Phytochemical Investigation of Aquilaria agallocha and Identification of a Diarylheptanoid Exhibiting Anti-Tau Aggregation Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
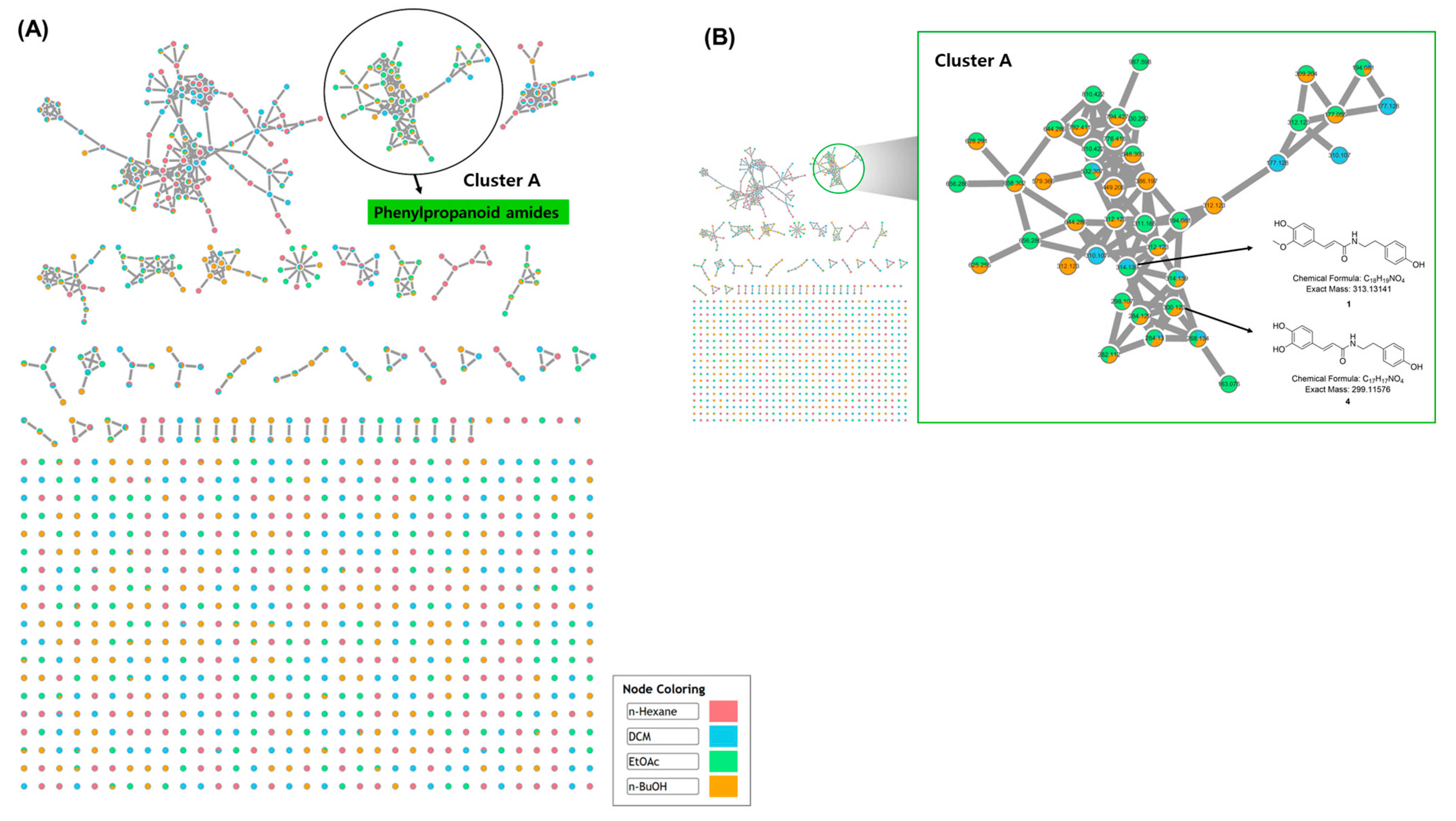
2.4. UHPLC–QTOF–MS/MS Analysis and GNPS Molecular Networking
2.5. 3D Visualization of LC/MS Data
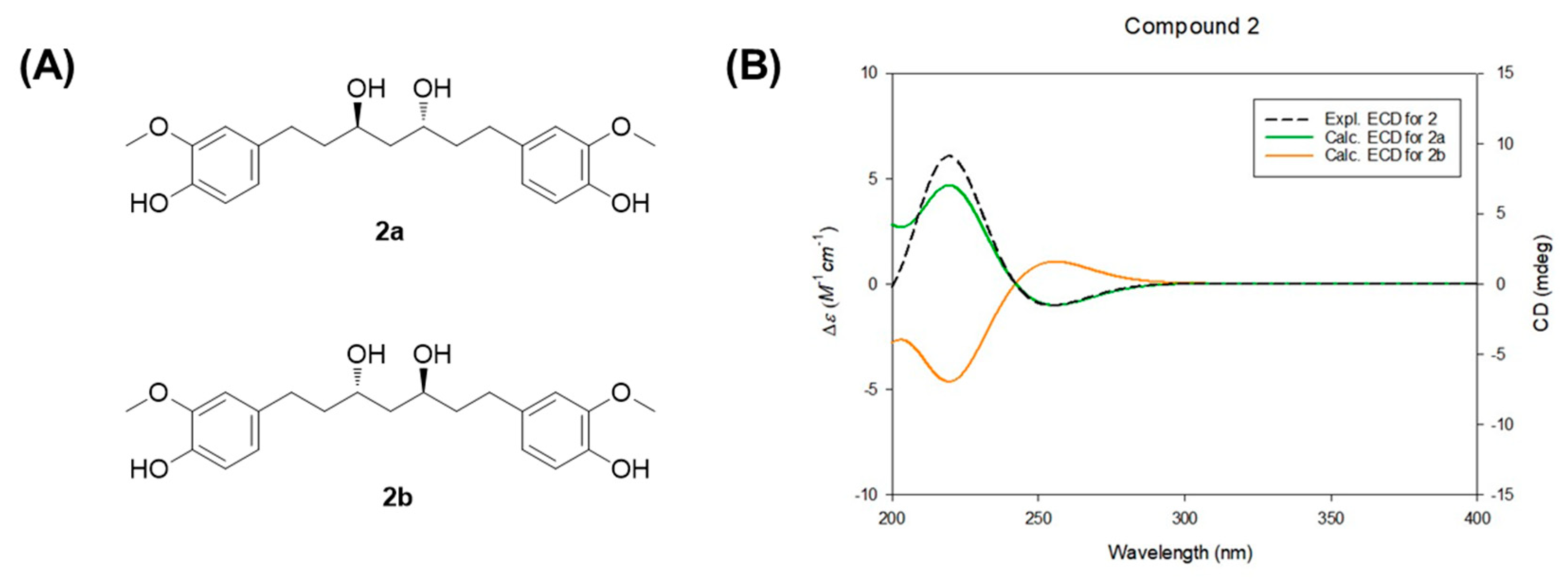
2.6. Computational Analysis of Electronic Circular Dichroism (ECD) Spectra
2.7. Tau-BiFC Cell Culture
2.8. Compound Treatment and Image Analysis
3. Results and Discussion
3.1. LC-MS/MS-Based Molecular Networking Analysis
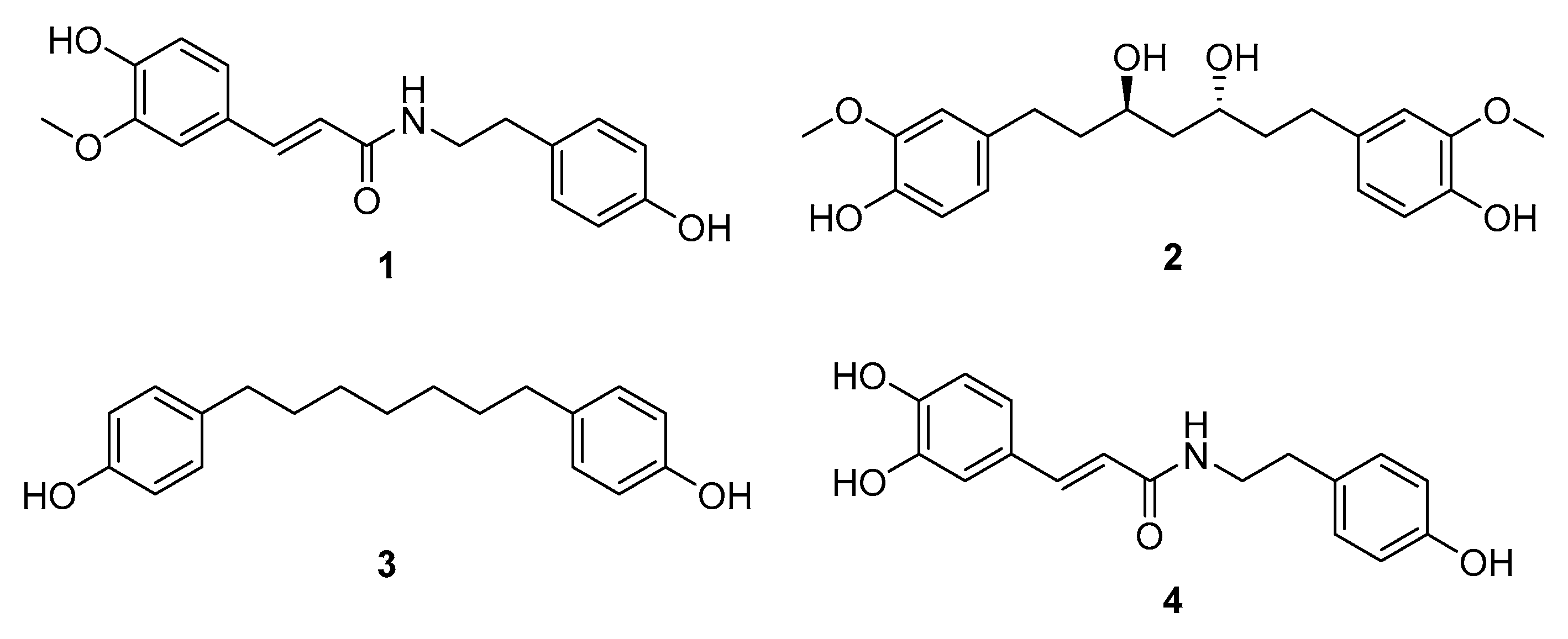
3.2. Isolation and Structural Elucidation of Compounds 1–4 from the EtOAc Fraction
3.3. Inhibition of TG-Induced Tau Oligomerization by Compound 2 in Tau-BiFC Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Chen, H.-Q.; Wang, H.; Mei, W.-L.; Dai, H.-F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the Matter: Agarwood Use and Trade and CITES Implementation for Aquilaria malaccensis; Traffic International: Cambridge, UK, 2000; pp. 17–18. [Google Scholar]
- Liu, Y.-Y.; Wei, J.-H.; Gao, Z.-H.; Zhang, Z.; Lyu, J.-C. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Dash, M.; Patra, J.K.; Panda, P.P. Phytochemical and antimicrobial screening of extracts of Aquilaria agallocha Roxb. Afr. J. Biotechnol. 2008, 7, 3531–3534. [Google Scholar]
- Aggarwal, G.; Sharma, M.; Singh, R.; Sharma, U. Ethnopharmacologically important highly subsidized Indian medicinal plants: Systematic review on their traditional uses, phytochemistry, pharmacology, quality control, conservation status and future prospective. J. Ethnopharmacol. 2024, 320, 117385. [Google Scholar]
