Assessment of T1 and T2 Relaxation-Time Changes in NMIBC Tissue After 5-ALA Photodynamic Therapy Using Quantitative Magnetic Resonance Imaging
Abstract
1. Introduction
1.1. Epidemiology of NMIBC and Clinical Classification
1.2. Modern Diagnostic Methods and Treatment
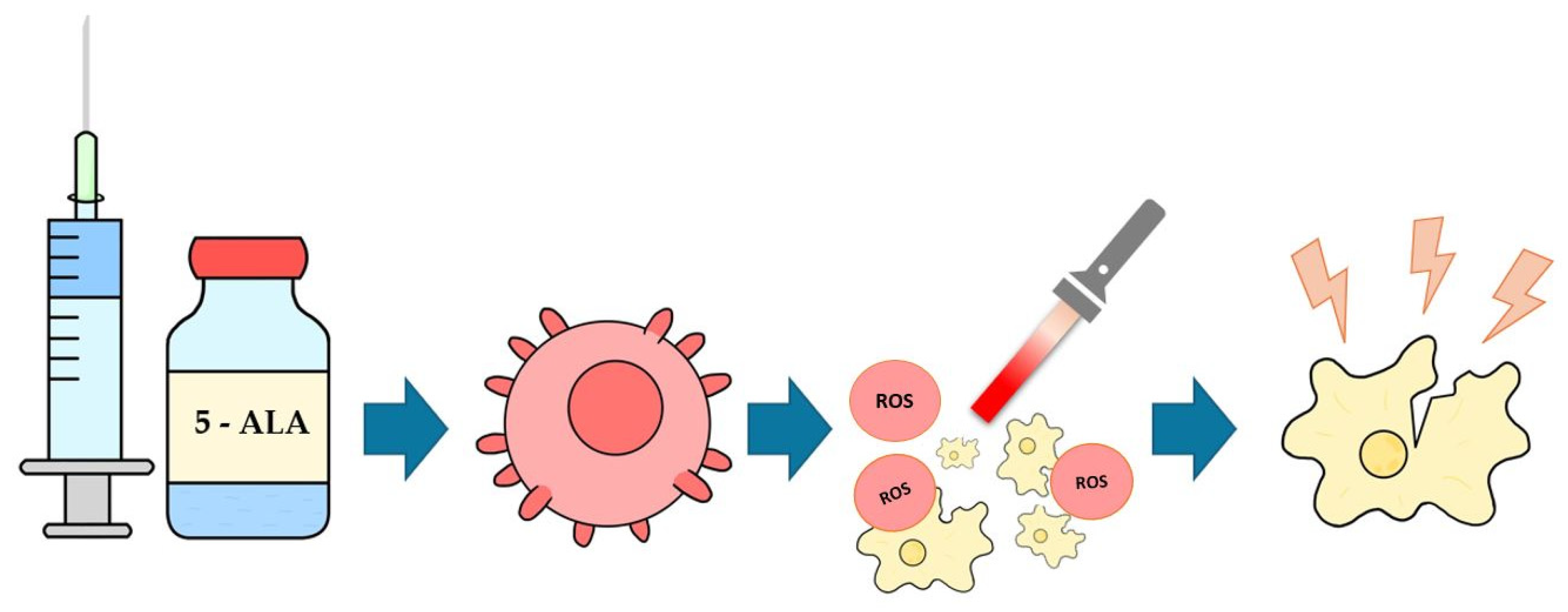
1.3. Photodynamic Therapy and 5-ALA Mechanism of Action
1.4. Magnetic Resonance Imaging a Tool for Therapeutic Response
2. Materials and Methods
2.1. Tissue Collection of NMIBC and Healthy Bladder
2.2. Sample Preparation
2.3. Photosensitizer
2.4. Photodynamic Protocol
2.5. Preparation for MRI
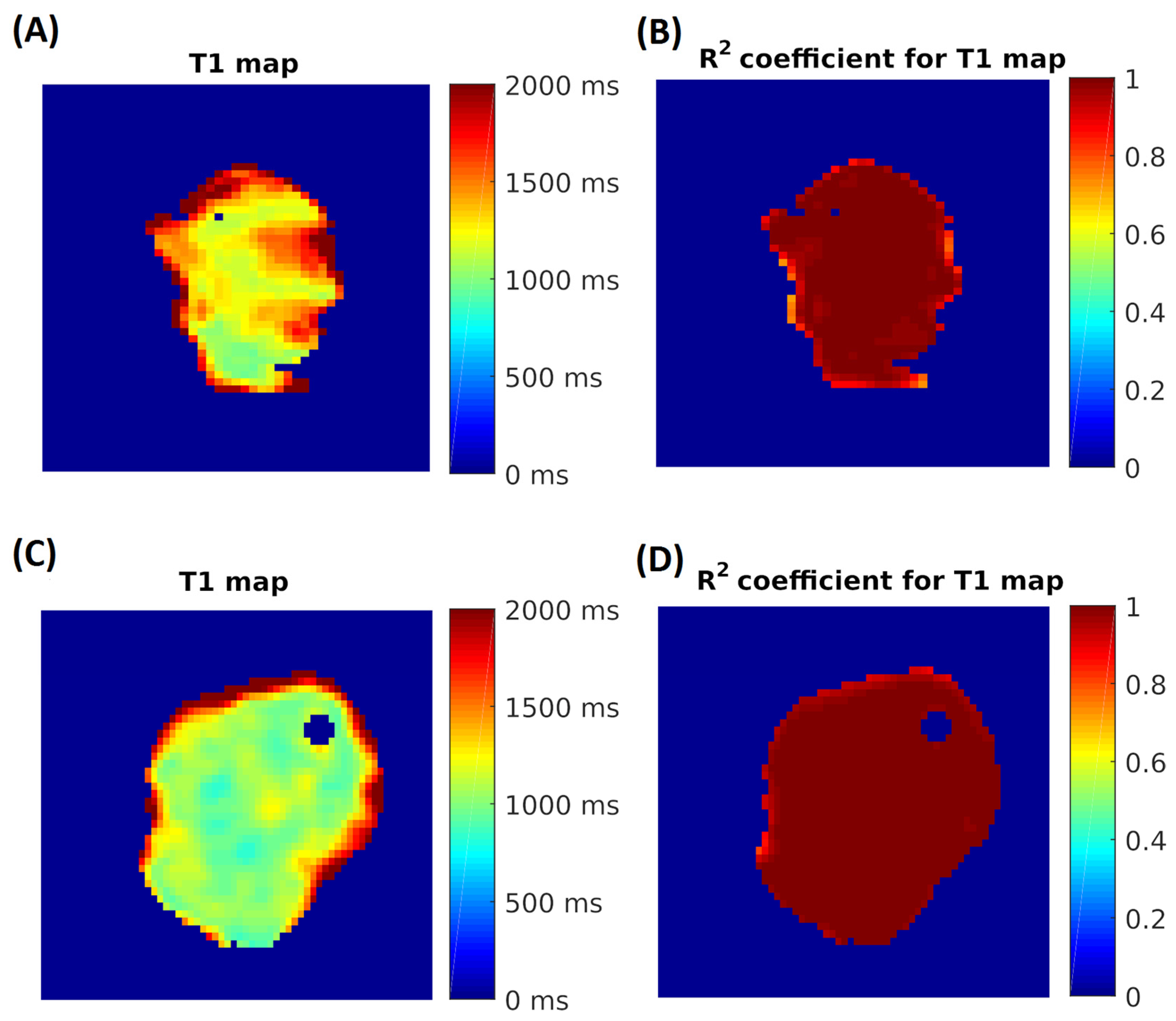
2.6. MRI Analysis of NMIBC and Bladder Tissue Samples
2.7. Statistical Analysis
3. Results
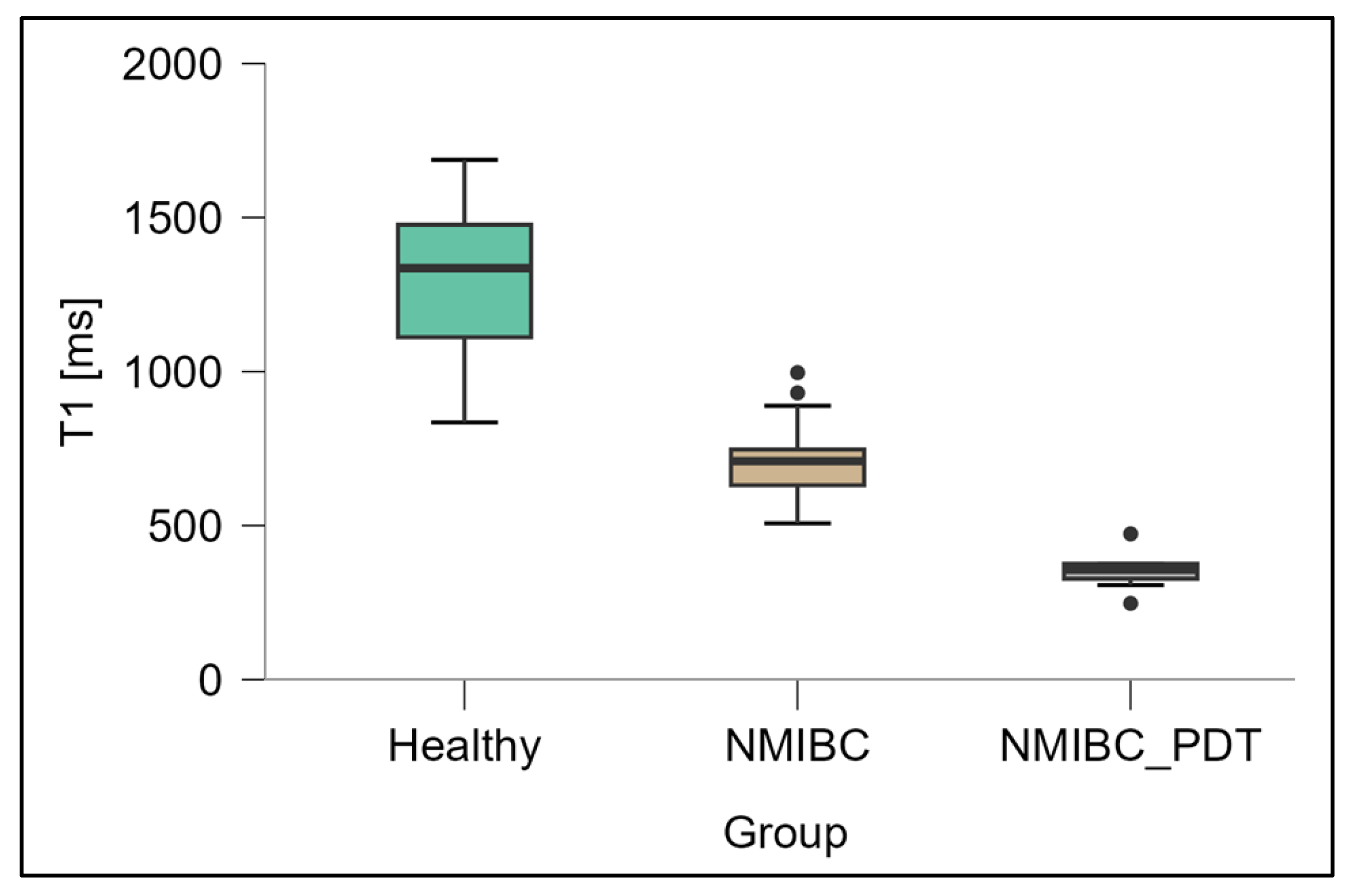
3.1. Measurement of T1 Relaxation Times
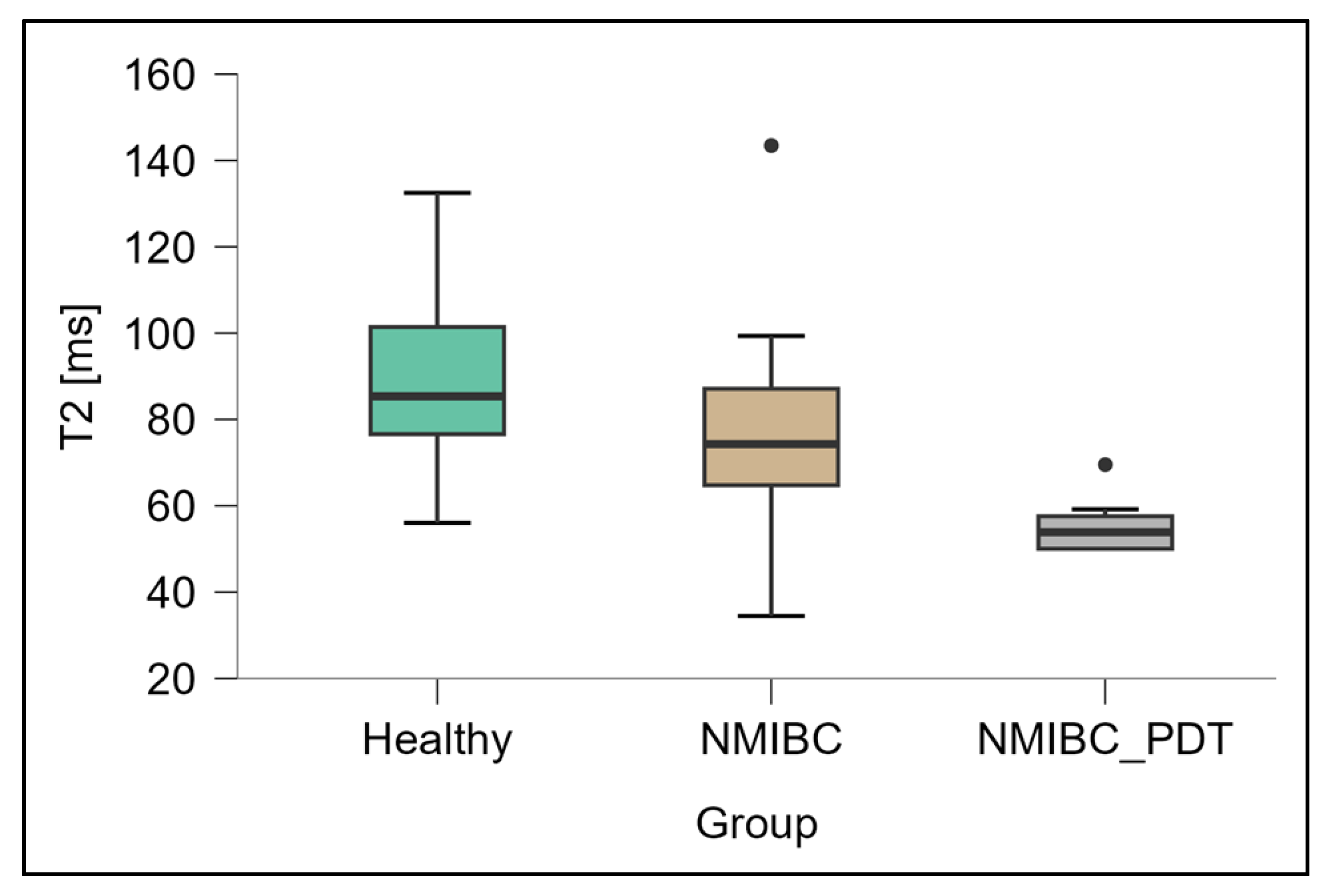
3.2. Measurements of T2 Relaxation Times
4. Discussion
4.1. Validation of Ex Vivo Finding in Clinical Settings
Future Challenges
4.2. Integration of Relaxometry with Broader Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ploeg, M.; Aben, K.K.; Hulsbergen-van de Kaa, C.A.; Schoenberg, M.P.; Witjes, J.A.; Kiemeney, L.A. Clinical Epidemiology of Nonurothelial Bladder Cancer: Analysis of the Netherlands Cancer Registry. J. Urol. 2010, 183, 915–920. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Aeddula, N.R. Bladder Cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Soares, A.; Bourlon, M.T.; Wong, A.; Joshi, A.; Jardim, D.; Korbenfeld, E.; Karak, F.E.; Orlandi, F.; Sze, H.; Ansari, J.; et al. Management of Metastatic Urothelial Carcinoma in Emerging Markets (EM): An Expert Opinion. Clin. Genitourin. Cancer 2024, 22, 467–475. [Google Scholar] [CrossRef]
- Aldousari, S.; Kassouf, W. Update on the Management of Non-Muscle Invasive Bladder Cancer. Can. Urol. Assoc. J. 2010, 4, 56–64. [Google Scholar] [CrossRef]
- Gontero, P.; Birtle, A.; Capoun, O.; Compérat, E.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Masson-Lecomte, A.; Mostafid, H.A.; Pradere, B.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—A Summary of the 2024 Guidelines Update. Eur. Urol. 2024, 86, 531–549. