Atezolizumab Plus Bevacizumab Combination Therapy in Unresectable Hepatocellular Carcinoma: An Institutional Experience
Abstract
1. Introduction
2. Methods
Study Design and Participants
3. Results
3.1. Patient Characteristics
3.2. Treatment Patterns
3.3. Bassline EGD Evaluation
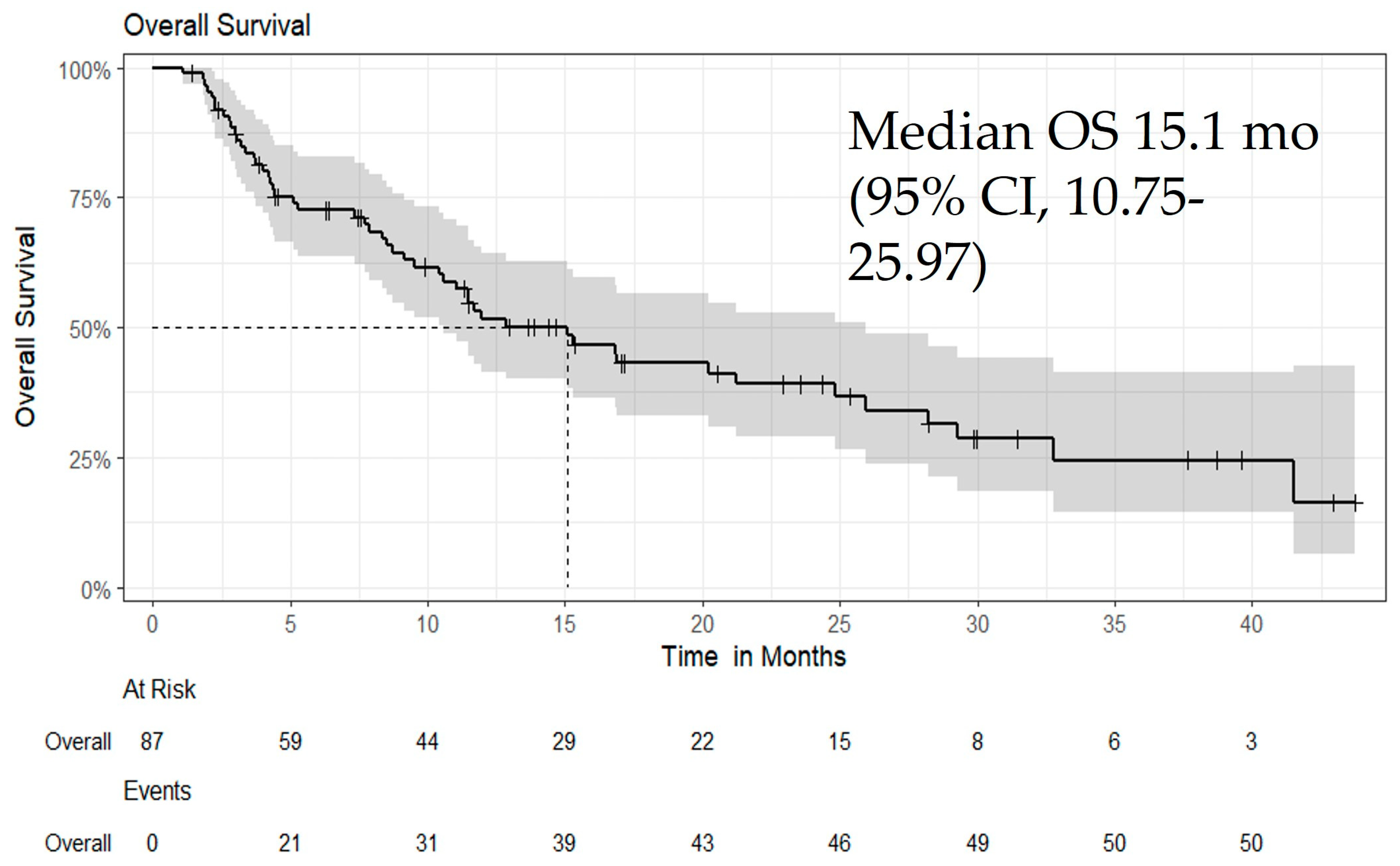
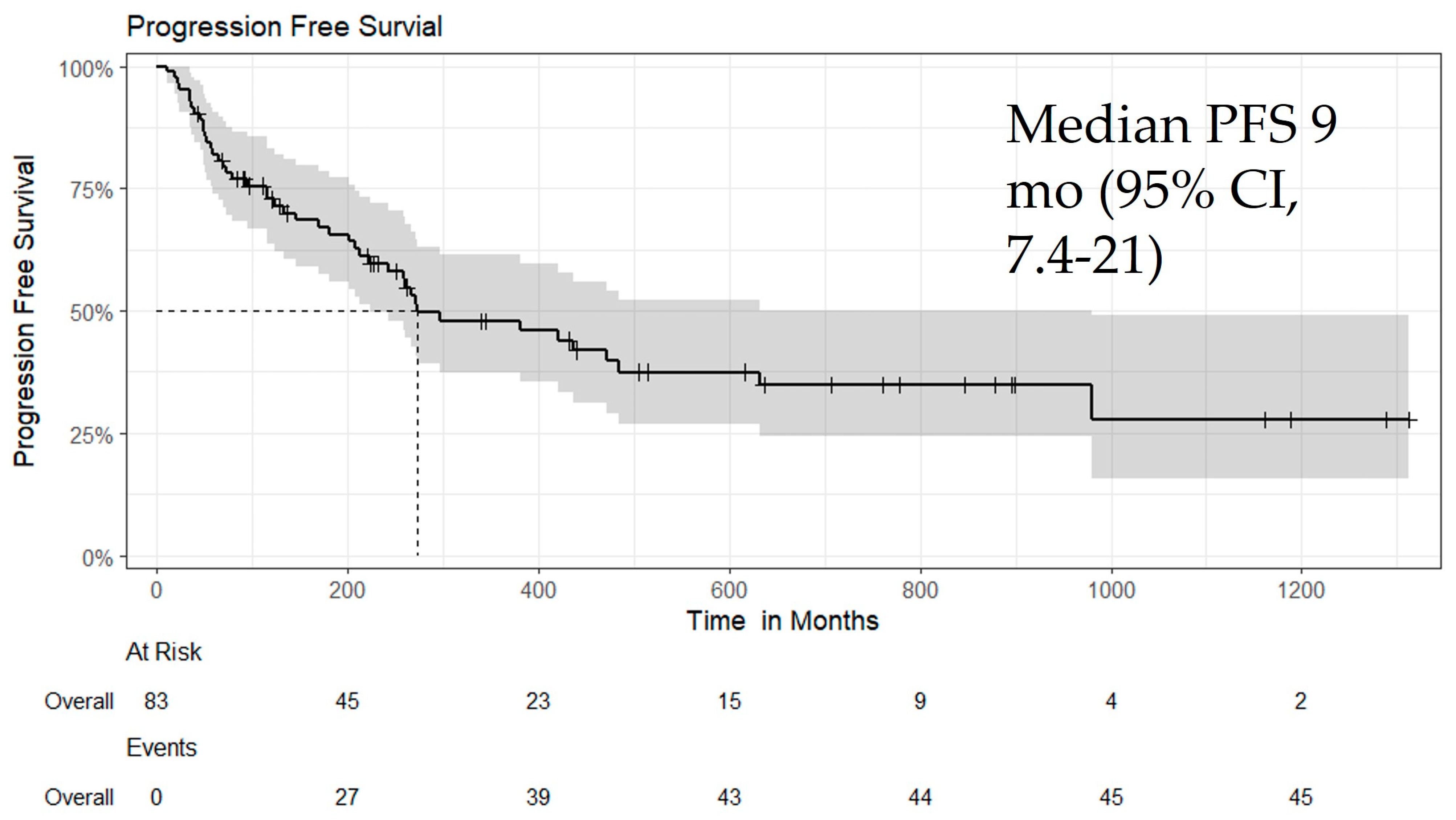
3.4. Efficacy
3.5. Subgroup Analysis
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| HCC | Hepatocellular carcinoma |
| uHCC | unresectable Hepatocellular carcinoma |
| Atezo/Bev | Atezolizumab plus bevacizumab |
| OS | overall survival |
| PFS | progression-free survival |
| EGD | esophagogastroduodenoscopy |
| BCLC | Barcelona Clinic Liver Cancer |
| CP | Child-Pugh |
| US | United States |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| DM | Diabetes Mellitus |
| NASH | alcohol, non-alcoholic steatohepatitis |
| NAFLD | non-alcoholic fatty liver disease |
| SEER | Surveillance Epidemiology and End Results |
| AFP | like Alpha-Fetoprotein |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| AI | Artificial Intelligence |
| LRT | Locoregional Therapies |
| OLT | Orthotopic Liver Transplant |
| RFA | Radiofrequency Ablation |
| MWA | Microwave Ablation |
| Y-90 | yttrium-90 radioembolization |
| SBRT | stereotactic body radiation therapy |
| TACE | and transarterial chemoembolization |
| ICPIs | Immune Checkpoint Inhibitors |
| FDA | Food and Drug Administration |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| VEGF | Vascular Endothelial Growth Factor |
| Durva/Treme | durvalumab plus tremelimumab |
| AST | Aspartate transaminase |
| ALT | Alanine transaminase |
| INR | International Normalized Ratio |
| PT | Prothrombin time |
| ALP | Alkaline Phosphatase |
| HTN | Hypertension |
| CKD | chronic kidney disease |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| UGIB | Upper GI Bleeding |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| CR | Complete response |
