Immaturity-Dependent Hippocampal Neurogenic Promotion and Fate Shift by Low-Dose Propofol in Neonatal Mice Revealed Through Single-Nuclei RNA-Sequencing
Abstract
1. Introduction
2. Methods
2.1. snRNA-Seq Data Processing
2.2. Pseudotime Analysis
2.3. Differentially Expressed Gene (DEG) Identification
2.4. Cell–Cell Communication Analysis
3. Results
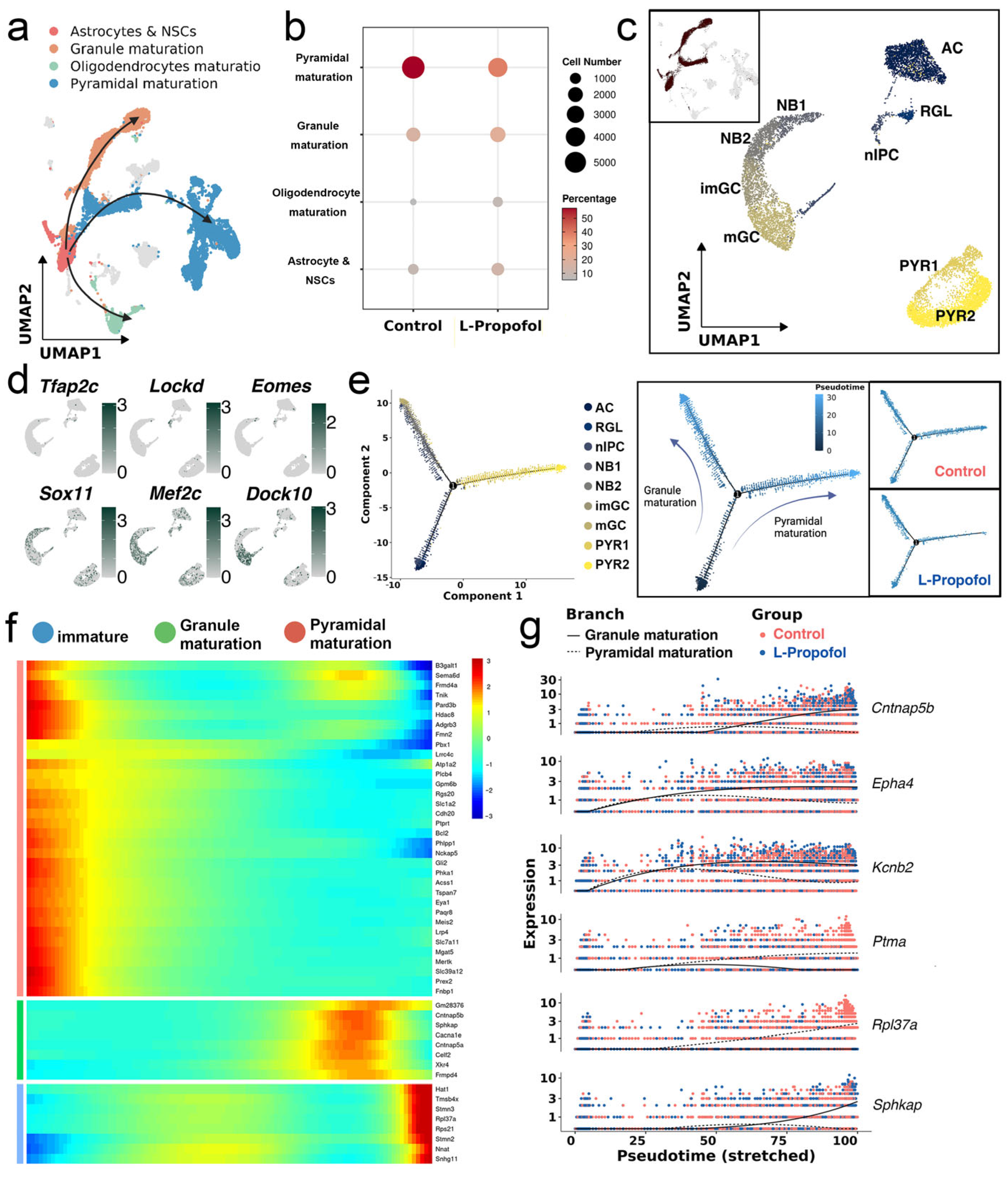
3.1. Cell Type Annotation of Single-Nucleus Transcriptomic Landscape in Neonatal Mouse Hippocampus
3.2. Low-Dose Propofol Alters the Direction of Neurogenic Lineage Differentiation
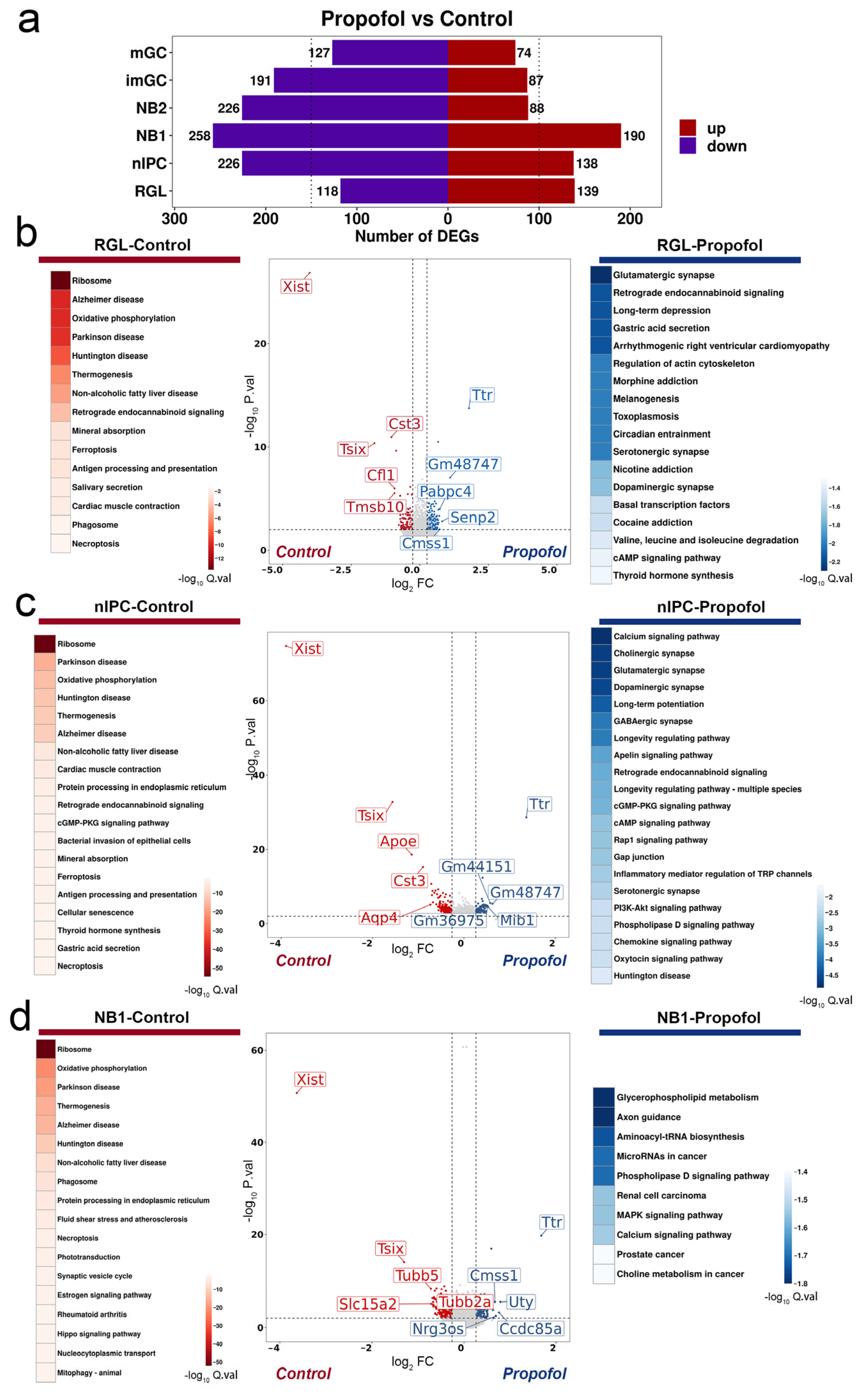
3.3. Propofol Cooperatively Regulates Neuronal Differentiation by Activating Synaptic Plasticity
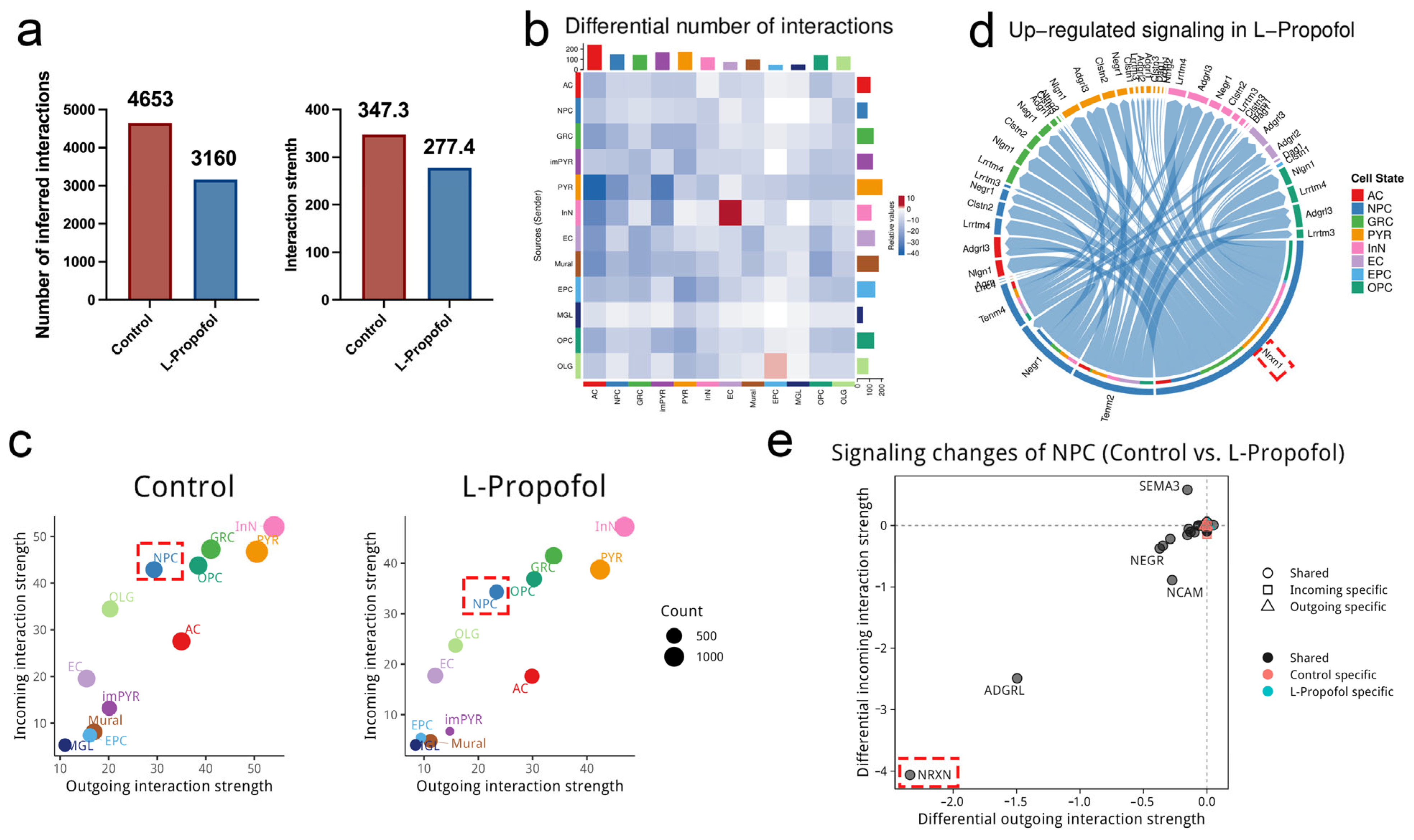
3.4. Cell–Cell Communication Analysis Highlights the Nrxn Signaling Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Astrocyte & neural stem cells |
| BEAM | Branch Expression Analysis Modeling |
| DEG | Differentially expressed gene |
| DG | Dentate gyrus |
| E12 | Embryo day 12 |
| EC | Endothelial cells |
| EPC | Ependymocytes |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| GRC | Granule cells |
| imGC | Immature granule cells |
| imPYR | Immature pyramidal neurons |
| InN | Interneurons |
| L-Propofo | low-dose propofol |
| MGL | Microglia |
| mGC | Mature granule cells |
| Mural | Mural cells |
| NB | Neuroblasts |
| NL1 | Neuroligin-1 |
| Nrxn | Neurexin |
| nIPC | Neural intermediate progenitor cells |
| NPC | Neural progenitor cells |
| NSCs | Neural stem cells |
| OLG | Oligodendrocytes |
| OPC | Oligodendrocyte precursor cells |
| PCA | Principal component analysis |
| PND | Postnatal day |
| PYR | Mature pyramidal neurons |
| RGL | Radial glia-like cells |
| snRNA-seq | Single-nucleus RNA-sequencing |
| UMAP | Uniform Manifold Approximation and Projection |
References
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 2018, 38, 10401–10410. [Google Scholar] [CrossRef] [PubMed]