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M.; et al. The TNM Classification of Malignant Tumours—Towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Anai, S.; Fujimoto, K.; Hirao, Y.; Furuse, H.; Kai, F.; Ozono, S.; Hara, T.; Matsuyama, H.; Oyama, M.; et al. Oral 5-Aminolevulinic Acid-Mediated Photodynamic Diagnosis Using Fluorescence Cystoscopy for Non-Muscle-Invasive Bladder Cancer: A Randomized, Double-Blind, Multicentre Phase II/III Study. Photodiagn. Photodyn. Ther. 2015, 12, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in Bladder Cancer Biology and Therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Rabien, A.; Rong, D.; Rabenhorst, S.; Schlomm, T.; Labonté, F.; Hofbauer, S.; Forey, N.; Le Calvez-Kelm, F.; Ecke, T.H. Diagnostic Performance of Uromonitor and TERTpm ddPCR Urine Tests for the Non-Invasive Detection of Bladder Cancer. Sci. Rep. 2024, 14, 30617. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.; Keane, K.G.; Gauci, R.; Hayne, D. Nuclear Medicine and Molecular Imaging in Urothelial Cancer: Current Status and Future Directions. Cancers 2025, 17, 232. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Soria, F.; Marra, G.; D’Andrea, D.; Gontero, P.; Shariat, S.F. The Rational and Benefits of the Second Look Transurethral Resection of the Bladder for T1 High Grade Bladder Cancer. Transl. Androl. Urol. 2019, 8, 46–53. [Google Scholar] [CrossRef]
- Liatsos, G.D.; Mariolis, I.; Hadziyannis, E.; Bamias, A.; Vassilopoulos, D. Review of BCG Immunotherapy for Bladder Cancer. Clin. Microbiol. Rev. 2025, 38, e00194-23. [Google Scholar] [CrossRef] [PubMed]
- Scilipoti, P.; Ślusarczyk, A.; de Angelis, M.; Soria, F.; Pradere, B.; Krajewski, W.; D’Andrea, D.; Mari, A.; Giudice, F.D.; Pichler, R.; et al. The Role of Mitomycin C in Intermediate-Risk Non–Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Oncol. 2024, 7, 1293–1302. [Google Scholar] [CrossRef]
- Chou, R.; Selph, S.; Buckley, D.I.; Fu, R.; Griffin, J.C.; Grusing, S.; Gore, J.L. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Roumiguié, M.; Kamat, A.M.; Shore, N.D.; Boormans, J.L.; Esen, A.A.; Lebret, T.; Kandori, S.; Bajorin, D.F.; Krieger, L.E.M.; et al. Pembrolizumab Monotherapy for High-Risk Non–Muscle-Invasive Bladder Cancer without Carcinoma in Situ and Unresponsive to BCG (KEYNOTE-057): A Single-Arm, Multicentre, Phase 2 Trial. Lancet Oncol. 2024, 25, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, R.S.; Steinberg, G.D. Non–Muscle-Invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar] [PubMed]