| PR | Partial response |
| SD | Stable Disease |
| POD | Progression of Disease |
| ORR | objective response rate |
| TSH | thyroid-stimulating hormone |
References
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gomaa, A.I.; A Khan, S.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Cruz-Ramón, V.; Chinchilla-López, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book. 2018, 38, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 1 May 2025).
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Abboud, Y.; Ismail, M.; Khan, H.; Medina-Morales, E.; Alsakarneh, S.; Jaber, F.; Pyrsopoulos, N.T. Hepatocellular Carcinoma Incidence and Mortality in the USA by Sex, Age, and Race: A Nationwide Analysis of Two Decades. J. Clin. Transl. Hepatol. 2024, 12, 172–181. [Google Scholar] [CrossRef]
- Yan, Q.; Sun, Y.-S.; An, R.; Liu, F.; Fang, Q.; Wang, Z.; Xu, T.; Chen, L.; Du, J. Application and progress of the detection technologies in hepatocellular carcinoma. Genes. Dis. 2023, 10, 1857–1869. [Google Scholar] [CrossRef]
- Yıldırım, H.; Kavgaci, G.; Chalabiyev, E.; Dizdar, O. Advances in the Early Detection of Hepatobiliary Cancers. Cancers 2023, 15, 3880. [Google Scholar] [CrossRef]
- Ayoub, W.S.; Steggerda, J.; Yang, J.D.; Kuo, A.; Sundaram, V.; Lu, S.C. Current status of hepatocellular carcinoma detection: Screening strategies and novel biomarkers. Ther. Adv. Med. Oncol. 2019, 11, 1758835919869120. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; Sayed, I.E.; Ali, M.M.; Wang, Y.; Baz, A.E.; Khalil, A.A.; Elarabany, N. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt. Liver J. 2024, 14, 2. [Google Scholar] [CrossRef]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma—A Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- He, C.; Zhang, W.; Zhao, Y.; Li, J.; Wang, Y.; Yao, W.; Wang, N.; Ding, W.; Wei, X.; Yang, R.; et al. Preoperative prediction model for macrotrabecular-massive hepatocellular carcinoma based on contrast-enhanced CT and clinical characteristics: A retrospective study. Front. Oncol. 