, K. Intracellular chloride activity increases in guinea pig ventricular muscle during simulated ischemia. Am. J. Physiol. 1998, 275 Pt 2, H1613–H1619. [Google Scholar] [CrossRef]
- Kawasaki, H.; Otani, H.; Mishima, K.; Imamura, H.; Inagaki, C. Involvement of anion exchange in the hypoxia/reoxygenation-induced changes in pH(i) and [Ca2+]i in cardiac myocyte. Eur. J. Pharmacol. 2001, 411, 35–43. [Google Scholar] [CrossRef]
- Ridley, P.D.; Curtis, M.J. Anion manipulation: A new antiarrhythmic approach. Action of substitution of chloride with nitrate on ischemia- and reperfusion-induced ventricular fibrillation and contractile function. Circ. Res. 1992, 70, 617–632. [Google Scholar] [CrossRef]
- Curtis, M.J.; Garlick, P.B.; Ridley, P.D. Anion manipulation, a novel antiarrhythmic approach: Mechanism of action. J. Mol. Cell Cardiol. 1993, 25, 417–436. [Google Scholar] [CrossRef]
- Liu, D.; He, H.; Li, G.L.; Chen, J.; Yin, D.; Liao, Z.P.; Tang, L.; Huang, Q.R.; Lai, Z.F.; He, M. Mechanisms of chloride in cardiomyocyte anoxia-reoxygenation injury: The involvement of oxidative stress and NF-kappaB activation. Mol. Cell Biochem. 2011, 355, 201–209. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wu, F.; Gu, X.M.; Jin, Z.X.; Kong, L.H.; Zhang, Y.; Zhou, J.J.; Gao, F. The Blockade of Transmembrane Cl− Flux Mitigates I/R-Induced Heart Injury via the Inhibition of Calpain Activity. Cell Physiol. Biochem. 2015, 35, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Asplin, J.R.; Evan, A.P.; Rajendran, V.M.; Velazquez, H.; Nottoli, T.P.; Binder, H.J.; Aronson, P.S. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat. Genet. 2006, 38, 474–478. [Google Scholar] [CrossRef]
- Sirish, P.; Thai, P.N.; Lee, J.H.; Yang, J.; Zhang, X.D.; Ren, L.; Li, N.; Timofeyev, V.; Lee, K.S.S.; Nader, C.E.; et al. Suppression of inflammation and fibrosis using soluble epoxide hydrolase inhibitors enhances cardiac stem cell-based therapy. Stem Cells Transl. Med. 2020, 9, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Thai, P.N.; Ren, L.; Timofeyev, V.; Jian, Z.; Park, S.; Ginsburg, K.S.; Overton, J.; Bossuyt, J.; Bers, D.M.; et al. Beat-to-beat dynamic regulation of intracellular pH in cardiomyocytes. iScience 2022, 25, 103624. [Google Scholar] [CrossRef]
- Steenbergen, C.; Deleeuw, G.; Rich, T.; Williamson, J.R. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ. Res. 1977, 41, 849–858. [Google Scholar] [CrossRef]
- Garlick, P.B.; Radda, G.K.; Seeley, P.J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem. J. 1979, 184, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Cascio, W.E.; Yan, G.X.; Kleber, A.G. Early changes in extracellular potassium in ischemic rabbit myocardium. The role of extracellular carbon dioxide accumulation and diffusion. Circ. Res. 1992, 70, 409–422. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Clasen, L.; Angendohr, S.; Becher, S.; Bartsch, B.; Enkel, S.; Meyer, C.; Kelm, M.; Makimoto, H.; Klocker, N. Cardiac ischemia and reperfusion in mice: A comprehensive hemodynamic, electrocardiographic and electrophysiological characterization. Sci. Rep. 2023, 13, 5693. [Google Scholar] [CrossRef]
- Verma, S.; Fedak, P.W.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.K.; Dhillon, B.; Yau, T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Galagudza, M.M.; Blokhin, I.O.; Shmonin, A.A.; Mischenko, K.A. Reduction of myocardial ischemia-reperfusion injury with pre- and postconditioning: Molecular mechanisms and therapeutic targets. Cardiovasc. Hematol. Disord. Drug Targets 2008, 8, 47–65. [Google Scholar] [CrossRef]
- Samaja, M.; Pagliaro, P. Editorial Commentary: Long and narrow road to win over myocardial ischemia-reperfusion injury. Trends Cardiovasc. Med. 2023, 33, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Lujain Alsadder, A.H. Cardiac Ischaemia–Reperfusion Injury: Pathophysiology, Therapeutic Targets and Future Interventions. Biomedicine 2025, 13, 2084. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J.H. The Fundamental Role of Bicarbonate Transporters and Associated Carbonic Anhydrase Enzymes in Maintaining Ion and pH Homeostasis in Non-Secretory Organs. Int. J. Mol. Sci. 2020, 21, 339. [Google Scholar] [CrossRef]