- Waidelich, R.; Stepp, H.; Baumgartner, R.; Weninger, E.; Hofstetter, A.; Kriegmair, M. Clinical Experience with 5-Aminolevulinic Acid and Photodynamic Therapy for Refractory Superficial Bladder Cancer. J. Urol. 2001, 165, 1904–1907. [Google Scholar] [CrossRef]
- Seo, K.W.; Kim, B.H.; Park, C.H.; Kim, C.I.; Chang, H.S. The Efficacy of the EORTC Scoring System and Risk Tables for the Prediction of Recurrence and Progression of Non–Muscle-Invasive Bladder Cancer after Intravesical Bacillus Calmette–Guérin Instillation. Korean J. Urol. 2010, 51, 165–170. [Google Scholar] [CrossRef][Green Version]
- Bansal, A.; Sankhwar, S.; Goel, A.; Kumar, M.; Purkait, B.; Aeron, R. Grading of Complications of Transurethral Resection of Bladder Tumor Using Clavien–Dindo Classification System. Indian J. Urol. 2016, 32, 232–237. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.G.; Witjes, J.A. Recurrence, Progression, and Follow-Up in Non–Muscle-Invasive Bladder Cancer. Eur. Urol. Suppl. 2009, 8, 556–562. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Oosterlinck, W.; van der Meijden, A.P. A Single Immediate Postoperative Instillation of Chemotherapy Decreases the Risk of Recurrence in Patients with Stage Ta T1 Bladder Cancer: A Meta-Analysis of Published Results of Randomized Clinical Trials. J. Urol. 2004, 171, 2186–2190. [Google Scholar] [CrossRef]
- Filson, C.P.; Montgomery, J.S.; Dailey, S.M.; Crossley, H.S.; Lentz, H.; Tallman, C.T.; He, C.; Weizer, A.Z. Complications Associated with Single-Dose, Perioperative Mitomycin-C for Patients Undergoing Bladder Tumor Resection. Urol. Oncol. 2014, 32, 40.e1–40.e8. [Google Scholar] [CrossRef]
- Decaestecker, K.; Oosterlinck, W. Managing the Adverse Events of Intravesical Bacillus Calmette–Guérin Therapy. Res. Rep. Urol. 2015, 7, 157–163. [Google Scholar] [CrossRef]
