PCSK9 Promotes Platelet Activation and NET Formation, Aggravating Pulmonary Microthrombosis in Sepsis-Induced Lung Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sepsis Model
2.2. Experimental Design and Drug Administration
2.3. Histopathology and Immunofluorescence
2.4. ELISA
2.5. Thromboelastography
2.6. Statistical Analysis
3. Results
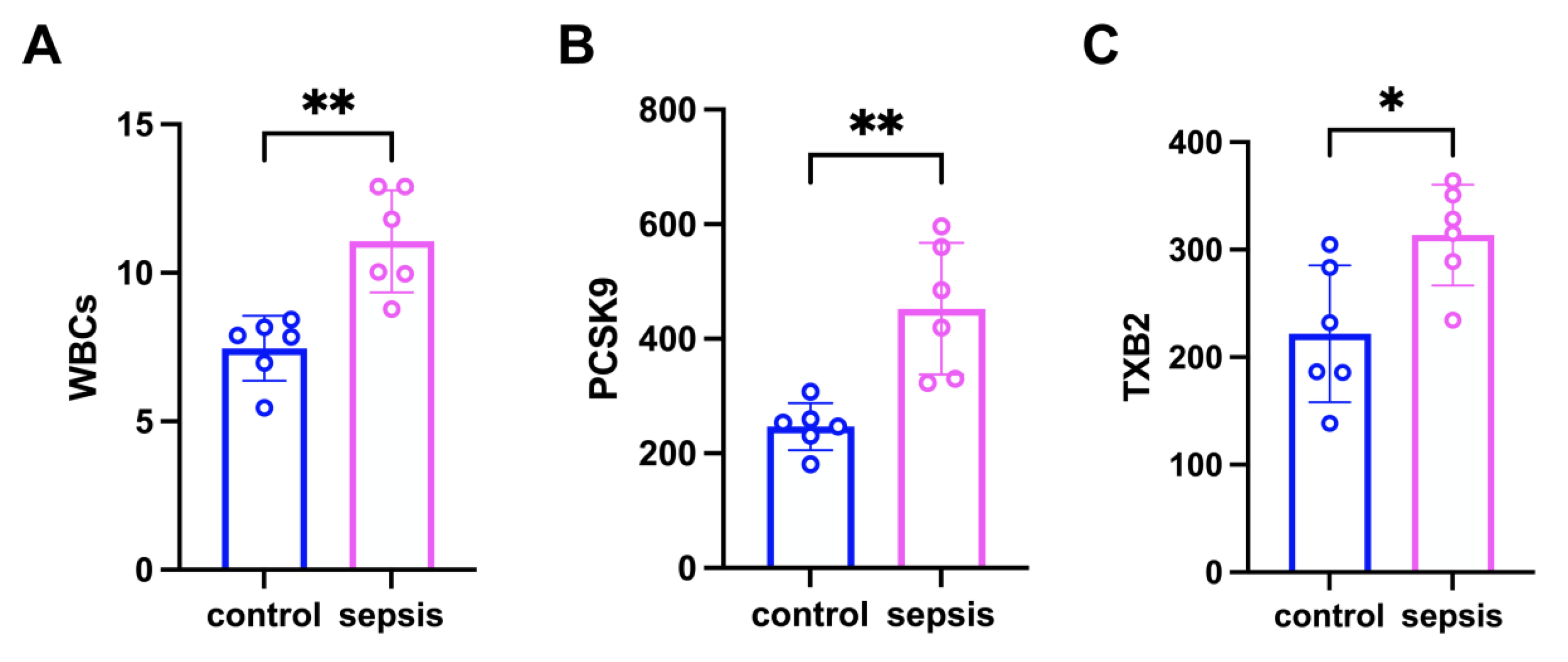
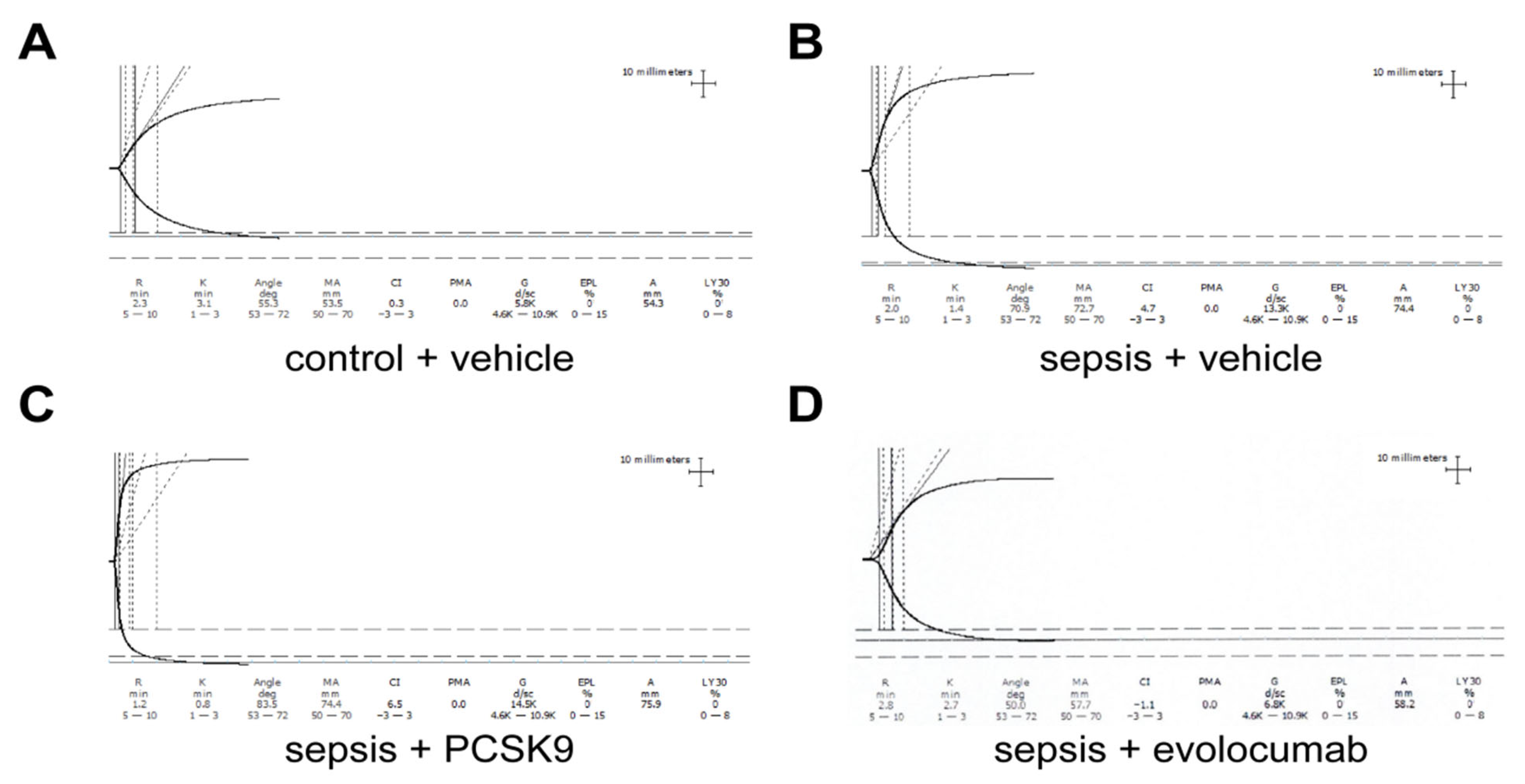
3.1. PCSK9 Enhances Platelet Aggregation in Sepsis Mice
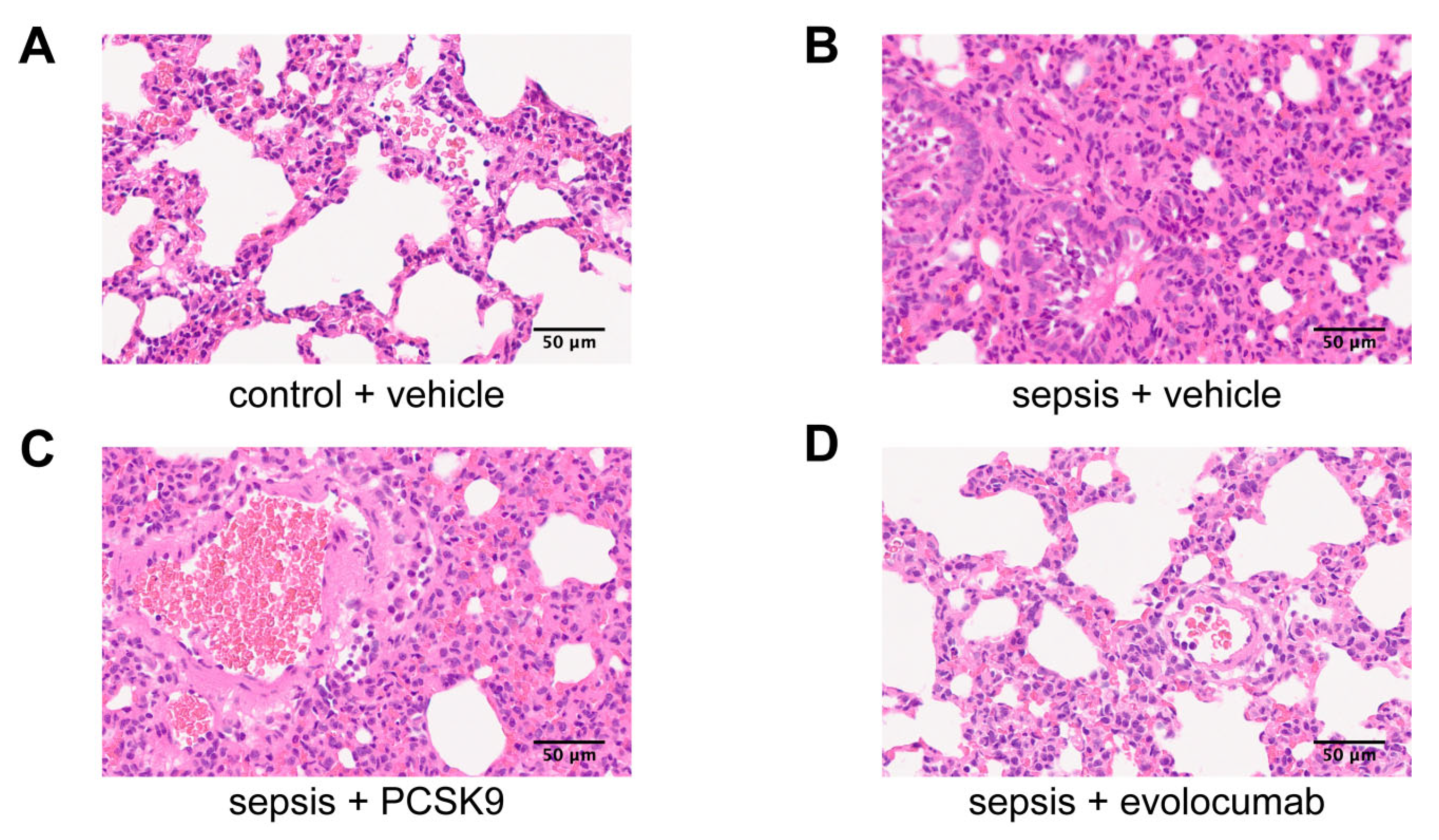
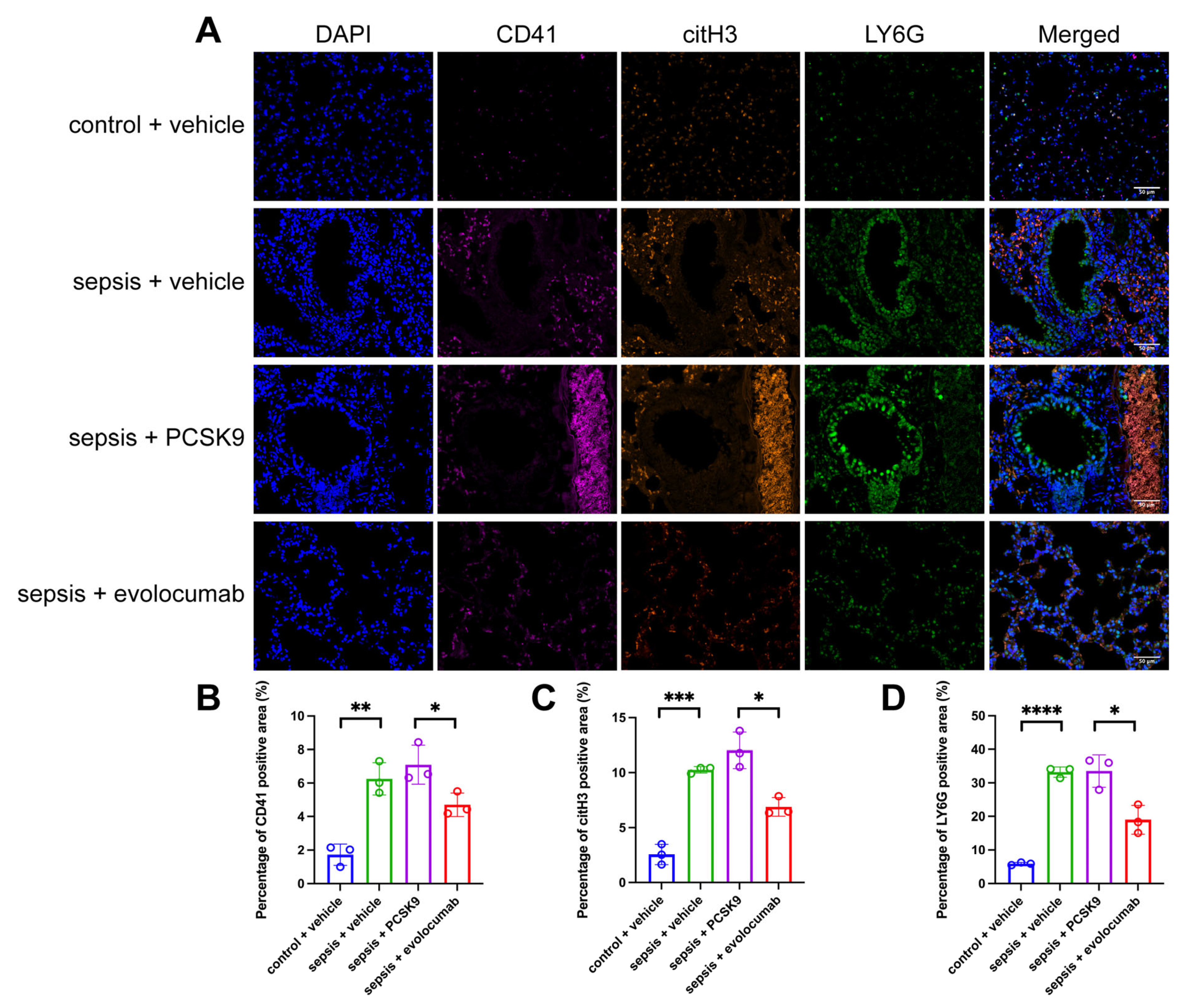
3.2. PCSK9 Accelerates Pulmonary Infiltration and Microthrombosis Formation
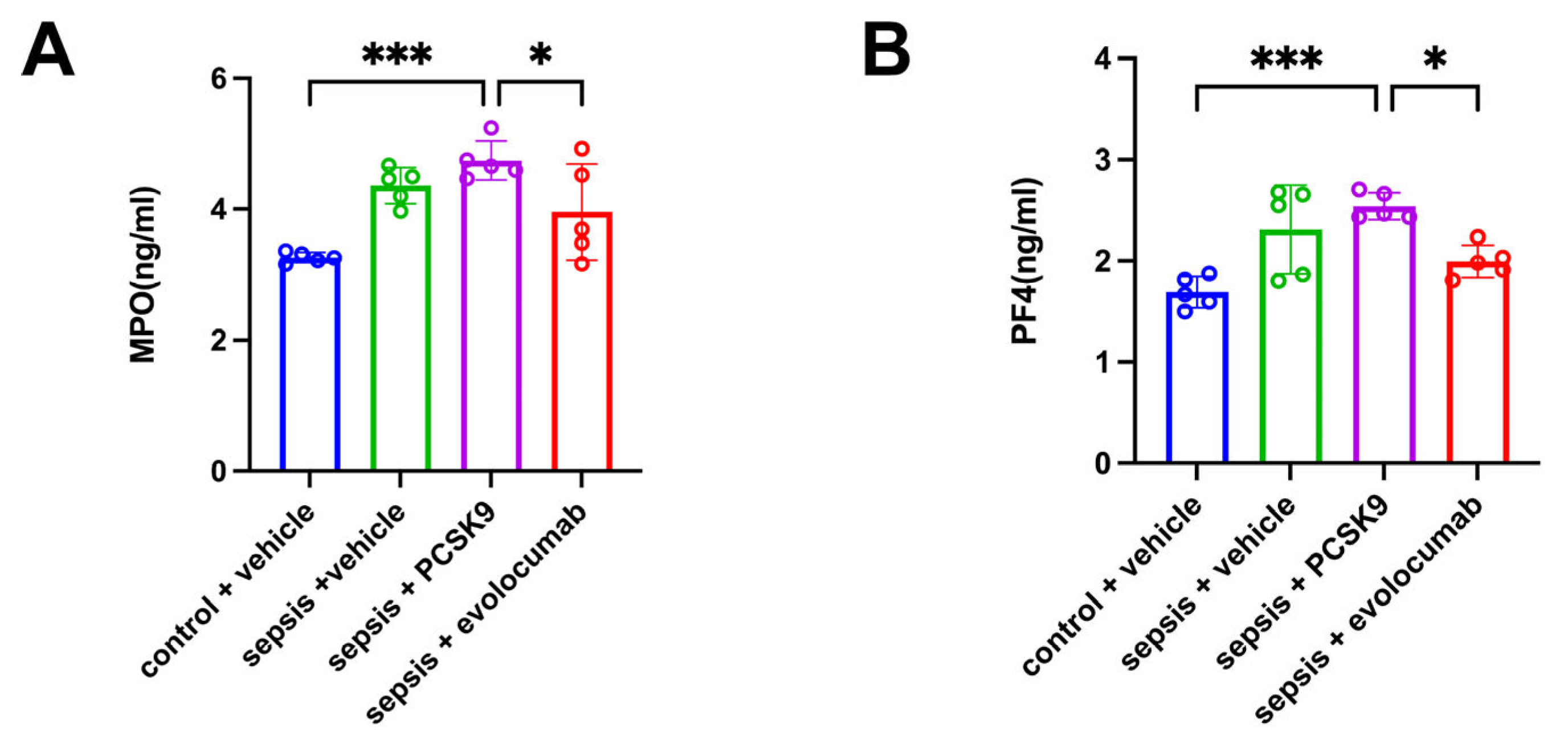
3.3. Platelet Activation-Induced NET Formation Drives Pulmonary Microthrombosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tidswell, R.; Singer, M. Sepsis—thoughtful management for the non-expert. Clin. Med. 2018, 18, 62–68. [Google Scholar] [CrossRef]
- Zou, S.; Jie, H.; Han, X.; Wang, J. The role of neutrophil extracellular traps in sepsis and sepsis-related acute lung injury. Int. Immunopharmacol. 2023, 124 Pt A, 110436. [Google Scholar] [CrossRef]