- Dalkılıç, S.; Dalkılıç, L.K.; İsbenov, E.; Uygur, L.; Taşdemir, C. Investigation of Cytotoxic, Antioxidant, Apoptotic/Necrotic Activity of Aquilaria agallocha Root Extract and Determination of Gene Expression Levels in HepG2, MCF-7 Cancer Cell Lines. Life 2025, 15, 651. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, L.; Li, C.; Yang, Y.; Zhang, S.; Xu, H.; Wang, Z.; Han, Z.; Yang, L. Eudesmane-type and agarospirane-type sesquiterpenes from agarwood of Aquilaria agallocha. Phytochem. 2021, 192, 112920. [Google Scholar] [CrossRef]
- Ueda, J.-Y.; Imamura, L.; Tezuka, Y.; Tran, Q.L.; Tsuda, M.; Kadota, S. New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorg. Med. Chem. 2006, 14, 3571–3574. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, Z.; Ye, K. Tau in neurodegenerative diseases: Molecular mechanisms, biomarkers, and therapeutic strategies. Transl. Neurodegener. 2024, 13, 40. [Google Scholar] [CrossRef]
- Li, C.; Götz, J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat. Rev. Drug Discov. 2017, 16, 863–883. [Google Scholar] [CrossRef]
- Basurto-Islas, G.; Diaz, M.C.; Ocampo, L.M.Z.; Martínez-Herrera, M.; López-Camacho, P.Y. Natural products against tau hyperphosphorylation-induced aggregates: Potential therapies for Alzheimer’s disease. Archiv. Pharm. 2025, 358, e2400721. [Google Scholar] [CrossRef] [PubMed]
- Nahar, J.; Boopathi, V.; Rupa, E.J.; Awais, M.; Valappil, A.K.; Morshed, M.N.; Murugesan, M.; Akter, R.; Yang, D.U.; Mathiyalagan, R. Protective effects of Aquilaria agallocha and Aquilaria malaccensis edible plant extracts against lung cancer, inflammation, and oxidative stress—In silico and in vitro study. Appl. Sci. 2023, 13, 6321. [Google Scholar] [CrossRef]
- Durairajan, S.S.; Selvarasu, K.; Bera, M.R.; Rajaram, K.; Iyaswamy, A.; Li, M. Alzheimer’s disease and other tauopathies: Exploring efficacy of medicinal plant-derived compounds in alleviating tau-mediated neurodegeneration. Curr. Mol. Pharmacol. 2022, 15, 361–379. [Google Scholar] [CrossRef]
