Evaluation of Thermal, Hematohistological, and Dermatological Biocompatibility of LED Devices for Neonatal Phototherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Wearable Device
2.2. In Vivo Study
2.3. Procedure
2.4. Treatment
2.5. Temperature Parameters
2.6. Serum Collection
2.7. Macroscopic Clinical Evaluation
2.8. Surgical Procedure
2.9. Histological Analysis
2.10. Statistical Treatment for Data Analysis
3. Results
3.1. Biochemical and Hematological Parameters
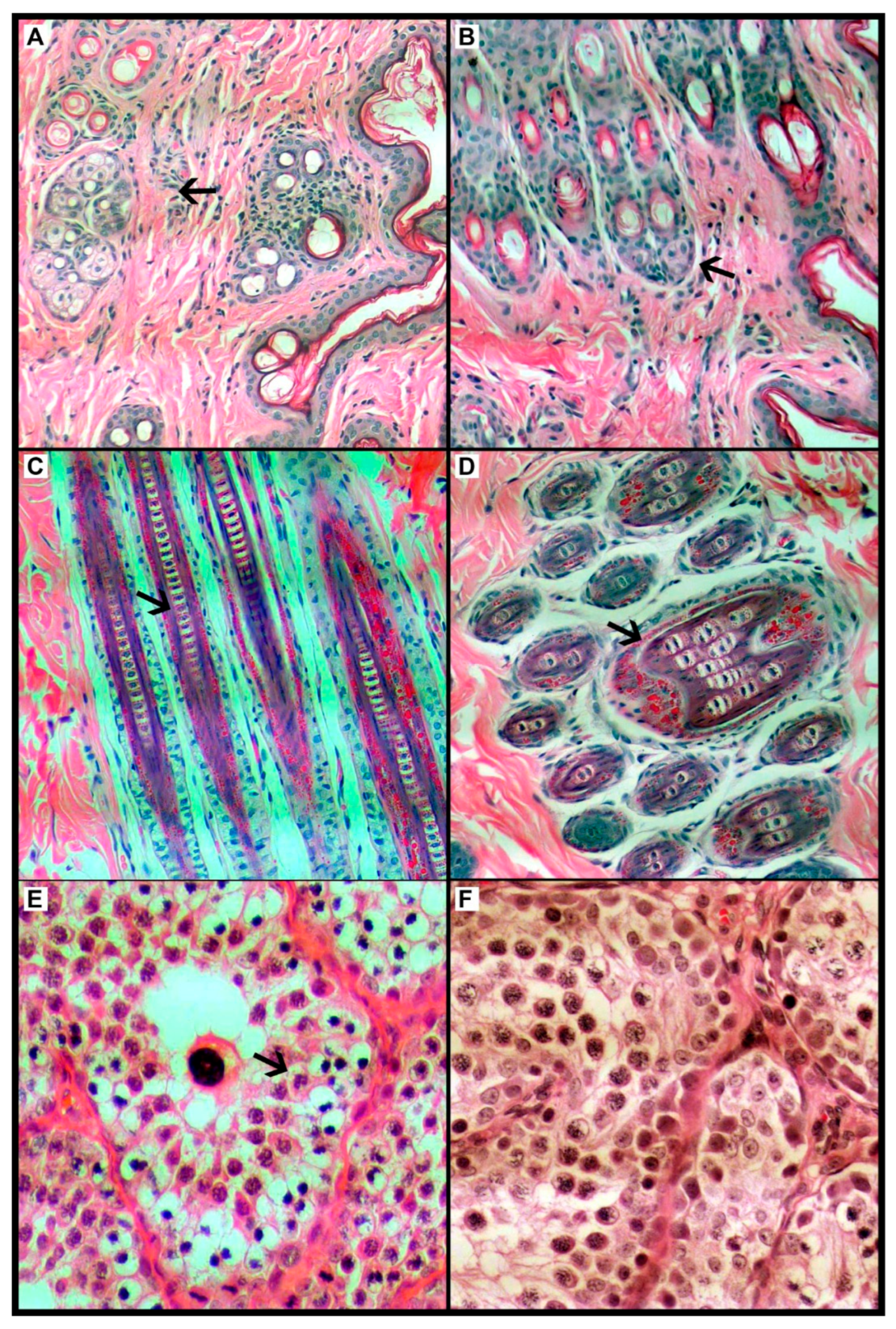
3.2. Dermatological and Histological Parameters of Skin and Gonads
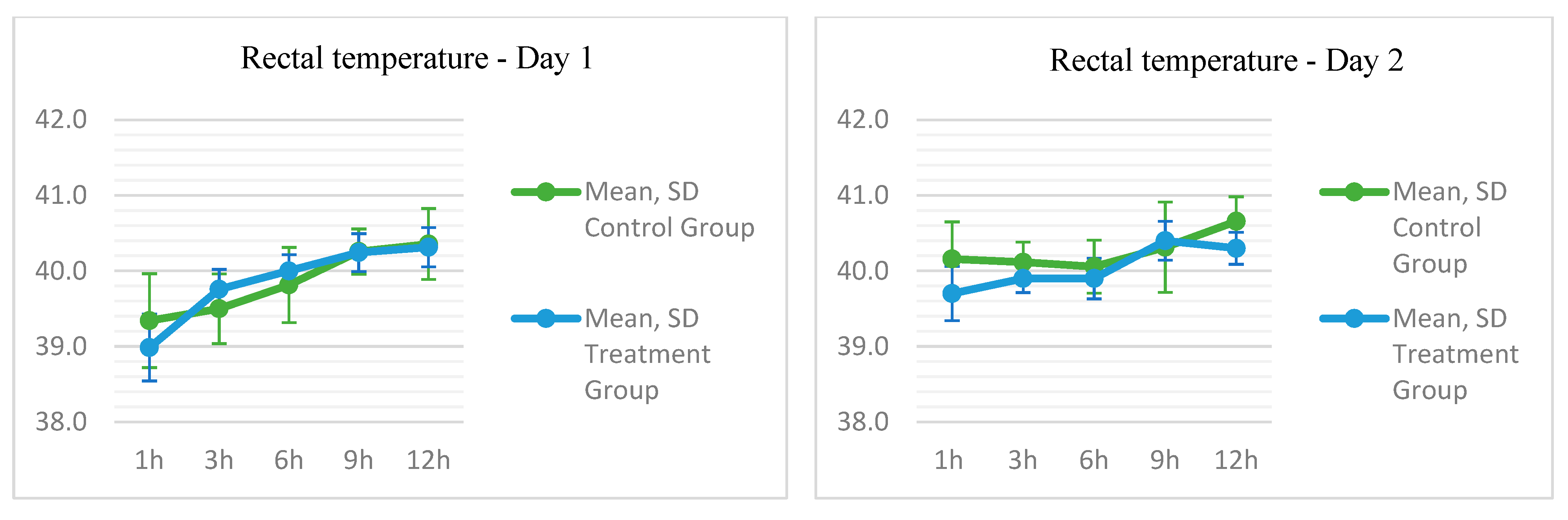
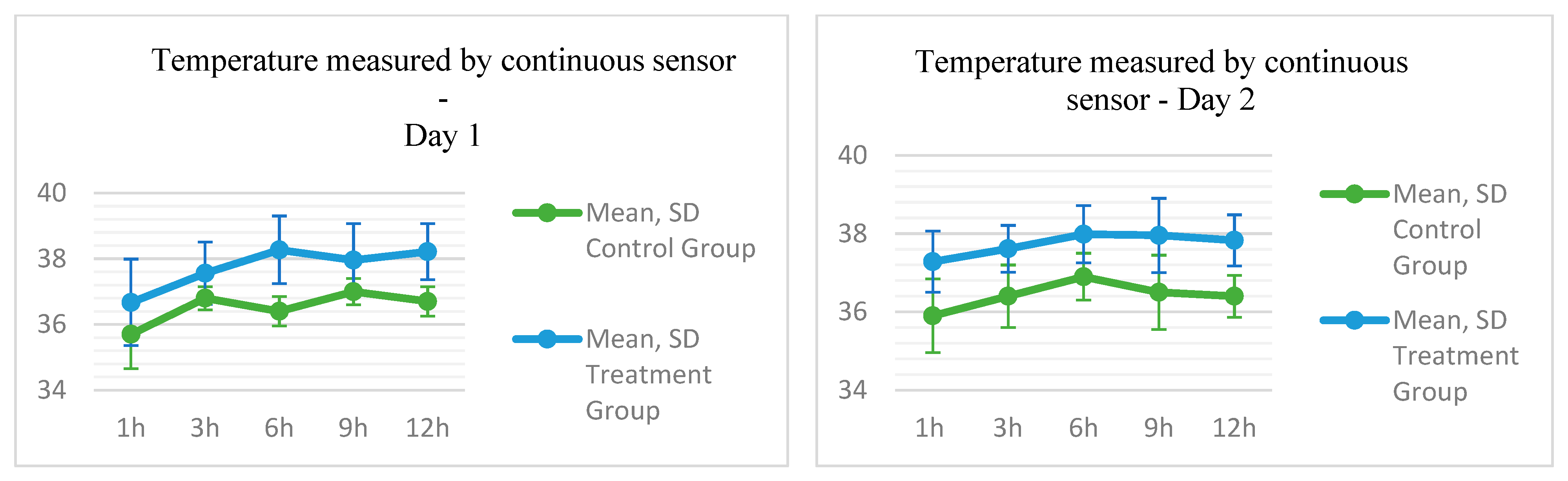
3.3. Thermal Parameters Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| ANOVA | Analysis of Variance |
| ANVISA | Agencia Nacional de Vigilância Sanitária |
| AP | Alkaline Phosphatase |
| AVMA | American Veterinary Medical Association |
| CG | Control Group |
| EDTA K3 | Ethylene-Diamine-Tetraacetic Acid |
| FDA | Food and Drug Administration |
| GGT | Gamma-Glutamyltransferase |
| H&E | Hematoxylin and Eosin |
| L | Liters |
| LED | Light-Emitting Diode |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| MCV | Mean Corpuscular Volume |
| MPV | Mean Platelet Volume |
| MRP2 | Transport Protein |
| NZW | New Zealand White |
| OLEDs | Organic Light-Emitting Diodes |
| PCV | Packed Cell Volume |
| PR | Paraná |
| PUVA | Psoralen Ultraviolet A Radiation |
| RBC | Red Blood Cell |
| RDW | Red Cell Distribution Width |
| SD | Standard Deviation |
| SP | São Paulo |
| T | Time |
| TG | Experimental Group |
| UGT1A1 | Glucuronosyltransferase |
| UI | International Unit |
| UNESP | Universidade Estadual Paulista |
| UV | Ultraviolet |
| UVB | Ultraviolet B |
| VLDL | Very-Low-Density Lipoprotein |
| cm2 | Square Centimeter(s) |
| cm3 | Cubic Centimeter(s) |
| dL | Deciliter |
| g/dL | Grams per Deciliter |
| h | Hours |
| kg | Kilograms |
| mL | Milliliter |
| mg | Milligrams |
| n | Sample Size |
| nm | Nanometer |
| p | p-Value |
| rpm | Revolutions per Minute |
| °C | Degree Celsius |
| µL | Microliters |
| µW | Microwatt |
Appendix A
| Control (n 7) | Treatment (n 7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Min. | Max. | Mean | Median | SD | Min. | Max. | p | |
| Temperature per sensor between device and skin in °C | |||||||||||
| Day 1 | |||||||||||
| Skin sensor T. D1T0 | 35.7 | 35.9 | 1.0404 | 33.5 | 36.8 | 36.7 | 36.4 | 1.3149 | 34.6 | 38.3 | 0.109 |