- Yang, H.H.; Duan, J.X.; Liu, S.K.; Xiong, J.B.; Guan, X.X.; Zhong, W.J.; Sun, C.C.; Zhang, C.Y.; Luo, X.Q.; Zhang, Y.F.; et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics 2020, 10, 4749–4761. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, B.; Yuan, S.; Zhang, J. The “Self-Sacrifice” of ImmuneCells in Sepsis. Front. Immunol. 2022, 13, 833479. [Google Scholar] [CrossRef]
- Williams, B.; Zou, L.; Pittet, J.-F.; Chao, W. Sepsis-Induced Coagulopathy: A Comprehensive Narrative Review of Pathophysiology, Clinical Presentation, Diagnosis, and Management Strategies. Anesth. Analg. 2024, 138, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Boggio, E.; Gigliotti, C.L.; Stoppa, I.; Sutti, S.; Giordano, M.; Dianzani, U.; Rolla, R. Platelets, Protean Cells with All-Around Functions and Multifaceted Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 4565. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Cox, D. Sepsis—It is all about the platelets. Front. Immunol. 2023, 14, 1210219. [Google Scholar] [CrossRef]
- Vardon-Bounes, F.; Ruiz, S.; Gratacap, M.-P.; Garcia, C.; Payrastre, B.; Minville, V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019, 20, 3494. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Davis, R.P.; Kim, S.-J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Ferri, N.; Sirtori, C.R.; Corsini, A.; Banach, M.; Ruscica, M. Proprotein Convertase Subtilisin/Kexin Type 9: A View beyond the Canonical Cholesterol-Lowering Impact. Am. J. Pathol. 2021, 191, 1385–1397. [Google Scholar] [CrossRef]