- Jitsanong, T.; Khanobdee, K.; Piyachaturawat, P.; Wongprasert, K. Diarylheptanoid 7-(3, 4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene from Curcuma comosa Roxb. protects retinal pigment epithelial cells against oxidative stress-induced cell death. Toxicol. In Vitro 2011, 25, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Vattanarongkup, J.; Piyachaturawat, P.; Tuchinda, P.; Sanvarinda, P.; Sanvarinda, Y.; Jantaratnotai, N. Protective effects of a diarylheptanoid from Curcuma comosa against hydrogen peroxide-induced astroglial cell death. Planta Med. 2016, 82, 1456–1462. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, Y.; Sun, H.; Chen, L.; Man, X. Curcumin inhibits endoplasmic reticulum stress induced by cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2017, 14, 4047–4052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharm. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.H.; Hong, J.-H.; Kim, S.H.; Park, K.-M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Kim, H.-J.; Kim, H.-M.; Sim, H.-Y.; Choi, W.; Lee, B.S.; Kim, K.H.; An, H.-J. Aster glehni ethanol extract inhibits inflammatory responses regulating skin barrier molecules in human keratinocytes. Nat. Prod. Sci. 2024, 30, 262–267. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jeong, S.Y.; Li, Z.; Kim, H.-Y.; Kim, H.-W.; Yoo, M.J.; Jang, H.J.; Kim, D.-K.; Cho, N.; Yoo, H.M. Development of a screening platform to discover natural products active against SARS-CoV-2 infection using lung organoid models. Biomater. Res. 2023, 27, 18. [Google Scholar] [CrossRef]
- You, C.-L.; Lee, S.-J.; Lee, J.; Vuong, T.A.; Lee, H.-Y.; Jeong, S.Y.; Alishir, A.; Walker, A.S.; Bae, G.-U.; Kim, K.H. Inonotus obliquus upregulates muscle regeneration and augments function through muscle oxidative metabolism. Int. J. Biol. Sci. 2023, 19, 4898. [Google Scholar] [CrossRef]
- Cho, C.H.; Chae, S.H.; Kim, S.H.; Kim, K.H. Phenolic compounds isolated from Juncus decipiens and their effects on osteoblast differentiation in the mouse mesenchymal stem cell line C3H10T1/2. Nat. Prod. Sci. 2024, 30, 135–142. [Google Scholar] [CrossRef]
