Tanshinone I Enhances the Pulmonary Immune Response of CD8+ T Cells by Promoting Memory Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Vaccination
2.4. Intravenous Labeling of T Cells and Lymphocyte Isolation
2.5. In Vitro Activation of Lymphocytes and Antigen Restimulation
2.6. Flow Cytometry
2.7. Statistics
3. Results
3.1. Treatment with TSN1 During Immunization Dictates the Capacity of CD8+ T Cells for Lung Homing
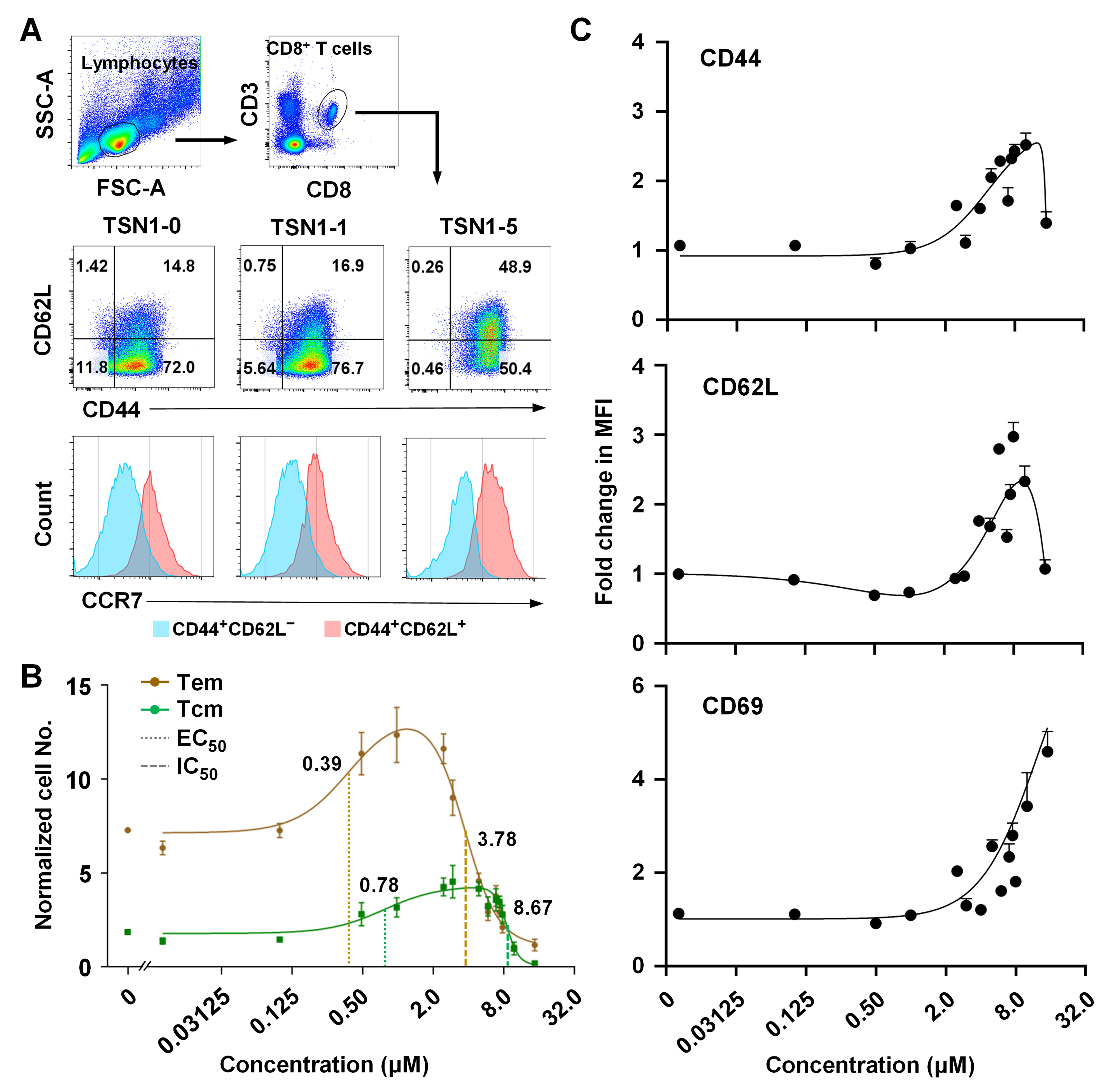
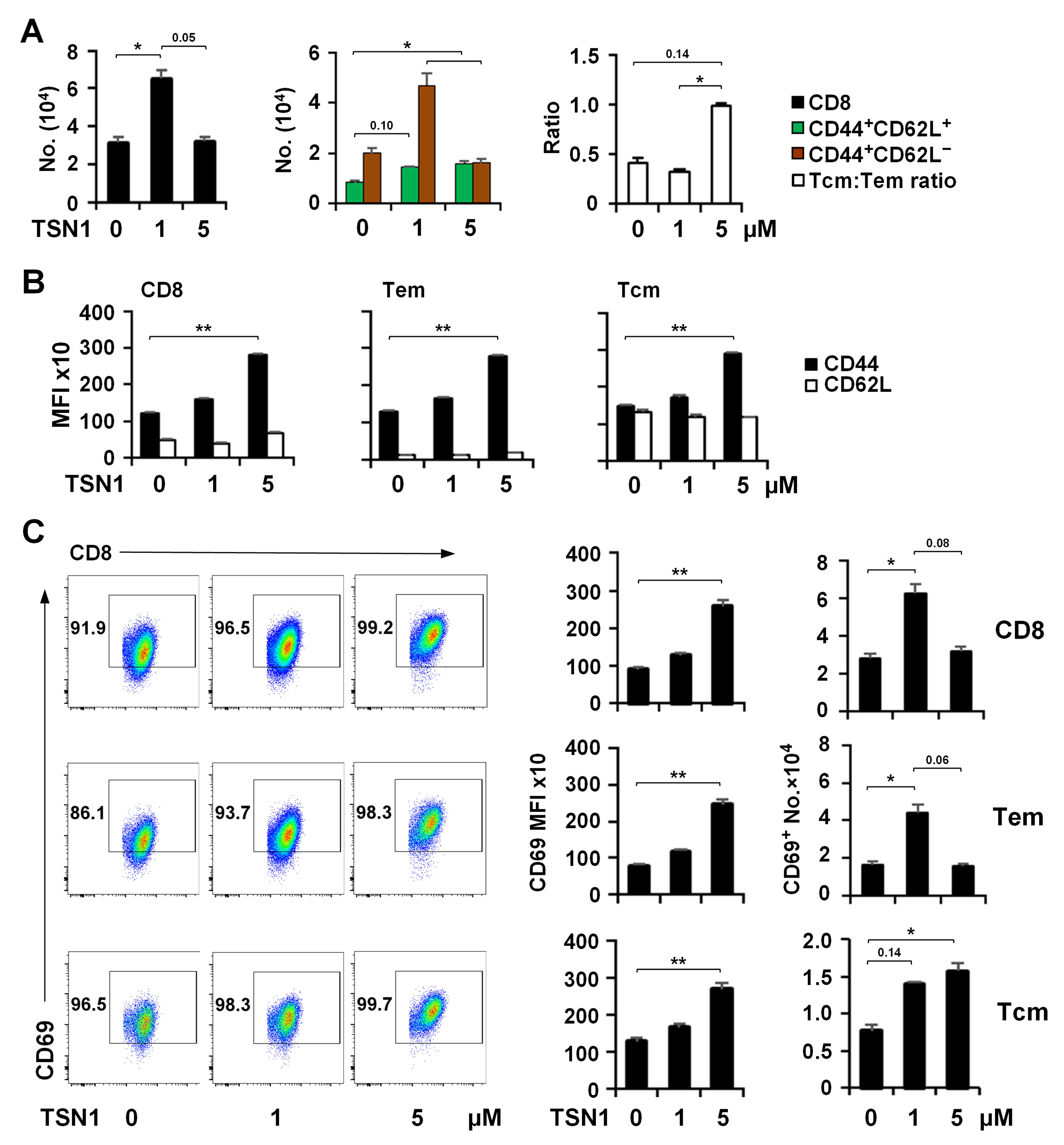
3.2. TSN1 Mediates Biphasic Regulation of the Memory Differentiation of T Cells
3.3. TSN1’s Differential Effect May Help Improve the Formation of Tem and Tcm
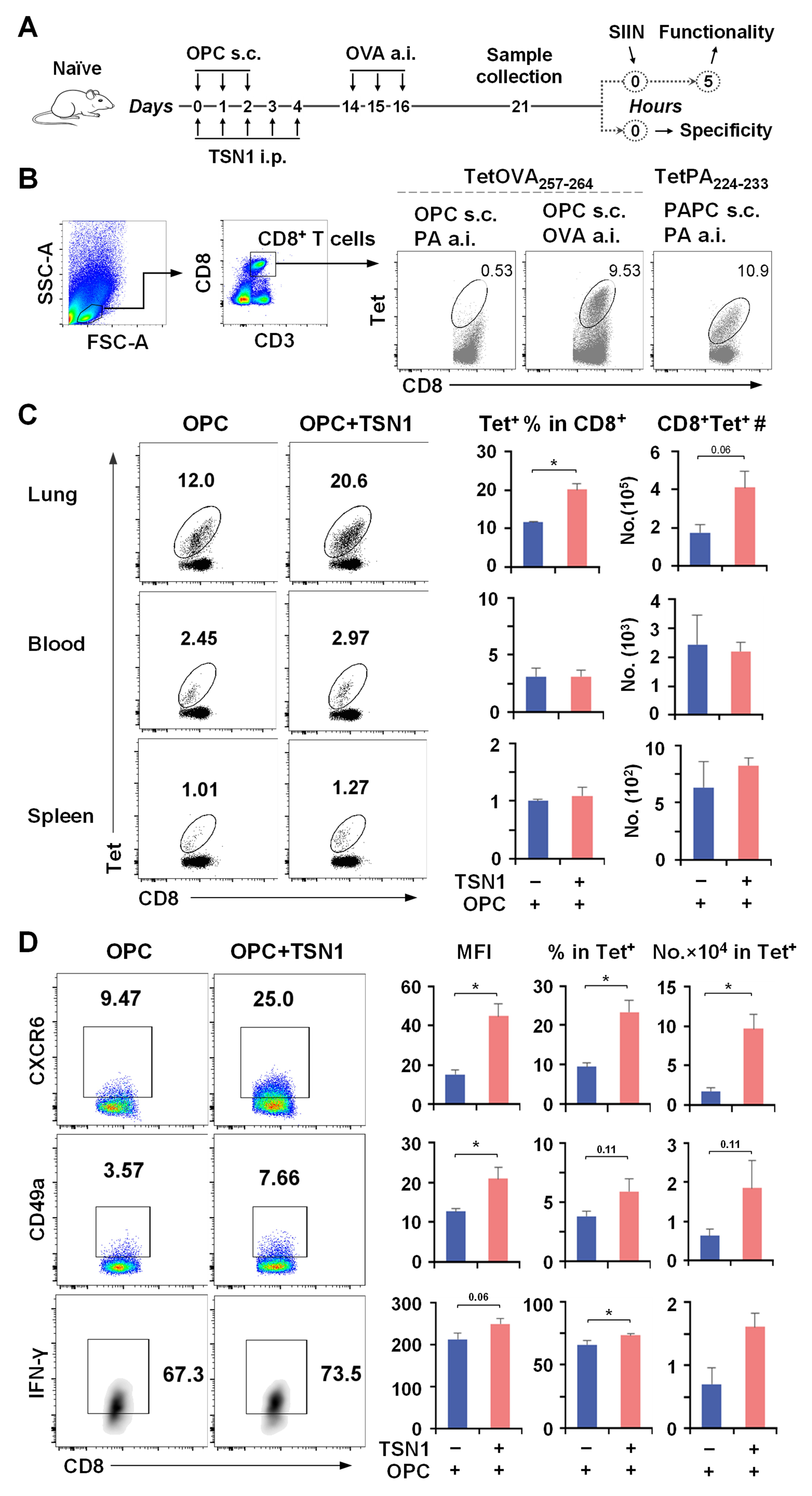
3.4. TSN1 Facilitates the Recruitment and Retention of Memory CD8+ T Cells in the Lung
4. Discussion
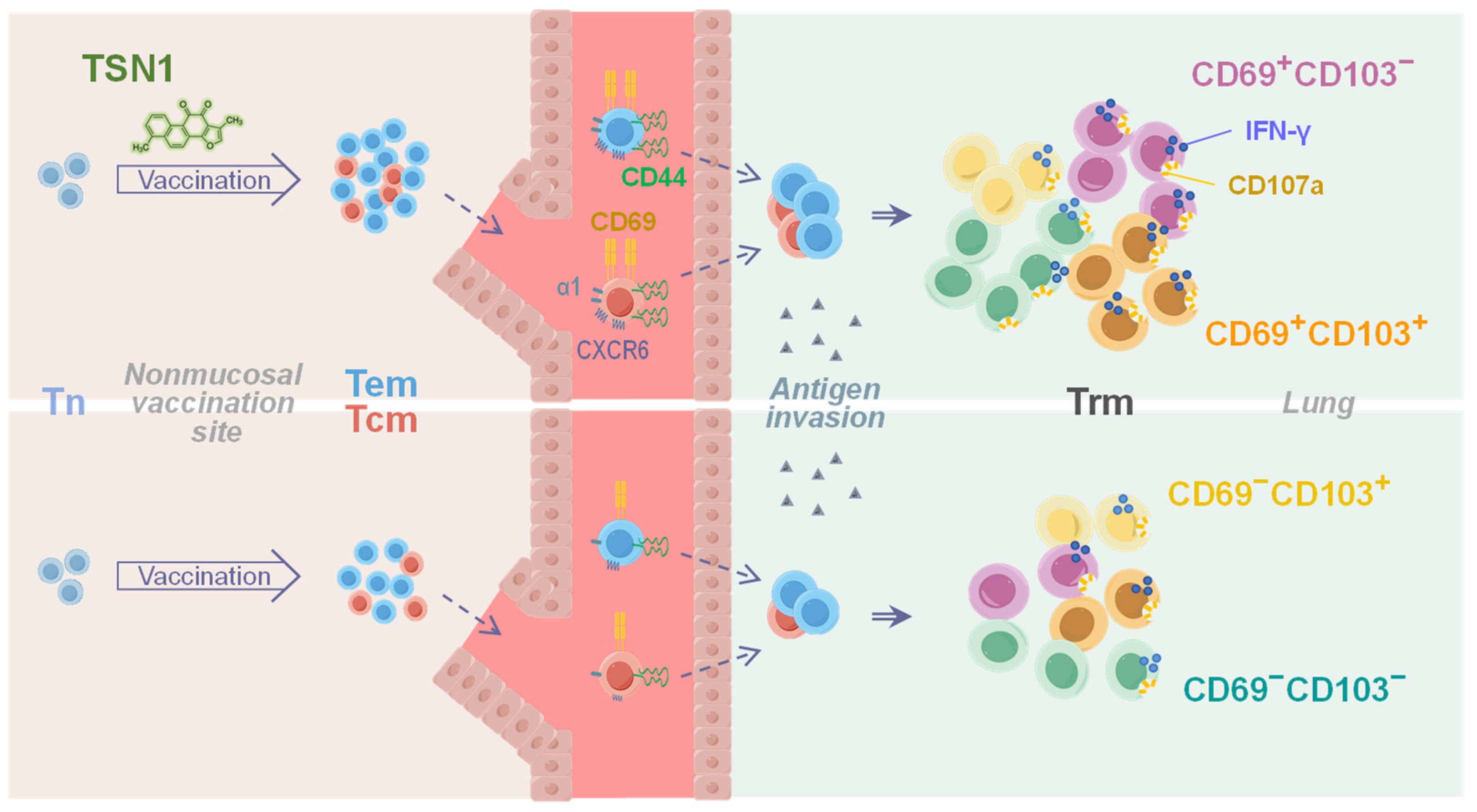
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishii, H.; Nomura, T.; Yamamoto, H.; Nishizawa, M.; Thu Hau, T.T.; Harada, S.; Seki, S.; Nakamura-Hoshi, M.; Okazaki, M.; Daigen, S.; et al. Neutralizing-antibody-independent SARS-CoV-2 control correlated with intranasal-vaccine-induced CD8+ T cell responses. Cell Rep. Med. 2022, 3, 100520. [Google Scholar] [CrossRef]
- Masopust, D.; Ha, S.J.; Vezys, V.; Ahmed, R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J. Immunol. 2006, 177, 831–839. [Google Scholar] [CrossRef]