- Franjic, D.; Skarica, M.; Ma, S.; Arellano, J.I.; Tebbenkamp, A.T.N.; Choi, J.; Xu, C.; Li, Q.; Morozov, Y.M.; Andrijevic, D.; et al. Transcriptomic Taxonomy and Neurogenic Trajectories of Adult Human, Macaque, and Pig Hippocampal and Entorhinal Cells. Neuron 2022, 110, 452–469.e14. [Google Scholar] [CrossRef] [PubMed]
- Kjell, J.; Fischer-Sternjak, J.; Thompson, A.J.; Friess, C.; Sticco, M.J.; Salinas, F.; Cox, J.; Martinelli, D.C.; Ninkovic, J.; Franze, K.; et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26, 277–293.e8. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Zhang, Q.; Zhang, Y. The Functions of Long Non-Coding RNAs in Neural Stem Cell Proliferation and Differentiation. Cell Biosci. 2020, 10, 74. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood Factors Transfer Beneficial Effects of Exercise on Neurogenesis and Cognition to the Aged Brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Jung, S.; Choe, S.; Woo, H.; Jeong, H.; An, H.-K.; Moon, H.; Ryu, H.Y.; Yeo, B.K.; Lee, Y.W.; Choi, H.; et al. Autophagic Death of Neural Stem Cells Mediates Chronic Stress-Induced Decline of Adult Hippocampal Neurogenesis and Cognitive Deficits. Autophagy 2020, 16, 512–530. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Kim, K.-M.; Choi, B.-M.; Park, S.-W.; Lee, S.-H.; Christensen, L.V.; Zhou, J.; Yoo, B.-H.; Shin, H.-W.; Bae, K.-S.; Kern, S.E.; et al. Pharmacokinetics and Pharmacodynamics of Propofol Microemulsion and Lipid Emulsion after an Intravenous Bolus and Variable Rate Infusion. Anesthesiology 2007, 106, 924–934. [Google Scholar] [CrossRef]
- Chang, C.; Bai, W.; Li, J.; Huo, S.; Wang, T.; Shao, J. Effects of Subchronic Propofol Administration on the Proliferation and Differentiation of Neural Stem Cells in Rat Hippocampus. Curr. Ther. Res. Clin. Exp. 2023, 98, 100691. [Google Scholar] [CrossRef]