- Komukai, K.; Brette, F.; Pascarel, C.; Orchard, C.H. Electrophysiological response of rat ventricular myocytes to acidosis. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H412–H422. [Google Scholar] [CrossRef]
- Jones, D.K.; Peters, C.H.; Tolhurst, S.A.; Claydon, T.W.; Ruben, P.C. Extracellular proton modulation of the cardiac voltage-gated sodium channel, Nav1.5. Biophys. J. 2011, 101, 2147–2156. [Google Scholar] [CrossRef]
- Saegusa, N.; Moorhouse, E.; Vaughan-Jones, R.D.; Spitzer, K.W. Influence of pH on Ca2+ current and its control of electrical and Ca2+ signaling in ventricular myocytes. J. Gen. Physiol. 2011, 138, 537–559. [Google Scholar] [CrossRef]
- Doering, A.E.; Lederer, W.J. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J. Physiol. 1993, 466, 481–499. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Kim, B.; Olcese, R.; Goldhaber, J.I.; Ottolia, M. Molecular determinants of pH regulation in the cardiac Na+-Ca2+ exchanger. J. Gen. Physiol. 2018, 150, 245–257. [Google Scholar] [CrossRef]
- Boyman, L.; Hagen, B.M.; Giladi, M.; Hiller, R.; Lederer, W.J.; Khananshvili, D. Proton-sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger. J. Biol. Chem. 2011, 286, 28811–28820. [Google Scholar] [CrossRef] [PubMed]
- Garciarena, C.D.; Youm, J.B.; Swietach, P.; Vaughan-Jones, R.D. H+-activated Na+ influx in the ventricular myocyte couples Ca2+-signalling to intracellular pH. J. Mol. Cell Cardiol. 2013, 61, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Jones, R.D. Cross-talking. Ca2+, H+ and nitric oxide. J. Physiol. 2014, 592, 3177–3178. [Google Scholar] [CrossRef]
- Swietach, P.; Spitzer, K.W.; Vaughan-Jones, R.D. Na+ ions as spatial intracellular messengers for co-ordinating Ca2+ signals during pH heterogeneity in cardiomyocytes. Cardiovasc. Res. 2015, 105, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A.; Fabiato, F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J. Physiol. 1978, 276, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Bountra, C.; Vaughan-Jones, R.D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J. Physiol. 1989, 418, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Jones, R.D.; Wu, M.L.; Bountra, C. Sodium-hydrogen exchange and its role in controlling contractility during acidosis in cardiac muscle. Mol. Cell Biochem. 1989, 89, 157–162. [Google Scholar] [CrossRef]
- Orchard, C.H.; Kentish, J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 1990, 258, C967–C981. [Google Scholar] [CrossRef]
- Harrison, S.M.; Frampton, J.E.; McCall, E.; Boyett, M.R.; Orchard, C.H. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am. J. Physiol. 1992, 262 Pt 1, C348–C357. [Google Scholar] [CrossRef]
- Wattanapermpool, J.; Reiser, P.J.; Solaro, R.J. Troponin I isoforms and differential effects of acidic pH on soleus and cardiac myofilaments. Am. J. Physiol. 1995, 268, C323–C330. [Google Scholar] [CrossRef]
- Yan, G.X.; Kleber, A.G. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ. Res. 1992, 71, 460–470. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Crampin, E.J.; Smith, N.P.; Langham, A.E.; Clayton, R.H.; Orchard, C.H. Acidosis in models of cardiac ventricular myocytes. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2006, 364, 1171–1186. [Google Scholar] [CrossRef]
- Theroux, P.; Chaitman, B.R.; Danchin, N.; Erhardt, L.; Meinertz, T.; Schroeder, J.S.; Tognoni, G.; White, H.D.; Willerson, J.T.; Jessel, A. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) Investigators. Circulation 2000, 102, 3032–3038. [Google Scholar] [CrossRef]
- Karmazyn, M. Mechanisms of protection of the ischemic and reperfused myocardium by sodium-hydrogen exchange inhibition. J. Thromb. Thrombolysis 1999, 8, 33–38. [Google Scholar] [CrossRef]
- Karmazyn, M.; Sostaric, J.V.; Gan, X.T. The myocardial Na+/H+ exchanger: A potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs 2001, 61, 375–389. [Google Scholar] [CrossRef]