- Hansen, S.G.; Ford, J.C.; Lewis, M.S.; Ventura, A.B.; Hughes, C.M.; Coyne-Johnson, L.; Whizin, N.; Oswald, K.; Shoemaker, R.; Swanson, T.; et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011, 473, 523–527. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Charles, T.P.; Joag, V.; Bollimpelli, V.S.; Scott, M.K.D.; Wimmers, F.; Burton, S.L.; Labranche, C.C.; Petitdemange, C.; Gangadhara, S.; et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 2020, 26, 932–940. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, S.; Badovinac, V.P.; Harty, J.T. Influenza-Specific Lung-Resident Memory CD8+ T Cells. Cold Spring Harb. Perspect. Biol. 2021, 13, a037978. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.R. Unique properties of tissue-resident memory T cells in the lungs: Implications for COVID-19 and other respiratory diseases. Nat. Rev. Immunol. 2023, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Fitch, M.; Edupuganti, S.; Yang, S.; Kissick, H.T.; Li, K.W.; Youngblood, B.A.; Abdelsamed, H.A.; McGuire, D.J.; Cohen, K.W.; et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 2017, 552, 362–367. [Google Scholar] [CrossRef]
- Stolley, J.M.; Johnston, T.S.; Soerens, A.G.; Beura, L.K.; Rosato, P.C.; Joag, V.; Wijeyesinghe, S.P.; Langlois, R.A.; Osum, K.C.; Mitchell, J.S.; et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 2020, 217, e20192197. [Google Scholar] [CrossRef]