- Huang, J.; Jing, S.; Chen, X.; Bao, X.; Du, Z.; Li, H.; Yang, T.; Fan, X. Propofol Administration during Early Postnatal Life Suppresses Hippocampal Neurogenesis. Mol. Neurobiol. 2016, 53, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Bulthuis, N.E.; Cameron, H.A. The Effects of Anesthesia on Adult Hippocampal Neurogenesis. Front. Neurosci. 2020, 14, 588356. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y. Propofol Addiction: The Mechanism Issues We Need to Know. Anesthesiol. Perioper. Sci. 2024, 2, 6. [Google Scholar] [CrossRef]
- Qiao, H.; Li, Y.; Xu, Z.; Li, W.; Fu, Z.; Wang, Y.; King, A.; Wei, H. Propofol Affects Neurodegeneration and Neurogenesis by Regulation of Autophagy via Effects on Intracellular Calcium Homeostasis. Anesthesiology 2017, 127, 490–501. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, F.; Chen, H.; Tang, Y.; Xing, T.; Luo, Q.; Yu, L.; Du, J.; Shen, J.; Zhang, L. Toxoplasma Gondii Dense Granule Protein 15 Induces Apoptosis in Choriocarcinoma JEG-3 Cells through Endoplasmic Reticulum Stress. Parasit. Vectors 2018, 11, 251. [Google Scholar] [CrossRef]
- Jiang, S.; Ge, D.; Song, B.; Deng, X.; Liu, Z.; He, J.; Sun, J.; Zhu, Z.; Meng, Z.; Zhu, Y. Subanesthetic Propofol Alleviates Chronic Stress-Induced Anxiety by Enhancing VTADA Neurons’ Activity. Neuropharmacology 2025, 265, 110264. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, X.; Peng, K.; Qin, H.; Yang, K.; Cai, F.; Hu, J.; Zhang, Y. Propofol Alleviates Anxiety-Like Behaviors Associated with Pain by Inhibiting the Hyperactivity of PVNCRH Neurons via GABAA Receptor Β3 Subunits. Adv. Sci. 2024, 11, e2309059. [Google Scholar] [CrossRef]
- Zacny, J.P.; Coalson, D.W.; Young, C.J.; Klafta, J.M.; Lichtor, J.L.; Rupani, G.; Thapar, P.; Apfelbaum, J.L. Propofol at Conscious Sedation Doses Produces Mild Analgesia to Cold Pressor-Induced Pain in Healthy Volunteers. J. Clin. Anesth. 1996, 8, 469–474. [Google Scholar] [CrossRef]
- Montgomery, J.E.; Sutherland, C.J.; Kestin, I.G.; Sneyd, J.R. Infusions of Subhypnotic Doses of Propofol for the Prevention of Postoperative Nausea and Vomiting. Anaesthesia 1996, 51, 554–557. [Google Scholar] [CrossRef]