- Malmström, P.U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette–Guérin for Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab Monotherapy for the Treatment of High-Risk Non–Muscle-Invasive Bladder Cancer Unresponsive to BCG (KEYNOTE-057): An Open-Label, Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Li, H.; Long, G.; Tian, J. Efficacy and Safety of Photodynamic Therapy for Non–Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2023, 13, 1255632. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic Therapy for Cancer: Role of Natural Products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Huang, Z.; Dallimore, I.; Moghissi, K. Tools of Clinical Photodynamic Therapy (PDT): A Mini Compendium. Photodiagn. Photodyn. Ther. 2024, 46, 104058. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Khaleghi Ghadiri, M.; Stummer, W.; Gorji, A. Enhancing 5-ALA-PDT Efficacy against Resistant Tumor Cells: Strategies and Advances. Life Sci. 2024, 351, 122808. [Google Scholar] [CrossRef]
- Fratz, E.J.; Hunter, G.A.; Ferreira, G.C. Expression of Murine 5-Aminolevulinate Synthase Variants Causes Protoporphyrin IX Accumulation and Light-Induced Mammalian Cell Death. PLoS ONE 2014, 9, e93078. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic Characterization of Tumor Cell-Specific Protoporphyrin IX Accumulation after Exposure to 5-Aminolevulinic Acid in Human Colonic Cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Fickweiler, S.; Wolfbeis, O.S.; Knuechel, R. Cell-Type Specific Protoporphyrin IX Metabolism in Human Bladder Cancer in Vitro. Photochem. Photobiol. 2000, 72, 226–233. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The Inhibition of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Photodynamic Action for Prostate Cancer. Photodiagn. Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef]
- Nm, N.; Cf, P.; Al, F.; Ne, A.; Am, B. Heme Biosynthesis in Human Breast Cancer—Mimetic “In Vitro” Studies and Some Heme Enzymic Activity Levels. Int. J. Biochem. 1990, 22, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, P.; de Rooij, F.W.; van Velthuysen, M.L.; Edixhoven, A.; van Hillegersberg, R.; Tilanus, H.W.; Wilson, J.H.; Siersema, P.D. Biochemical Basis of 5-Aminolaevulinic Acid-Induced Protoporphyrin IX Accumulation: A Study in Patients with (Pre)Malignant Lesions of the Oesophagus. Br. J. Cancer 1998, 78, 679–682. [Google Scholar] [CrossRef]