2023, 13, 1124069. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Li, H.-Y.; Wang, Z.-H.; Wu, J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim. Biophys Acta Rev. Cancer 2020, 1874, 188441. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021, 7, 113–123. [Google Scholar] [CrossRef]
- Cosgrove, D.; Tan, R.; Osterland, A.J.; Hernandez, S.; Ogale, S.; Mahrus, S.; Murphy, J.; Wilson, T.; Patton, G.; Loaiza-Bonilla, A.; et al. Atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma: Real-world experience from a US Community Oncology Network. J. Hepatocell. Carcinoma 2025, 12, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Kim, R.D.; Arora, S.P.; Arshad, J.; Kournoutas, I.A.; O’donnell, C.D.; Totev, T.I.; Tan, A.; Mu, F.; et al. Real-World Experiences Using Atezolizumab+ Bevacizumab for the Treatment of Unresectable Hepatocellular Carcinoma: A Multicenter Study. Cancers 2025, 17, 1814. [Google Scholar] [CrossRef]
- Yiu, D.C.; Chan, B.L.H.; Wong, A.C.F.; Feng, M.Y.; Chan, S.L. Real-world experiences of atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma in Hong Kong. Liver Cancer Int. 2023, 4, 121–126. [Google Scholar] [CrossRef]
- Himmelsbach, V.; Pinter, M.; Scheiner, B.; Venerito, M.; Sinner, F.; Zimpel, C.; Marquardt, J.U.; Trojan, J.; Waidmann, O.; Finkelmeier, F. Efficacy and safety of atezolizumab and bevacizumab in the real-world treatment of advanced hepatocellular carcinoma: Experience from four tertiary centers. Cancers 2022, 14, 1722. [Google Scholar] [CrossRef]