- Feldman, D.A.; Jones, K.G.; Vonesh, L.C.; Jacobs, R.; Hoffman, N.; Lybbert, C.; Huang, J.; Kuck, K.; Odell, D.; Tadler, S.C.; et al. Immediate Effects of Propofol on Mood: A Randomized Comparison of Two Doses in a Cohort with Depression. Psychopharmacology 2025, 242, 481–495. [Google Scholar] [CrossRef]
- Chen, K.; Lu, D.; Yang, X.; Zhou, R.; Lan, L.; Wu, Y.; Wang, C.; Xu, X.; Jiang, M.H.; Wei, M.; et al. Enhanced Hippocampal Neurogenesis Mediated by PGC-1α-Activated OXPHOS after Neonatal Low-Dose Propofol Exposure. Front. Aging Neurosci. 2022, 14, 925728. [Google Scholar] [CrossRef]
- Kim, I.T. Adjustable Strabismus Surgery under Intravenous Anesthesia with Propofol and Fentanyl. J. Korean Ophthalmol. Soc. 2007, 48, 1522–1526. [Google Scholar] [CrossRef]
- Upton, D.H.; Popovic, K.; Fulton, R.; Kassiou, M. Anaesthetic-Dependent Changes in Gene Expression Following Acute and Chronic Exposure in the Rodent Brain. Sci. Rep. 2020, 10, 9366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, X.; Li, S. Propofol Promotes Migration, Alleviates Inflammation, and Apoptosis of Lipopolysaccharide-Induced Human Pulmonary Microvascular Endothelial Cells by Activating PI3K/AKT Signaling Pathway via Upregulating APOM Expression. Drug Dev. Res. 2022, 83, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Song, R.; Pang, Q.; Liu, Y.; Zhuang, J.; Chen, Y.; Hu, J.; Hu, J.; Liu, Y.; Liu, Z.; et al. Propofol Inhibits Parthanatos via ROS-ER-Calcium-Mitochondria Signal Pathway in Vivo and Vitro. Cell Death Dis. 2018, 9, 932. [Google Scholar] [CrossRef]
- Tabnak, P.; Masrouri, S.; Geraylow, K.R.; Zarei, M.; Esmailpoor, Z.H. Targeting MiRNAs with Anesthetics in Cancer: Current Understanding and Future Perspectives. Biomed. Pharmacother. 2021, 144, 112309. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, D.A.; Cryan, J.F.; Nolan, Y.M. Born This Way: Hippocampal Neurogenesis across the Lifespan. Aging Cell 2019, 18, e13007. [Google Scholar] [CrossRef]