- Avkiran, M.; Marber, M.S. Na+/H+ exchange inhibitors for cardioprotective therapy: Progress, problems and prospects. J. Am. Coll. Cardiol. 2002, 39, 747–753. [Google Scholar] [CrossRef]
- Yellon, D.M.; Baxter, G.F. Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: Distant dream or near reality? Heart 2000, 83, 381–387. [Google Scholar] [CrossRef]
- Scholz, W.; Albus, U.; Counillon, L.; Gogelein, H.; Lang, H.J.; Linz, W.; Weichert, A.; Scholkens, B.A. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc. Res. 1995, 29, 260–268. [Google Scholar] [CrossRef]
- Jung, I.S.; Lee, S.H.; Yang, M.K.; Park, J.W.; Yi, K.Y.; Yoo, S.E.; Kwon, S.H.; Chung, H.J.; Choi, W.S.; Shin, H.S. Cardioprotective effects of the novel Na+/H+ exchanger-1 inhibitor KR-32560 in a perfused rat heart model of global ischemia and reperfusion: Involvement of the Akt-GSK-3beta cell survival pathway and antioxidant enzyme. Arch. Pharm. Res. 2010, 33, 1241–1251. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, F.; Xia, Q. Cariporide attenuates myocardial ischaemia, reperfusion injury and apoptosis in isolated rat hearts. Acta Cardiol. 2006, 61, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.H.; Long, C.; Liu, J.; Liu, B. Inhibition of the Na+/H+ exchanger protects the immature rabbit myocardium from ischemia and reperfusion injury. Pediatr. Cardiol. 2008, 29, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moore, P.G. KATP channel blocker does not abolish the protective effect of Na+/H+ exchange 1 inhibition against ischaemia/reperfusion in aged myocardium. Eur. J. Anaesthesiol. 2010, 27, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Avkiran, M.; Cook, A.R.; Cuello, F. Targeting Na+/H+ exchanger regulation for cardiac protection: A RSKy approach? Curr. Opin. Pharmacol. 2008, 8, 133–140. [Google Scholar] [CrossRef]
- Fantinelli, J.C.; Orlowski, A.; Aiello, E.A.; Mosca, S.M. The electrogenic cardiac sodium bicarbonate co-transporter (NBCe1) contributes to the reperfusion injury. Cardiovasc. Pathol. 2014, 23, 224–230. [Google Scholar] [CrossRef]
- Lu, M.; Jia, M.; Wang, Q.; Guo, Y.; Li, C.; Ren, B.; Qian, F.; Wu, J. The electrogenic sodium bicarbonate cotransporter and its roles in the myocardial ischemia-reperfusion induced cardiac diseases. Life Sci. 2021, 270, 119153. [Google Scholar] [CrossRef]
- Yamamoto, T.; Swietach, P.; Rossini, A.; Loh, S.H.; Vaughan-Jones, R.D.; Spitzer, K.W. Functional diversity of electrogenic Na+-HCO3− cotransport in ventricular myocytes from rat, rabbit and guinea pig. J. Physiol. 2005, 562 Pt 2, 455–475. [Google Scholar] [CrossRef]
- Khandoudi, N.; Albadine, J.; Robert, P.; Krief, S.; Berrebi-Bertrand, I.; Martin, X.; Bevensee, M.O.; Boron, W.F.; Bril, A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc. Res. 2001, 52, 387–396. [Google Scholar] [CrossRef]
- Ch’en, F.F.; Villafuerte, F.C.; Swietach, P.; Cobden, P.M.; Vaughan-Jones, R.D. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br. J. Pharmacol. 2008, 153, 972–982. [Google Scholar] [CrossRef]
- Vairamani, K.; Prasad, V.; Wang, Y.; Huang, W.; Chen, Y.; Medvedovic, M.; Lorenz, J.N.; Shull, G.E. NBCe1 Na+-HCO3− cotransporter ablation causes reduced apoptosis following cardiac ischemia-reperfusion injury in vivo. World J. Cardiol. 2018, 10, 97–109. [Google Scholar] [CrossRef]
- Aiello, E.A.; Casey, J.R.; Alvarez, B.V. Cl−/HCO3− Exchanger slc26a6: A pH Regulator Shapes the Cardiac Action Potential. Circ. Arrhythm. Electrophysiol. 2017, 10, e005812. [Google Scholar] [CrossRef]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar] [CrossRef]
- Voronkov, N.S.; Maslov, L.N.; Vyshlov, E.V.; Mukhomedzyanov, A.V.; Ryabov, V.V.; Derkachev, I.A.; Kan, A.; Gusakova, S.V.; Gombozhapova, A.E.; Panteleev, O.O. Do platelets protect the heart against ischemia/reperfusion injury or exacerbate cardiac ischemia/reperfusion injury? The role of PDGF, VEGF, and PAF. Life Sci. 2024, 347, 122617. [Google Scholar] [CrossRef]
- Jedlitschky, G.; Greinacher, A.; Kroemer, H.K. Transporters in human platelets: Physiologic function and impact for pharmacotherapy. Blood 2012, 119, 3394–3402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).