| Skin sensor T. D1T1 | 36.8 | 36.8 | 0.3498 | 36.2 | 37.2 | 37.6 | 37.8 | 0.9502 | 35.7 | 38.6 | 0.046 |
| Skin sensor T. D1T2 | 36.4 | 36.5 | 0.4488 | 35.8 | 37.1 | 38.3 | 38.3 | 1.0291 | 36.5 | 39.9 | 0.006 |
| Skin sensor T. D1T3 | 37.0 | 36.9 | 0.4018 | 36.5 | 37.7 | 38.0 | 38.5 | 1.1103 | 35.8 | 38.9 | 0.047 |
| Skin sensor T. D1T4 | 36.7 | 36.8 | 0.4488 | 35.8 | 37.1 | 38.2 | 38.8 | 0.8552 | 36.8 | 38.9 | 0.008 |
| Day 2 | |||||||||||
| Skin sensor T. D2T0 | 35.9 | 35.9 | 0.9414 | 34.3 | 37.0 | 37.3 | 37.3 | 0.7798 | 36.1 | 38.3 | 0.011 |
| Skin sensor T. D2T1 | 36.4 | 36.5 | 0.8000 | 35.5 | 37.4 | 37.6 | 37.5 | 0.5984 | 36.9 | 38.5 | 0.015 |
| Skin sensor T. D2T2 | 36.9 | 36.6 | 0.5984 | 36.3 | 37.8 | 38.0 | 38.3 | 0.7335 | 36.9 | 38.6 | 0.012 |
| Skin sensor T. D2T3 | 36.5 | 36.6 | 0.9517 | 35.4 | 38.3 | 38.0 | 38.5 | 0.9484 | 36.5 | 38.7 | 0.029 |
| Skin sensor T. D2T4 | 36.4 | 36.5 | 0.5407 | 35.4 | 37.0 | 37.8 | 38.0 | 0.6525 | 36.8 | 38.7 | 0.004 |
| Rectal Temperature | |||||||||||
| Day 1 | |||||||||||
| Rectal T. D1T0 | 39.3 | 39.4 | 0.6214 | 38.4 | 40.4 | 39.0 | 38.9 | 0.4413 | 38.6 | 39.9 | 0.200 |
| Rectal T. D1T1 | 39.5 | 39.5 | 0.4619 | 38.7 | 40.1 | 39.8 | 39.8 | 0.2637 | 39.2 | 40.0 | 0.244 |
| Rectal T. D1T2 | 39.8 | 39.9 | 0.4981 | 39.1 | 40.4 | 40.0 | 40.0 | 0.2160 | 39.7 | 40.3 | 0.520 |
| Rectal T. D1T3 | 40.3 | 40.3 | 0.2992 | 39.9 | 40.7 | 40.2 | 40.1 | 0.2507 | 40.0 | 40.6 | 0.846 |
| Rectal T. D1T4 | 40.4 | 40.1 | 0.4685 | 39.9 | 41.1 | 40.3 | 40.3 | 0.2610 | 40.0 | 40.8 | 0.796 |
| Day 2 | |||||||||||
| Rectal T. D2T0 | 40.2 | 40.0 | 0.4928 | 39.6 | 40.9 | 39.7 | 39.7 | 0.3592 | 39.1 | 40.1 | 0.096 |
| Rectal T. D2T1 | 40.1 | 40.1 | 0.2673 | 39.6 | 40.5 | 40.0 | 39.9 | 0.1864 | 39.7 | 40.2 | 0.294 |
| Rectal T. D2T2 | 40.1 | 40.1 | 0.3505 | 39.6 | 40.6 | 39.9 | 39.9 | 0.2690 | 39.6 | 40.4 | 0.480 |
| Rectal T. D2T3 | 40.3 | 40.2 | 0.5984 | 39.7 | 41.5 | 40.4 | 40.4 | 0.2573 | 40.0 | 40.6 | 0.440 |
| Rectal T. D2T4 | 40.7 | 40.7 | 0.3259 | 40.1 | 41.1 | 40.3 | 40.3 | 0.2116 | 40.1 | 40.7 | 0.039 |
Appendix B
| Groups | Control (n 7) | Treatment (n 7) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin Assessment | R2 | R4 | R6 | R8 | R10 | R12 | R14 | R4 | R6 | R5 | R7 | R9 | R11 | R13 | |