- Parga-Vidal, L.; van Gisbergen, K. Area under Immunosurveillance: Dedicated Roles of Memory CD8 T-Cell Subsets. Cold Spring Harb. Perspect. Biol. 2020, 12, a037796. [Google Scholar] [CrossRef]
- Milner, J.J.; Nguyen, H.; Omilusik, K.; Reina-Campos, M.; Tsai, M.; Toma, C.; Delpoux, A.; Boland, B.S.; Hedrick, S.M.; Chang, J.T.; et al. Delineation of a molecularly distinct terminally differentiated memory CD8 T cell population. Proc. Natl. Acad. Sci. USA 2020, 117, 25667–25678. [Google Scholar] [CrossRef]
- Szabo, P.A. Axes of heterogeneity in human tissue-resident memory T cells. Immunol. Rev. 2023, 316, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Miron, M.; Meng, W.; Rosenfeld, A.M.; Dvorkin, S.; Poon, M.M.L.; Lam, N.; Kumar, B.V.; Louzoun, Y.; Luning Prak, E.T.; Farber, D.L. Maintenance of the human memory T cell repertoire by subset and tissue site. Genome Med. 2021, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.M.L.; Caron, D.P.; Wang, Z.; Wells, S.B.; Chen, D.; Meng, W.; Szabo, P.A.; Lam, N.; Kubota, M.; Matsumoto, R.; et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat. Immunol. 2023, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host Microbe 2023, 31, 146–157. [Google Scholar] [CrossRef]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
- Zhu, X.; Gebo, K.A.; Abraham, A.G.; Habtehyimer, F.; Patel, E.U.; Laeyendecker, O.; Gniadek, T.J.; Fernandez, R.E.; Baker, O.R.; Ram, M.; et al. Dynamics of inflammatory responses after SARS-CoV-2 infection by vaccination status in the USA: A prospective cohort study. Lancet Microbe 2023, 4, e692–e703. [Google Scholar] [CrossRef]