- Boyd, J.H.; Fjell, C.D.; Russell, J.A.; Sirounis, D.; Cirstea, M.S.; Walley, K.R. Increased Plasma PCSK9 Levels Are Associated with Reduced Endotoxin Clearance and the Development of Acute Organ Failures during Sepsis. J. Innate Immun. 2016, 8, 211–220. [Google Scholar] [CrossRef]
- Genga, K.R.; Lo, C.; Cirstea, M.S.; Filho, F.S.L.; Walley, K.R.; Russell, J.A.; Linder, A.; Francis, G.A.; Boyd, J.H. Impact of PCSK9 loss-of-function genotype on 1-year mortality and recurrent infection in sepsis survivors. EBioMedicine 2018, 38, 257–264. [Google Scholar] [CrossRef]
- Navarese, E.P.; Gurbel, P.A.; Andreotti, F.; Tantry, U.; Jeong, Y.H.; Kozinski, M.; Engstrøm, T.; Di Pasquale, G.; Kochman, W.; Ardissino, D.; et al. Optimal timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 158, 261–270. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.-T.; Li, T.-H.; Wang, Z.; Wei, D.-H.; Liu, L.-S.; Zheng, X.-L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef]
- Hally, K.E.; Parker, O.M.; Brunton-O'Sullivan, M.M.; Harding, S.A.; Larsen, P.D. Linking Neutrophil Extracellular Traps and Platelet Activation: A Composite Biomarker Score for Predicting Outcomes after Acute Myocardial Infarction. Thromb. Haemost. 2021, 121, 1637–1649. [Google Scholar] [CrossRef]