- Lee, S.R.; Schalk, F.; Schwitalla, J.W.; Guo, H.; Yu, J.S.; Song, M.; Jung, W.H.; De Beer, Z.W.; Beemelmanns, C.; Kim, K.H. GNPS-Guided Discovery of Madurastatin Siderophores from the Termite-Associated Actinomadura sp. RB99. Chem. Eur. J. 2022, 28, e202200612. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a styrylpyrone-fused steroid with a hexacyclic 6/5/6/6/6/5 skeleton from a mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Alishir, A.; Zhang, S.; Zhang, Y.; Choi, S.; Pang, C.; Bae, H.Y.; Jung, W.H.; Kim, K.H. Identification of Obscurolide-Type Metabolites and Antifungal Metabolites from the Termite-Associated Streptomyces neopeptinius BYF101. J. Nat. Prod. 2023, 86, 1891–1900. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Zeng, H.; Locatelli, M.; Bardelli, C.; Amoruso, A.; Coisson, J.D.; Travaglia, F.; Arlorio, M.; Brunelleschi, S. Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: Evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. J. Agric. Food Chem. 2011, 59, 5342–5350. [Google Scholar] [CrossRef]
- Wang, C.; Eskiw, C.H. Cytoprotective effects of Avenathramide C against oxidative and inflammatory stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 2932. [Google Scholar] [CrossRef]
- Kim, H.R.; Min, H.-Y.; Jeong, Y.H.; Lee, S.K.; Lee, N.S.; Seo, E.-K. Cytotoxic constituents from the whole plant of Corydalis pallida. Arch. Pharm. Res. 2005, 28, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Mimaki, Y.; Sakagami, H.; Sashida, Y. New diarylheptanoids and diarylheptanoid glucosides from the rhizomes of Tacca chantrieri and their cytotoxic activity. J. Nat. Prod. 2002, 65, 283–289. [Google Scholar] [CrossRef]
- Kawasaki, I.; Matsuda, K.; Kaneko, T. Preparation of 1, 7-bis (p-hydroxyphenyl) heptane. Bull. Chem. Soc. Jpn. 1971, 44, 1986–1987. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Usuguchi, J.; Nakatani, N. Constitutents of Zingiberaceae I. Diarylheptanoids from the rhizomes of ginger (Zingiber officinale Roscoe). Chem. Pharm. Bull. 1991, 39, 120–122. [Google Scholar] [CrossRef]