| T 0 Day 1 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 1 Day 1 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 2 Day 2 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 3 Day 2 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 4 Day 3 | Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 5 Day 5 | Erythema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T 6 Day 7 | Erythema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vesiculobullous | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Exulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ulcerations | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
References
- Lin, Q.; Zhu, D.; Chen, C.; Feng, Y.; Shen, F.; Wu, Z. Risk factors for neonatal hyperbilirubinemia: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Seung Lee, J.; Kim, J.; Ye, Y.; Kim, T. Materials and device design for advanced phototherapy systems. Adv. Drug Deliv. Rev. 2022, 186, 114339. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L.; Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Medicina Interna de Harrison, 20th ed.; AMGH: Porto Alegre, Brazil, 2020. [Google Scholar]
- Martinez, J.B.; Dantas, M.; Voltarelli, J.C. Semiologia Geral e Especializada; Guanabara Koogan: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Wang, Y.; Wei, R.; Zhao, W.; Zhao, C. Bilirubin Removal by Polymeric Adsorbents for Hyperbilirubinemia Therapy. Macromol. Biosci. 2023, 23, 2200567. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.H.; Huaman, K.; Caballero, P. Light-Emitting Diode (LED) Phototherapy Versus Non-LED Phototherapy Devices for Hyperbilirubinemia in Neonates: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2022, 40, 1618–1628. [Google Scholar] [CrossRef]
- Nascimento, T.F.; de Avila, M.A.G.; Bocchi, S.C.M. From suffering to resignation: Grounded Theory approach to maternal experience with newborn in phototherapy. Rev. Bras. Saúde Matern. Infant. 2018, 18, 143–151. [Google Scholar] [CrossRef]
- Føreland, A.M.; Rosenberg, L.; Johannessen, B. Nurses’ experiences using conventional overhead phototherapy versus fibreoptic blankets for the treatment of neonatal hyperbilirubinemia. J. Neonatal Nurs. 2016, 22, 108–114. [Google Scholar] [CrossRef]
- Shahriarpanah, S.; Haji Ebrahim Tehrani, F.; Davati, A.; Ansari, I. Effect of Phototherapy on Serum Level of Calcium, Magnesium and Vitamin D in Infants with Hyperbilirubinemia. Iran. J. Pathol. 2018, 13, 357–362. [Google Scholar]
- Shawky, A.H.; Refaee, A. Risk factors for short-term side effects of phototherapy in neonatal jaundice. Arch. Dis. Child. 2021, 106, A124–A125. [Google Scholar] [CrossRef]