- Ely, K.H.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 2006, 176, 537–543. [Google Scholar] [CrossRef]
- Marzo, A.L.; Yagita, H.; Lefrancois, L. Cutting edge: Migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 2007, 179, 36–40. [Google Scholar] [CrossRef]
- Charlton, J.J.; Chatzidakis, I.; Tsoukatou, D.; Boumpas, D.T.; Garinis, G.A.; Mamalaki, C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J. Immunol. 2013, 190, 6104–6114. [Google Scholar] [CrossRef]
- Kang, X.; Chen, J.; Qin, Q.; Wang, F.; Wang, Y.; Lan, T.; Xu, S.; Wang, F.; Xia, J.; Ekberg, H.; et al. Isatis tinctoria L. combined with co-stimulatory molecules blockade prolongs survival of cardiac allografts in alloantigen-primed mice. Transpl. Immunol. 2010, 23, 34–39. [Google Scholar] [CrossRef]
- Eikawa, S.; Nishida, M.; Mizukami, S.; Yamazaki, C.; Nakayama, E.; Udono, H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. USA 2015, 112, 1809–1814. [Google Scholar] [CrossRef]
- Meng, M.; Gao, R.; Liu, Z.; Liu, F.; Du, S.; Song, Y.; He, J. Ginsenosides, potential TMPRSS2 inhibitors, a trade-off between the therapeutic combination for anti-PD-1 immunotherapy and the treatment of COVID-19 infection of LUAD patients. Front. Pharmacol. 