- Schauder, A.; Feuerstein, T.; Malik, Z. The Centrality of PBGD Expression Levels on ALA-PDT Efficacy. Photochem. Photobiol. Sci. 2011, 10, 1310–1317. [Google Scholar] [CrossRef]
- Pustogarov, N.; Panteleev, D.; Goryaynov, S.A.; Ryabova, A.V.; Rybalkina, E.Y.; Revishchin, A.; Potapov, A.A.; Pavlova, G. Hiding in the Shadows: CPOX Expression and 5-ALA Induced Fluorescence in Human Glioma Cells. Mol. Neurobiol. 2017, 54, 5699–5708. [Google Scholar] [CrossRef]
- Sinha, A.K.; Anand, S.; Ortel, B.J.; Chang, Y.; Mai, Z.; Hasan, T.; Maytin, E.V. Methotrexate Used in Combination with Aminolaevulinic Acid for Photodynamic Killing of Prostate Cancer Cells. Br. J. Cancer 2006, 95, 485–495. [Google Scholar] [CrossRef]
- Uchida, T.; Togashi, H.; Kuroda, Y.; Yamashita, A.; Itoh, N.; Haga, K.; Sadahiro, M.; Kayama, T. In vivo analysis of redox status in organs—From bench to bedside. Free Radic Res. 2020, 54, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Khoo, V.S.; Joon, D.L. New Developments in MRI for Target Volume Delineation in Radiotherapy. Br. J. Radiol. 2006, 79, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Colafati, G.S.; Voicu, I.P.; Carducci, C.; Miele, E.; Carai, A.; Di Loreto, S.; Marrazzo, A.; Cacchione, A.; Cecinati, V.; Tornesello, A.; et al. MRI Features as a Helpful Tool to Predict the Molecular Subgroups of Medulloblastoma: State of the Art. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418775375. [Google Scholar] [CrossRef] [PubMed]
- Kulik, M.; Nedelcu, C.; Martin, F.; Lebdai, S.; Rousselet, M.C.; Azzouzi, A.R.; Aubé, C. Post-Treatment MRI Aspects of Photodynamic Therapy for Prostate Cancer. Insights Imaging 2014, 5, 697–713. [Google Scholar] [CrossRef]
- Aebisher, D.; Osuchowski, M.; Bartusik-Aebisher, D.; Krupka-Olek, M.; Dynarowicz, K.; Kawczyk-Krupka, A. An Analysis of the Effects of In Vitro Photodynamic Therapy on Prostate Cancer Tissue by Histopathological Examination and Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 11354. [Google Scholar] [CrossRef]