- Cavalieri, D.; Angelova, A.; Islah, A.; Lopez, C.; Bocchio, M.; Bollmann, Y.; Baude, A.; Cossart, R. CA1 Pyramidal Cell Diversity Is Rooted in the Time of Neurogenesis. Elife 2021, 10, e69270. [Google Scholar] [CrossRef]
- Subramanian, L.; Tole, S. Mechanisms Underlying the Specification, Positional Regulation, and Function of the Cortical Hem. Cereb. Cortex 2009, 19, i90–i95. [Google Scholar] [CrossRef]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef]
- Hochgerner, H.; Zeisel, A.; Lönnerberg, P.; Linnarsson, S. Conserved Properties of Dentate Gyrus Neurogenesis across Postnatal Development Revealed by Single-Cell RNA Sequencing. Nat. Neurosci. 2018, 21, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Muñoz-Manchado, A.B.; Codeluppi, S.; Lönnerberg, P.; La Manno, G.; Juréus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Brain Structure. Cell Types in the Mouse Cortex and Hippocampus Revealed by Single-Cell RNA-Seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dai, S.; Zhu, R.; Tan, J.; Zhao, Q.; Yin, Y.; Sun, J.; Du, X.; Ge, L.; Xu, J.; et al. Deciphering Molecular Heterogeneity and Dynamics of Human Hippocampal Neural Stem Cells at Different Ages and Injury States. Elife 2024, 12, RP89507. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.B.; Fan, Y.; Zhang, Y.; Tokolyi, A.; Murphy, A.N.; Kammourh, S.; Deans, P.J.M.; Ghorbani, S.; Onatzevitch, R.; Pero, A.; et al. Phenotypic Complexities of Rare Heterozygous Neurexin-1 Deletions. Nature 2025, 642, 710–720. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, X.; Zhang, Z.; Li, M. Genetic Insights and Neurobiological Implications from NRXN1 in Neuropsychiatric Disorders. Mol. Psychiatry 2019, 24, 1400–1414. [Google Scholar] [CrossRef]
- Li, Y.E.; Preissl, S.; Hou, X.; Zhang, Z.; Zhang, K.; Qiu, Y.; Poirion, O.B.; Li, B.; Chiou, J.; Liu, H.; et al. An Atlas of Gene Regulatory Elements in Adult Mouse Cerebrum. Nature 2021, 598, 129–136. [Google Scholar] [CrossRef]