- Chou, M.-L.; Babamale, A.O.; Walker, T.L.; Cognasse, F.; Blum, D.; Burnouf, T. Blood–brain crosstalk: The roles of neutrophils, platelets, and neutrophil extracellular traps in neuropathologies. Trends Neurosci. 2023, 46, 764–779. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Haas, M.E.; Levenson, A.E.; Sun, X.; Liao, W.-H.; Rutkowski, J.M.; De Ferranti, S.D.; A Schumacher, V.; E Scherer, P.; Salant, D.J.; Biddinger, S.B. The Role of Proprotein Convertase Subtilisin/Kexin Type 9 in Nephrotic Syndrome-Associated Hypercholesterolemia. Circulation 2016, 134, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction During Sepsis Via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation 2023, 46, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.A.; Cook, J.A.; Wise, W.C.; Halushka, P.V. Role of thromboxane, prostaglandins and leukotrienes in endotoxic and septic shock. Intensiv. Care Med. 1986, 12, 116–126. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Sepsis-induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology 2020, 132, 1238–1245. [Google Scholar] [CrossRef]
- Schultz, M.; van der Poll, T.; Levi, M. Sepsis and thrombosis. Semin. Thromb. Hemost. 2013, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, M.; Choe, K.; Song, E.; Seo, H.; Hwang, Y.; Ahn, J.; Lee, S.-H.; Lee, J.H.; Jo, Y.H.; et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur. Respir. J. 2019, 53, 1800786. [Google Scholar] [CrossRef]
- Sevransky, J.E.; Levy, M.M.; Marini, J.J. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: An evidence-based review. Crit. Care Med. 2004, 32 (Suppl. 11), S548–S553. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Levi, M.; Sivapalaratnam, S. Disseminated intravascular coagulation: An update on pathogenesis and diagnosis. Expert Rev. Hematol. 2018, 11, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Plasma concentration of c-reactive protein and risk of developing peripheral vascular disease. Circulation 1998, 97, 425–428. [Google Scholar] [CrossRef]
- Humar, A.; Jessurun, J.; Sharp, H.L.; Gruessner, R.W. Thrombotic microangiopathy after liver–small bowel transplant. Clin. Transplant. 1998, 12, 600–601. [Google Scholar] [CrossRef]
- Sharma, S.; Tyagi, T.; Antoniak, S. Platelet in thrombo-inflammation: Unraveling new therapeutic targets. Front. Immunol. 2022, 13, 1039843. [Google Scholar] [CrossRef] [PubMed]