- Barnaś, E.; Ostańska, E.; Bartusik-Aebisher, D.; Dynarowicz, K.; Skręt-Magierło, J.; Aebisher, D. Breast cancer tissue treated using photodynamic therapy. Acta Pol. Pharm. Drug Res. 2021, 78, 835–843. [Google Scholar] [CrossRef]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, E.V.; Kaprin, A.D.; Alekseev, B.Y.; Apolikhin, O.I.; Slovokhodov, E.K.; Ivano-va-Radkevich, V.I.; Urlova, A.N. 5-aminolevulinic acid in intraoperative photodynamic therapy of bladder cancer (results of a multicenter study). Photodiagn. Photodyn Ther. 2016, 16, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Gultekin, M.H.; Erdogan, A.; Cankaya, B.Y. Signal Abnormalities of the Bladder Wall Detected by Native T1 Mapping in Patients with Overactive Bladder. NMR Biomed. 2022, 35, e4748. [Google Scholar] [CrossRef]
- Baba, Y.; Lerch, M.M.; Stark, D.D.; Tanimoto, A.; Kreft, B.P.; Zhao, L.; Saluja, A.K.; Takahashi, M. Time after Excision and Temperature Alter Ex Vivo Tissue Relaxation Time Measurements. J. Magn. Reson. Imaging 1994, 4, 647–651. [Google Scholar] [CrossRef]
- Bjørnerud, A.; Johansson, L.O.; Briley-Sæbø, K.; Ahlström, H.K. Assessment of T1 and T Effects in Vivo and Ex Vivo Using Iron Oxide Nanoparticles in Steady State—Dependence on Blood Volume and Water Exchange. Magn. Reson. Med. 2002, 47, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Hao, X.; Liu, F.; Xu, D.; Liu, J.; Peterson, B.S. The Effects of Changing Water Content, Relaxation Times, and Tissue Contrast on Tissue Segmentation and Measures of Cortical Anatomy in MR Images. Magn. Reson. Imaging 2013, 31, 1709–1730. [Google Scholar] [CrossRef]
- Cai, Q.; Wen, Z.; Huang, Y.; Li, M.; Ouyang, L.; Ling, J.; Qian, L.; Guo, Y.; Wang, H. Investigation of Synthetic Magnetic Resonance Imaging Applied in the Evaluation of the Tumor Grade of Bladder Cancer. J. Magn. Reson. Imaging 2021, 54, 1989–1997. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, C.; Sun, F.; Yang, J.; Chen, Z.; Ao, H.; Cui, C.; Yang, Z.; Huang, W. Insights into the organic semiconducting photosensitizers for hypoxia-tolerant type I photodynamic therapy. Nano TransMed 2022, 1, e9130010. [Google Scholar] [CrossRef]
- Lv, J.; Qiu, Y.; Pan, L.; Zhang, X.; Li, M.; Yin, X. Photothermal/photodynamic antibacterial hydrogel embedded with copper carbon dots and Au nanoparticles. Nano TransMed 2024, 3, 100034. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, W.; Liu, H. Tumor microenvironment responsive nanozymes for multimodal imaging of tumors. Nano TransMed 2024, 3, 100032. [Google Scholar] [CrossRef]