- Mizrak, D.; Levitin, H.M.; Delgado, A.C.; Crotet, V.; Yuan, J.; Chaker, Z.; Silva-Vargas, V.; Sims, P.A.; Doetsch, F. Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep. 2019, 26, 394–406.e5. [Google Scholar] [CrossRef]
- Baptista, P.; Andrade, J.P. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front. Neuroanat. 2018, 12, 44. [Google Scholar] [CrossRef]
- Akers, K.G.; Martinez-canabal, A.; Restivo, L.; Yiu, A.P.; Cristofaro, A.D.; Hsiang, H.L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal Neurogenesis Regulates Forgetting During Adulthood and Infancy. Science 2014, 598, 598–603. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Salta, E.; Lazarov, O.; Fitzsimons, C.P.; Tanzi, R.; Lucassen, P.J.; Choi, S.H. Adult Hippocampal Neurogenesis in Alzheimer’s Disease: A Roadmap to Clinical Relevance. Cell Stem Cell 2023, 30, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Tosoni, G.; Ayyildiz, D.; Bryois, J.; Macnair, W.; Fitzsimons, C.P.; Lucassen, P.J.; Salta, E. Mapping Human Adult Hippocampal Neurogenesis with Single-Cell Transcriptomics: Reconciling Controversy or Fueling the Debate? Neuron 2023, 111, 1714–1731.e3. [Google Scholar] [CrossRef]
- Sell, G.L.; Barrow, S.L.; McAllister, A.K. Glutamate Signaling and Neuroligin/Neurexin Adhesion Play Opposing Roles That Are Mediated by Major Histocompatibility Complex I Molecules in Cortical Synapse Formation. J. Neurosci. 2024, 44, e0797242024. [Google Scholar] [CrossRef]
- Cheung, A.; Konno, K.; Imamura, Y.; Matsui, A.; Abe, M.; Sakimura, K.; Sasaoka, T.; Uemura, T.; Watanabe, M.; Futai, K. Neurexins in Serotonergic Neurons Regulate Neuronal Survival, Serotonin Transmission, and Complex Mouse Behaviors. Elife 2023, 12, e85058. [Google Scholar] [CrossRef]