- Xiong, T.; Qu, Y.; Cambier, S.; Mu, D. The side effects of phototherapy for neonatal jaundice: What do we know? What should we do? Eur. J. Pediatr. 2011, 170, 1247–1255. [Google Scholar] [CrossRef]
- Jahanshahifard, S.; Ahmadpour-Kacho, M.; Pasha, Y. Effects of phototherapy on Cytokines′ levels and white blood cells in term neonate with hyperbilirubinemia. J. Clin. Neonatol. 2012, 1, 139. [Google Scholar] [CrossRef]
- Zarkesh, M.; Dalili, S.; Fallah, M.; Heidarzadeh, A.; Rad, A. The effect of neonatal phototherapy on serum level of interlukin-6 and white blood cells′ count. J. Clin. Neonatol. 2016, 5, 189. [Google Scholar] [CrossRef]
- Mrkaić, L.; Kamenov, B.; Najman, S.; Dimitrijević, H.; Mitrović, V.; Maglajlić, S. Neonatal immune system changes caused by phototherapy. Srp. Arh. Celok. Lek. 1994, 122 (Suppl. 1), 36–37. [Google Scholar] [PubMed]
- Yurdakök, M. Phototherapy in the newborn: What’s new? J. Pediatr. Neonatal Individ. Med. 2015, 4, e040255. [Google Scholar] [CrossRef]
- Eghbalian, F.; Shabani, S.; Faradmal, J.; Jenabi, E. Effects of Phototherapy on the Serum Magnesium Level in Neonates with Indirect Hyperbilirubinemia: A Prospective Cohort Study. Int. J. Pediatr. 2022, 2022, 5439630. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, K.; Mani, S.; Vasudevan, N.; Preethi, S.; Krishnamoorthy, N.; Pratibha, R.K.; Sundar, S. Effect of Light-Emitting Diode Phototherapy on Serum Calcium Levels in Neonates with Jaundice. Cureus 2022, 14, e23938. [Google Scholar] [CrossRef]
- Bouceiro Mendes, R.; Alpalhão, M.; Filipe, P. UVB phototherapy in the treatment of vitiligo: State of the art and clinical perspectives. Photodermatol. Photoimmunol. Photomed. 2022, 38, 215–223. [Google Scholar] [CrossRef]
- Stern, R.S. Genital Tumors among Men with Psoriasis Exposed to Psoralens and Ultraviolet A Radiation (PUVA) and Ultraviolet B Radiation. N. Engl. J. Med. 1990, 322, 1093–1097. [Google Scholar] [CrossRef]
- Tkacs, N.C.; Thompson, H.J. From Bedside to Bench and Back Again: Research Issues in Animal Models of Human Disease. Biol. Res. Nurs. 2006, 8, 78–88. [Google Scholar] [CrossRef]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for Neonatal Jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef]
- Lacerda, G.S. Sistema Fototerápico Vestível para Tratamento Contínuo da Icterícia Neonatal. Ph.D. Thesis, Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2019. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, M.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Jain, N. Schalm’s Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1986; pp. 20–86. [Google Scholar]