2023, 14, 1085509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.; Tian, Y.; Cao, L.; Gao, Q.; Zhang, C.; Zhang, K.; Shen, C.; Ping, Y.; Maimela, N.R.; et al. Metformin Enhances the Antitumor Activity of CD8+ T Lymphocytes via the AMPK-miR-107-Eomes-PD-1 Pathway. J. Immunol. 2020, 204, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Cho, Y.W.; Woo, Y.J.; Baek, Y.A.; Kim, E.J.; Cho, Y.; Kim, J.Y.; Kim, B.S.; Song, J.J.; Ha, S.J. Metabolic Reprogramming by the Excessive AMPK Activation Exacerbates Antigen-Specific Memory CD8+ T Cell Differentiation after Acute Lymphocytic Choriomeningitis Virus Infection. Immune Netw. 2019, 19, e11. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Zhang, L.; Gao, C.; Li, J.J.; Jiang, J.; Zhu, Q. Berberine improves central memory formation of CD8+ T cells: Implications for design of natural product-based vaccines. Acta Pharm. Sin. B 2023, 13, 2259–2268. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.G.; Wang, Y.; Wang, M.; Li, M.; Gao, C.; Zhang, L.; Li, Y.; Liu, Y.; Dajic Stevanovic, Z.; et al. Teucrium montanum extract drives effector and memory differentiation of CD8+ T cells. Biomed. Res. Ther. 2023, 10, 6023–6034. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, T.C.; Chang, H.W.; Son, K.H.; Kang, S.S.; Kim, H.P. Effects of tanshinone I isolated from Salvia miltiorrhiza bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytother. Res. 2002, 16, 616–620. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Lee, G.W.; Lee, J.S.; Cho, M.K.; Son, K.H.; Jeon, S.J.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis 2008, 29, 1885–1892. [Google Scholar] [CrossRef]