- Glerup, S.; Schulz, R.; Laufs, U.; Schlüter, K.-D. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic Res. Cardiol. 2017, 112, 32. [Google Scholar] [CrossRef] [PubMed]
- Ziogos, E.; Chelko, S.P.; Harb, T.; Engel, M.; A Vavuranakis, M.; Landim-Vieira, M.; Walsh, E.M.; Williams, M.S.; Lai, S.; Halushka, M.K.; et al. Platelet activation and endothelial dysfunction biomarkers in acute coronary syndrome: The impact of PCSK9 inhibition. Eur. Hear. J.-Cardiovasc. Pharmacother. 2023, 9, 636–646. [Google Scholar] [CrossRef]
- Andrews, R.K.; Arthur, J.F.; Gardiner, E.E. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb. Haemost. 2014, 112, 659–665. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- Moschonas, I.C.; Tselepis, A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019, 288, 9–16. [Google Scholar] [CrossRef]
- Walley, K.R. Role of lipoproteins and proprotein convertase subtilisin/kexin type 9 in endotoxin clearance in sepsis. Curr. Opin. Crit. Care 2016, 22, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Wang, J.; Guo, C.; Kleiman, K.; Meng, H.; Knight, J.S.; Eitzman, D.T. Proprotein convertase subtilisin/kexin type 9 (PCSK9) Deficiency is Protective Against Venous Thrombosis in Mice. Sci. Rep. 2017, 7, 14360. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Scavalli, A.S.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef]