- Brecher, G.; Cronkite, E.P. Morphology and Enumeration of Human Blood Platelets. J. Appl. Physiol. 1950, 3, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.H. Dermal toxicity. Apprais. Safe Chem. Foods Drugs Cosmet. 1959, 46–59. [Google Scholar]
- Leary, S.L. American Veterinary Medical Association Guidelines for the Euthanasia of Animals—Version 20; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Nascimento, T.F.; Cristina Mangini Bocchi, S.; César Lyra, J.; Fernando Bianchi, R.; Silveira Lacerda, G.; Patrícia Fernandes Abadde, L.; Rocha, N.S.; Vieira, S.E.; Langoni, H.; do Nascimento, C.N.; et al. Evaluation of Thermal; Hemato-Histological and Dermatological Biocompatibility of a LED Device for Neonatal Phototherapy. Mendeley Data 2023. [Google Scholar] [CrossRef]
- Altuntas, N.; Dogan, O.; Kislal, F. Effect of Phototherapy on Neutrophil VCS Parameters and White Blood Cells. J. Coll. Physicians Surg. Pak. 2019, 29, 453–455. [Google Scholar] [CrossRef]
- Souza, C.D.; Eren, M.V. Monocytes and Macrophages and Their Disorders. In Schalm’s Veterinary Hematology; Wiley: Hoboken, NJ, USA, 2022; pp. 386–394. [Google Scholar] [CrossRef]
- Suneja, S.; Rajani, K.; Saxena, R. Effect of phototherapy on biochemical parameters in neonatal hyperbilirubinemia patients: A clinical insight. Indian J. Neonatal Med. Res. 2018, 6, 13–18. [Google Scholar]
- Akpinar Gozetici, M.; Kizilelma Yigit, A.; Beser, O.F. Effect of Phototherapy on Serum Electrolyte Levels. Cerrahpasa Med. J. 2021, 45, 16–20. [Google Scholar] [CrossRef]
- Cetinkursun, S.; Demirbag, S.; Cincik, M.; Baykal, B.; Gunal, A. Effects of phototherapy on newborn rat testicles. Arch. Androl. 2006, 52, 61–70. [Google Scholar] [CrossRef]
- Koç, H.; Altunhan, H.; Dilsiz, A.; Kaymakçi, A.; Duman, S.; Oran, B.; Erkul, I. Testicular Changes in Newborn Rats Exposed to Phototherapy. Pediatr. Dev. Pathol. 1999, 2, 333–336. [Google Scholar] [CrossRef]
- Savedra, R.M.L.; Fonseca, A.M.T.; Silva, M.M.; Bianchi, R.F.; Siqueira, M.F. White LED phototherapy as an improved treatment for neonatal jaundice. Rev. Sci. Instrum. 2021, 92, 064101. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.R.; Tannure, A.M.; Cardoso, L.C.; Siqueira, M.F.; Bianchi, A.C.G.; Bianchi, R.F. Colorimetric dosimeter to promote most efficient use of neonatal phototherapy. Sens. Actuators B Chem. 2016, 240, 1003–1008. [Google Scholar] [CrossRef]