- Lee, C.L.; Freeman, M.; Burak, K.W.; Moffat, G.T.; O’donnell, C.D.J.; Ding, P.Q.; Lyubetska, H.; Meyers, B.M.; Gordon, V.; Kosyachkova, E.; et al. Real-World Outcomes of Atezolizumab with Bevacizumab Treatment in Hepatocellular Carcinoma Patients: Effectiveness, Esophagogastroduodenoscopy Utilization and Bleeding Complications. Cancers 2024, 16, 2878. [Google Scholar] [CrossRef] [PubMed]
- Hosui, A.; Hayata, N.; Kurahashi, T.; Namiki, A.; Okamoto, A.; Aochi, K.; Ashida, M.; Tanimoto, T.; Murai, H.; Ohnishi, K.; et al. Efficacy of Adding Locoregional Therapy in ATZ/BEV-Treated Patients with Stable HCC. Cancers 2025, 17, 185. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) or Median Range |
|---|---|
| Age at Initiation (years) | Median (Q1–Q3): 68 (61–73); Range: 29–91 |
| Sex | |
| Female | 19 (22%) |
| Male | 68 (78%) |
| Ethnicity | |
| Hispanic or Latino | 86 (99%) |
| Unknown | 1 (1%) |
| Race | |
| American Indian or Alaska Native | 1 (1.2%) |
| Asian | 11 (13%) |
| Black | 12 (14%) |
| White | 60 (71%) |
| Unknown | 3 (3%) |
| Vital Status | |
| Alive | 36 (41%) |
| Deceased | 51 (59%) |
| Disease Progression | |
| No Progression | 38 (45%) |
| Progression | 46 (55%) |
| Child–Pugh Score | |
| 5 | 24 (28%) |
| 6 | 28 (33%) |
| 7 | 13 (15%) |
| 8 | 12 (14%) |
| 9 | 7 (8.2%) |
| 10 | 1 (1.1%) |
| Child–Pugh Class | A: 52 (60%); B: 34 (39%); C: 1 (1.1%) |
| BCLC Stage | A: 8 (9.2%); B: 18 (21%); C: 61 (70%) |
| ECOG Performance Status | 0: 21 (24%); 1: 56 (64%); 2: 9 (10%); 3: 1 (1.1%) |
| Total (N = 87) | |
|---|---|
| Category | EGD Performed (N = 79) |
| Patient with Varices | 26 |
| Treatment for Varices | 7 |
| Band ligation | 6 |
| Beta-blockers | 1 |
| No treatment required | 13 |
| Characteristic | n (%) or Median (Range) |
|---|---|
| Best Overall Response | |
| Complete response | 6 (7.2%) |
| Disease progression | 13 (16%) |
| Partial response | 21 (25%) |
| Stable disease | 43 (52%) |
| Follow-up Time (months) | |
| Median (Q1, Q3) | 10 (Total N = 44) |
| Min, Max | 1, 44 |