- Walley, K.R.; Thain, K.R.; Russell, J.A.; Reilly, M.P.; Meyer, N.J.; Ferguson, J.F.; Christie, J.D.; Nakada, T.-A.; Fjell, C.D.; Thair, S.A.; et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014, 6, 258ra143. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Xiong, W.; Jiang, L. PCSK9 Promotes Platelet Activation and NET Formation, Aggravating Pulmonary Microthrombosis in Sepsis-Induced Lung Injury. Biomedicines 2025, 13, 2843. https://doi.org/10.3390/biomedicines13122843
Lv X, Xiong W, Jiang L. PCSK9 Promotes Platelet Activation and NET Formation, Aggravating Pulmonary Microthrombosis in Sepsis-Induced Lung Injury. Biomedicines. 2025; 13(12):2843. https://doi.org/10.3390/biomedicines13122843
Chicago/Turabian StyleLv, Xin, Wanxia Xiong, and Linghui Jiang. 2025. "PCSK9 Promotes Platelet Activation and NET Formation, Aggravating Pulmonary Microthrombosis in Sepsis-Induced Lung Injury" Biomedicines 13, no. 12: 2843. https://doi.org/10.3390/biomedicines13122843
APA StyleLv, X., Xiong, W., & Jiang, L. (2025). PCSK9 Promotes Platelet Activation and NET Formation, Aggravating Pulmonary Microthrombosis in Sepsis-Induced Lung Injury. Biomedicines, 13(12), 2843. https://doi.org/10.3390/biomedicines13122843