- Wu, R.; Wen, L. Meta-analysis of the efficacy of different blue light therapy methods for neonatal jaundice. J. Matern. -Fetal Neonatal Med. 2024, 38, 1. [Google Scholar] [CrossRef]
- Treesirichod, A.; Eiamkulbutr, S.; Laohathai, P.; Vongbhavit, K.; Panburana, J. The efficacy of infrared filter window film to prevent hyperthermia in neonatal hyperbilirubinemia with conventional phototherapy: A randomized control trial. Pediatr. Neonatol. 2022, 63, 489–495. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, Y.; Kwon, J.H.; Ihm, C.; Kim, S.Y.; Choi, K.C. Wearable Photomedicine for Neonatal Jaundice Treatment Using Blue Organic Light-Emitting Diodes (OLEDs): Toward Textile-Based Wearable Phototherapeutics. Adv. Sci. 2022, 9, 2204622. [Google Scholar] [CrossRef]

| Measure | Before Starting the Experiment (T0) | ||
|---|---|---|---|
| Control (n 7) | Treatment (n 7) | p-Value | |
| M SD | M SD | ||
| Urea (mg/dL−1) | 38.1 ± 7.9042 | 31.1 ± 4.0178 | 0.059 |
| Creatinine (mg/dL−1) | 0.8 ± 0.1604 | 0.7 ± 0.0770 | 0.249 |
| ALT—alanine aminotransferase (UI-L) | 51.6 ± 10.7371 | 54.9 ± 15.3126 | 0.650 |
| ALP—alkaline phosphatase (UI-L) | 165.9 ± 66.5919 | 177.6 ± 30.7401 | 0.680 |
| Gamma glutamyl transferase (UI-L) | 3.7 ± 2.6475 | 4.5 ± 1.5703 | 0.475 |
| Serum total protein (UI-L) | 5.8 ± 0.1397 | 5.7 ± 0.4923 | 0.566 |
| Albumin (gdL−1) | 4.0 ± 0.2440 | 4.1 ± 0.1864 | 0.550 |
| Globulin (gdL-1) | 1.8 ± 0.3309 | 1.6 ± 0.3891 | 0.259 |
| Total bilirubin (mg/dL−1) | 0.1 ± 0.0549 | 0.1 ± 0.0787 | 0.276 |
| Direct bilirubin (mg/dL−1) | 0.0 ± 0.0488 | 0.0 ± 0.0378 | 0.552 |
| Indirect bilirubin (mg/dL−1) | 0.1 ± 0.0756 | 0.1 ± 0.0951 | 0.237 |
| Measure | After treatment (T2) | ||
| Urea (mg/dL−1) | 32.9 ± 8.3152 | 28.4 ± 5.1916 | 0.255 |
| Creatinine (mg/dL−1) | 0.8 ± 0.1941 | 0.8 ± 0.1243 | 0.577 |
| ALT—alanine aminotransferase (UI-L) | 58.0 ± 14.8773 | 59.4 ± 13.6364 | 0.855 |
| ALP—alkaline phosphatase (UI-L) | 158.3 ± 44.4512 | 195.7 ± 46.8640 | 0.151 |
| Gamma glutamyl transferase (UI-L) | 3.9 ± 2.5844 | 4.5 ± 1.6359 | 0.622 |
| Serum total protein (UI-L) | 6.0 ± 0.3101 | 5.9 ± 0.5460 | 0.598 |
| Albumin (gdL−1) | 4.2 ± 0.2289 | 4.2 ± 0.2160 | 0.814 |
| Globulin (gdL−1) | 1.6 ± 0.7616 | 1.7 ± 0.4276 | 0.865 |
| Total bilirubin (mg/dL−1) | 0.1 ± 0.0378 | 0.1 ± 0.0690 | 0.356 |
| Direct bilirubin (mg/dL−1) | 0.0 ± 0.0378 | 0.0 ± 0.0690 | 1.000 |
| Indirect bilirubin (mg/dL−1) | 0.1 ± 0.0577 | 0.1 ± 0.0577 | 1.000 |