- Tromp, A.; Mowry, B.; Giacomotto, J. Neurexins in Autism and Schizophrenia-a Review of Patient Mutations, Mouse Models and Potential Future Directions. Mol. Psychiatry 2021, 26, 747–760. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Li, H.-L.; Kassabov, S.R.; Jin, I.; Puthanveettil, S.V.; Karl, K.A.; Lu, Y.; Kim, J.-H.; Bailey, C.H.; Kandel, E.R. Neurexin-Neuroligin Transsynaptic Interaction Mediates Learning-Related Synaptic Remodeling and Long-Term Facilitation in Aplysia. Neuron 2011, 70, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Liakath-Ali, K.; Polepalli, J.S.; Lee, S.-J.; Cloutier, J.-F.; Südhof, T.C. Transsynaptic Cerebellin 4-Neogenin 1 Signaling Mediates LTP in the Mouse Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2022, 119, e2123421119. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lan, L.; Xu, X.; Chen, K.; Yang, X.; Feng, X.; Lu, D. Immaturity-Dependent Hippocampal Neurogenic Promotion and Fate Shift by Low-Dose Propofol in Neonatal Mice Revealed Through Single-Nuclei RNA-Sequencing. Biomedicines 2025, 13, 2806. https://doi.org/10.3390/biomedicines13112806
Zhang W, Lan L, Xu X, Chen K, Yang X, Feng X, Lu D. Immaturity-Dependent Hippocampal Neurogenic Promotion and Fate Shift by Low-Dose Propofol in Neonatal Mice Revealed Through Single-Nuclei RNA-Sequencing. Biomedicines. 2025; 13(11):2806. https://doi.org/10.3390/biomedicines13112806
Chicago/Turabian StyleZhang, Wen, Liangtian Lan, Xuanxian Xu, Keyu Chen, Xiaoyu Yang, Xia Feng, and Dihan Lu. 2025. "Immaturity-Dependent Hippocampal Neurogenic Promotion and Fate Shift by Low-Dose Propofol in Neonatal Mice Revealed Through Single-Nuclei RNA-Sequencing" Biomedicines 13, no. 11: 2806. https://doi.org/10.3390/biomedicines13112806
APA StyleZhang, W., Lan, L., Xu, X., Chen, K., Yang, X., Feng, X., & Lu, D. (2025). Immaturity-Dependent Hippocampal Neurogenic Promotion and Fate Shift by Low-Dose Propofol in Neonatal Mice Revealed Through Single-Nuclei RNA-Sequencing. Biomedicines, 13(11), 2806. https://doi.org/10.3390/biomedicines13112806