| Time to Best Response | |
| Median (Q1, Q3) | 3.4 (1.9, 7.6) |
| Min, Max | 0.1, 27.7 |
| Adverse Event | Yes, n (%) | No, n (%) |
|---|---|---|
| Any Atezo/Bev Toxicity | 12 (14%) | 75 (86%) |
| Abnormal Electrolytes | 2 (2.3%) | 85 (98%) |
| Upper GI Bleeding | 3 (3.4%) | 84 (97%) |
| Intracerebral Hemorrhage | 1 (1.1%) | 86 (99%) |
| Elevated TSH | 1 (1.1%) | 86 (99%) |
| Grade 3 Hepatitis | 1 (1.1%) | 86 (99%) |
| Elevated Liver Enzymes | 1 (1.1%) | 86 (99%) |
| Poor Appetite & Fatigue | 3 (3.4%) | 84 (97%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Hamdaneh, Y.; Khasawneh, B.; Al-Rawi, M.; Al-Najjar, E.; Dhillon, V.; Alhaj, A.; Rayyan, Y.; Abdelrahim, M. Atezolizumab Plus Bevacizumab Combination Therapy in Unresectable Hepatocellular Carcinoma: An Institutional Experience. Biomedicines 2025, 13, 2844. https://doi.org/10.3390/biomedicines13122844
Esmail A, Hamdaneh Y, Khasawneh B, Al-Rawi M, Al-Najjar E, Dhillon V, Alhaj A, Rayyan Y, Abdelrahim M. Atezolizumab Plus Bevacizumab Combination Therapy in Unresectable Hepatocellular Carcinoma: An Institutional Experience. Biomedicines. 2025; 13(12):2844. https://doi.org/10.3390/biomedicines13122844
Chicago/Turabian StyleEsmail, Abdullah, Yazan Hamdaneh, Bayan Khasawneh, Maryam Al-Rawi, Ebtesam Al-Najjar, Vikram Dhillon, Ahmad Alhaj, Yaser Rayyan, and Maen Abdelrahim. 2025. "Atezolizumab Plus Bevacizumab Combination Therapy in Unresectable Hepatocellular Carcinoma: An Institutional Experience" Biomedicines 13, no. 12: 2844. https://doi.org/10.3390/biomedicines13122844
APA StyleEsmail, A., Hamdaneh, Y., Khasawneh, B., Al-Rawi, M., Al-Najjar, E., Dhillon, V., Alhaj, A., Rayyan, Y., & Abdelrahim, M. (2025). Atezolizumab Plus Bevacizumab Combination Therapy in Unresectable Hepatocellular Carcinoma: An Institutional Experience. Biomedicines, 13(12), 2844. https://doi.org/10.3390/biomedicines13122844