| Variable | pMauchly | pANOVA | Multiple Comparisons |
|---|---|---|---|
| Urea (mg/dL−1) | 0.073 | <0.001 | M0 > M1, M2 |
| Creatinine (mg/dL−1) | 0.25 | 0.031 | M1 < M2 |
| ALT—alanine aminotransferase (UI-L) | 0.183 | 0.066 | M0 = M1 = M2 |
| ALP—alkaline phosphatase (UI-L) | 0.454 | 0.806 | M0 = M1 = M2 |
| Gamma glutamyl transferase (UI-L) | 0.087 | 0.291 | M0 = M1 = M2 |
| Serum total protein (UI-L) | <0.001 | 0.352 | M0 = M1 = M2 |
| Albumin (gdL−1) | <0.001 | 0.330 | M0 = M1 = M2 |
| Globulin (gdL−1) | <0.001 | 0.420 | M0 = M1 = M2 |
| Total bilirubin (mg/dL−1) | 0.634 | 0.546 | M0 = M1 = M2 |
| Direct bilirubin (mg/dL−1) | 0.022 | 0.706 | M0 = M1 = M2 |
| Indirect bilirubin (mg/dL−1) | 0.158 | 0.753 | M0 = M1 = M2 |
| Measure | Before (T0) | ||
|---|---|---|---|
| Control (n 7 a) | Treatment (n 7) | p-Value | |
| M DP | M DP | ||
| Red blood cells (µL) | 5.9 ± 0.6770 | 5.9 ± 0.6625 | 0.991 |
| Hemoglobin (g/dL) | 11.4 ± 0.9661 | 11.6 ± 1.1838 | 0.753 |
| Hematocrit (%) | 39.3 ± 2.6277 | 40.0 ± 3.0000 | 0.644 |
| MCV (fL) | 66.8 ± 5.3232 | 67.9 ± 5.0340 | 0.692 |
| MCHC (%) | 29.1 ± 2.8644 | 29.0 ± 2.4478 | 0.938 |
| PT (Plasma) (g/dL) | 5.9 ± 0.2545 | 5.9 ± 0.4860 | 0.788 |
| RDW (%) a | 14.8 ± 1.6669 | 14.9 ± 1.5394 | 0.930 |
| Platelets (µL) (105) | 3.17 ± 0.64 | 3.73 ± 0.10 | 0.289 |
| Leukocytes (µL) | 7814.3 ± 5148.2776 | 6557.1 ± 1739.5949 | 0.552 |
| Relative neutrophils (%) | 41.3 ± 17.4520 | 45.0 ± 12.8452 | 0.658 |
| Absolute neutrophils (µL) | 3804.7 ± 4231.2037 | 3011.4 ± 1505.5346 | 0.649 |
| Relat lymphocytes (%) | 51.3 ± 17.2889 | 47.4 ± 15.1091 | 0.665 |
| Abs lymphocytes (µL) | 3515.0 ± 1331.9323 | 3084.7 ± 1187.8752 | 0.536 |
| Relat eosinophils (%) | 1.7 ± 0.7559 | 2.3 ± 1.6036 | 0.410 |
| Abs eosinophils (µL) | 115.7 ± 53.3439 | 145.0 ± 125.1919 | 0.580 |
| Relat basophils (%) | 0.1 ± 0.3780 | 0.0 ± 0.0000 | 0.337 |
| Abs basophils (µL) | 4.6 ± 12.0949 | 0.0 ± 0.0000 | 0.337 |
| Relat monocytes (%) | 5.4 ± 4.2762 | 5.6 ± 3.9940 | 0.950 |
| Abs monocytes (µL) | 363.7 ± 358.9562 | 336.6 ± 167.4344 | 0.859 |
| Measure | After (T2) | ||
| Red blood cells (µL) | 6.2 ± 0.5029 | 6.1 ± 0.2829 | 0.858 |
| Hemoglobin (g/dL) | 12.1 ± 0.6630 | 12.1 ± 0.8789 | 0.973 |
| Hematocrit (%) | 40.9 ± 2.1931 | 40.7 ± 1.7043 | 0.894 |
| MCV (fL) | 66.5 ± 3.9352 | 66.5 ± 2.9648 | 0.975 |
| MCHC (%) | 29.8 ± 2.0244 | 29.8 ± 1.5261 | 0.999 |
| PT (Plasma) (g/dL) | 6.2 ± 0.1799 | 6.0 ± 0.5589 | 0.532 |
| RDW (%) b | 14.4 ± 1.1545 | 15.0 ± 1.4318 | 0.438 |