- Lin, A.L.; Qin, Q.; Zhao, X.; Duong, T.Q. Blood longitudinal (T1) and transverse (T2) relaxation time constants at 11.7 Tesla. Magn. Reson. Mater. Phy. 2012, 25, 245–249. [Google Scholar] [CrossRef]
- Ye, L.; Wang, Y.; Xiang, W.; Yao, J.; Liu, J.; Song, B. Radiomic Analysis of Quantitative T2 Mapping and Conventional MRI in Predicting Histologic Grade of Bladder Cancer. J. Clin. Med. 2023, 12, 5900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Jiang, Z.; Shen, W.; Liu, H. Deep learning in bladder cancer imaging: A review. Front. Oncol. 2022, 12, 930917. [Google Scholar] [CrossRef]
- Arita, Y.; Kwee, T.C.; Akin, O.; Shigeta, K.; Paudyal, R.; Roest, C.; Ueda, R.; Lema-Dopico, A.; Nalavenkata, S.; Ruby, L.; et al. Multiparametric MRI and artificial intelligence in predicting and monitoring treatment response in bladder cancer. Insights Imaging 2025, 16, 7. [Google Scholar] [CrossRef] [PubMed]
| Treatment Method | Treatment Efficacy | Adverse Events | References |
|---|---|---|---|
| TURBT (alone, without intravesical therapy) | After TURBT alone, NMIBC recurrence is estimated in 48–70% of patients; the risk of progression may reach up to 17% at 1 year and up to 45% within 5 years. | The most common complications include bleeding, 2.8%, and bladder perforation, 1.3%; overall morbidity is estimated at 14% in large series. | [21,22,23] |
| TURBT+single, immediate intravesical chemotherapy | Meta-analysis of 7 RCTs (n = 1476) showed a 39% reduction in recurrence risk vs. TURBT alone (OR 0.61, p < 0.0001). | The most frequent adverse events were irritative symptoms such as dysuria in 17% and lower urinary tract symptoms (LUTS) in 7%, which required treatment; the remaining adverse events were transient and mild. | [24,25] |
| TURBT + BCG | IPD meta-analysis of 9 RCTs: with maintenance BCG—32% lower risk of recurrence vs. mitomycin; without maintenance, BCG performs worse (↑28% risk of recurrence). | Common adverse events include cystitis and urinary frequency; systemic effects are rare. | [26,27] |
| Immunotherapy (pembrolizumab, for BCG-unresponsive CIS ± Ta/T1) | In KEYNOTE-057 (Cohort A): CR 41% at 3 months; 46% of responses last ≥12 months. In Cohort B (without CIS): 12-month DFS 43.5%. | Adverse events include immune-related reactions; Grade 3–4: 13%. No deaths were reported as directly attributable to therapy in the study. | [28] |