- Puente, A.R.; Nagabhushanam, K.; Ganjihal, S.; Majeed, M.; Polavarapu, P.L. Chiroptical spectroscopic studies for the absolute configuration determination of hexahydrocurcumin and octahydrocurcumin. Chirality 2022, 34, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.; Haque, M.M.; Kim, M.J.; Lee, J.H.; Baik, J.-H.; Kim, Y.; Kim, D.J.; Grailhe, R.; Kim, Y.K. Bimolecular fluorescence complementation; lighting-up tau-tau interaction in living cells. PLoS ONE 2013, 8, e81682. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-Q.; Yang, Y.; Song, J.; Jiang, Q.; Liu, Z.-C.; Wang, Q.; Zhu, L.-Q.; Wang, J.-Z.; Tian, Q. LiCl attenuates thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3β in vivo and in vitro. J. Alzheimer’s Dis. 2010, 21, 1107–1117. [Google Scholar] [CrossRef]
- Lim, S.; Shin, S.; Sung, Y.; Lee, H.E.; Kim, K.H.; Song, J.Y.; Lee, G.-H.; Aziz, H.; Lukianenko, N.; Kang, D.M. Levosimendan inhibits disulfide tau oligomerization and ameliorates tau pathology in TauP301L-BiFC mice. Exp. Mol. Med. 2023, 55, 612–627. [Google Scholar] [CrossRef] [PubMed]



| Experimental Procedure | Equipment | |
|---|---|---|
| Optical rotations | JASCO P-2000 polarimeter (JASCO, Easton, MD, USA) | |
| Ultraviolet (UV) spectra | Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) | |
| Nuclear magnetic resonance (NMR) spectra | Bruker AVANCE III HD 850 NMR spectrometer (Bruker Corporation, Billerica, MA, USA) with a 5 mm TCI CryoProbe operating at 850 MHz (1H) and 212.5 MHz (13C) | |
| HR-ESIMS |
| |
| Preparative HPLC | Waters 1525 Binary HPLC pump with a Waters 996 Photodiode Array Detector (Waters Corporation, Milford, MA, USA) and a Hector C18 column (250 × 21.2 mm, 5 μm; flow rate: 5 mL/min; Rstech Corporation, Daejeon, Republic of Korea) | |