| Platelets (µL) (105) | 327,285.7 ± 98,915.8037 | 359,878.6 ± 63,324.7359 | 0.477 |
| Leukocytes (µL) | 7371.4 ± 3233.0142 | 6028.6 ± 832.0943 | 0.308 |
| Relative segmented (%) | 42.1 ± 13.2844 | 49.4 ± 11.2229 | 0.289 |
| Absolute segmented (µL) | 3368.0 ± 2695.3135 | 3015.0 ± 918.9525 | 0.749 |
| Relative lymphocytes (%) | 48.9 ± 13.2467 | 44.0 ± 11.3578 | 0.476 |
| Absolute lymphocytes (µL) | 3339.1 ± 701.1746 | 2641.0 ± 732.8708 | 0.094 |
| Relative eosinophils (%) | 1.1 ± 1.4639 | 1.7 ± 1.7995 | 0.527 |
| Absolute eosinophils (µL) | 100.1 ± 158.5564 | 114.4 ± 94.9664 | 0.841 |
| Relative basophils (%) | 0.0 ± 0.0000 | 0.0 ± 0.0000 | |
| Absolute basophils (µL) | 0.0 ± 0.0000 | 0.0 ± 0.0000 | |
| Relative monocytes (%) | 7.9 ± 4.4132 | 4.9 ± 3.1320 | 0.168 |
| Absolute monocytes (µL) | 564.1 ± 310.0137 b | 278.4 ± 152.5766 b | 0.049 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, T.F.; Bocchi, S.C.M.; Lyra, J.C.; Bianchi, R.F.; Junior, L.d.A.D.; Lacerda, G.S.; Abadde, L.P.F.; Rocha, N.S.; Vieira, S.E.; Langoni, H.; et al. Evaluation of Thermal, Hematohistological, and Dermatological Biocompatibility of LED Devices for Neonatal Phototherapy. Biomedicines 2025, 13, 2826. https://doi.org/10.3390/biomedicines13112826
Nascimento TF, Bocchi SCM, Lyra JC, Bianchi RF, Junior LdAD, Lacerda GS, Abadde LPF, Rocha NS, Vieira SE, Langoni H, et al. Evaluation of Thermal, Hematohistological, and Dermatological Biocompatibility of LED Devices for Neonatal Phototherapy. Biomedicines. 2025; 13(11):2826. https://doi.org/10.3390/biomedicines13112826
Chicago/Turabian StyleNascimento, Tayomara Ferreira, Silvia Cristina Mangini Bocchi, João Cesar Lyra, Rodrigo Fernando Bianchi, Lauro de Assis Duarte Junior, Giselle Silveira Lacerda, Luciana Patrícia Fernandes Abadde, Noeme Sousa Rocha, Susana Eduardo Vieira, Hélio Langoni, and et al. 2025. "Evaluation of Thermal, Hematohistological, and Dermatological Biocompatibility of LED Devices for Neonatal Phototherapy" Biomedicines 13, no. 11: 2826. https://doi.org/10.3390/biomedicines13112826
APA StyleNascimento, T. F., Bocchi, S. C. M., Lyra, J. C., Bianchi, R. F., Junior, L. d. A. D., Lacerda, G. S., Abadde, L. P. F., Rocha, N. S., Vieira, S. E., Langoni, H., Nascimento, C. N. d., & Jensen, R. (2025). Evaluation of Thermal, Hematohistological, and Dermatological Biocompatibility of LED Devices for Neonatal Phototherapy. Biomedicines, 13(11), 2826. https://doi.org/10.3390/biomedicines13112826