| Photodynamic therapy (PDT) | Meta-analysis of 28 studies: CR 68% in unresectable NMIBC; RFS at 12 months: 71%, at 24 months: 38%. After resection: RFS at 12 months: 81%, at 24 months: 56%. Also effective after BCG failure (1-year RFS: 68%, 2-year: 56%). | Severe events were rare; the most common were photosensitivity and LUTS/bladder irritation. | [29] |
| Sample Group | T1 (ms), Mean ± SD | T2 (ms), Mean ± SD |
|---|---|---|
| Healthy tissue | 1351.7 ± 271.1 | 93.5 ± 20.3 |
| NMIBC | 727.7 ± 145.0 | 78.5 ± 20.4 |
| NMIBC after PDT | 368.9 ± 65.2 | 55.7 ± 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewski, D.; Dynarowicz, K.; Truszkiewicz, A.; Osuchowski, M.; Kubrak, T.; Bartusik-Aebisher, D.; Przygórzewska, A.; Szpara, J.; Aebisher, D. Assessment of T1 and T2 Relaxation-Time Changes in NMIBC Tissue After 5-ALA Photodynamic Therapy Using Quantitative Magnetic Resonance Imaging. Biomedicines 2025, 13, 2867. https://doi.org/10.3390/biomedicines13122867
Godlewski D, Dynarowicz K, Truszkiewicz A, Osuchowski M, Kubrak T, Bartusik-Aebisher D, Przygórzewska A, Szpara J, Aebisher D. Assessment of T1 and T2 Relaxation-Time Changes in NMIBC Tissue After 5-ALA Photodynamic Therapy Using Quantitative Magnetic Resonance Imaging. Biomedicines. 2025; 13(12):2867. https://doi.org/10.3390/biomedicines13122867
Chicago/Turabian StyleGodlewski, Dominik, Klaudia Dynarowicz, Adrian Truszkiewicz, Michał Osuchowski, Tomasz Kubrak, Dorota Bartusik-Aebisher, Agnieszka Przygórzewska, Jakub Szpara, and David Aebisher. 2025. "Assessment of T1 and T2 Relaxation-Time Changes in NMIBC Tissue After 5-ALA Photodynamic Therapy Using Quantitative Magnetic Resonance Imaging" Biomedicines 13, no. 12: 2867. https://doi.org/10.3390/biomedicines13122867
APA StyleGodlewski, D., Dynarowicz, K., Truszkiewicz, A., Osuchowski, M., Kubrak, T., Bartusik-Aebisher, D., Przygórzewska, A., Szpara, J., & Aebisher, D. (2025). Assessment of T1 and T2 Relaxation-Time Changes in NMIBC Tissue After 5-ALA Photodynamic Therapy Using Quantitative Magnetic Resonance Imaging. Biomedicines, 13(12), 2867. https://doi.org/10.3390/biomedicines13122867