| Semi-preparative HPLC | Waters 1525 Binary HPLC pump with a Waters 996 Photodiode Array Detector (Waters Corporation, Milford, CT, USA)
| |
| LC/MS analysis | Agilent 1200 Series HPLC system equipped with a diode array detector and 6130 Series ESI mass spectrometer using an analytical Kinetex C18 100 Å column (100 × 2.1 mm, 5 μm; flow rate: 0.3 mL/min; Phenomenex, Torrance, CA, USA). | |
| Column chromatography |
| |
| Thin-layer chromatography (TLC) | pre-coated silica gel F254 plates and RP-C18 F254s plates (Merck, Darmstadt, Germany); spots were detected under UV light or by heating following spraying with anisaldehyde-sulfuric acid. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.R.; Kim, J.; Kim, B.; Kang, D.M.; Kim, Y.K.; Kim, J.-C.; Lim, S.; Kim, K.H. Phytochemical Investigation of Aquilaria agallocha and Identification of a Diarylheptanoid Exhibiting Anti-Tau Aggregation Activity. Biomedicines 2025, 13, 2855. https://doi.org/10.3390/biomedicines13122855
Cho YR, Kim J, Kim B, Kang DM, Kim YK, Kim J-C, Lim S, Kim KH. Phytochemical Investigation of Aquilaria agallocha and Identification of a Diarylheptanoid Exhibiting Anti-Tau Aggregation Activity. Biomedicines. 2025; 13(12):2855. https://doi.org/10.3390/biomedicines13122855
Chicago/Turabian StyleCho, Yeo Rang, Jiyeon Kim, Bora Kim, Dong Min Kang, Yun Kyung Kim, Jin-Chul Kim, Sungsu Lim, and Ki Hyun Kim. 2025. "Phytochemical Investigation of Aquilaria agallocha and Identification of a Diarylheptanoid Exhibiting Anti-Tau Aggregation Activity" Biomedicines 13, no. 12: 2855. https://doi.org/10.3390/biomedicines13122855
APA StyleCho, Y. R., Kim, J., Kim, B., Kang, D. M., Kim, Y. K., Kim, J.-C., Lim, S., & Kim, K. H. (2025). Phytochemical Investigation of Aquilaria agallocha and Identification of a Diarylheptanoid Exhibiting Anti-Tau Aggregation Activity. Biomedicines, 13(12), 2855. https://doi.org/10.3390/biomedicines13122855