- Roth, A.; Zhao, P.; Soukup, S.T.; Guigas, C.; Starke, J.; Kulling, S.E.; Diel, P. Chemical Stability and Bioactivity of tanshinone I, tanshinone IIA, cryptotanshinone and dihydrotanshinone in in vitro test systems. Toxicol. Lett. 2023, 375, 21–28. [Google Scholar] [CrossRef]
- Elmaaty, A.A.; Darwish, K.M.; Khattab, M.; Elhady, S.S.; Salah, M.; Hamed, M.I.A.; Al-Karmalawy, A.A.; Saleh, M.M. In a search for potential drug candidates for combating COVID-19: Computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J. Biomol. Struct. Dyn. 2022, 40, 8866–8893. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Wu, Y.; Lu, J.; Gao, X.; Zhu, X.; Yang, J.; Liu, S.; Xiao, G.; Pan, X. Identification of tanshinone I as cap-dependent endonuclease inhibitor with broad-spectrum antiviral effect. J. Virol. 2023, 97, e0079623. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.R.; Goggins, J.A.; Sabbagh, L.M.; Freytag, L.C.; Clements, J.D.; McLachlan, J.B. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4+ T cells following parenteral immunization. Mucosal. Immunol. 2018, 11, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.T.; Limbert, V.M.; Horowitz, R.M.; D’Souza, S.J.; Bachnak, L.; Godwin, M.S.; Bauer, D.L.; Harrell, J.E.; Morici, L.A.; Taylor, J.J.; et al. Establishment of isotype-switched, antigen-specific B cells in multiple mucosal tissues using non-mucosal immunization. NPJ Vaccines 2023, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Pizzolla, A.; Nguyen, T.H.; Sant, S.; Jaffar, J.; Loudovaris, T.; Mannering, S.I.; Thomas, P.G.; Westall, G.P.; Kedzierska, K.; Wakim, L.M. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Investig. 2018, 128, 721–733. [Google Scholar] [CrossRef]
- Thome, J.J.; Yudanin, N.; Ohmura, Y.; Kubota, M.; Grinshpun, B.; Sathaliyawala, T.; Kato, T.; Lerner, H.; Shen, Y.; Farber, D.L. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 2014, 159, 814–828. [Google Scholar] [CrossRef]
- Reilly, E.C.; Lambert Emo, K.; Buckley, P.M.; Reilly, N.S.; Smith, I.; Chaves, F.A.; Yang, H.; Oakes, P.W.; Topham, D.J. T(RM) integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 12306–12314. [Google Scholar] [CrossRef]
- Hayward, S.L.; Scharer, C.D.; Cartwright, E.K.; Takamura, S.; Li, Z.T.; Boss, J.M.; Kohlmeier, J.E. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8+ T cells. Nat. Immunol. 2020, 21, 309–320. [Google Scholar] [CrossRef]
- Weeden, C.E.; Gayevskiy, V.; Marceaux, C.; Batey, D.; Tan, T.; Yokote, K.; Ribera, N.T.; Clatch, A.; Christo, S.; Teh, C.E.; et al. Early immune pressure initiated by tissue-resident memory T cells sculpts tumor evolution in non-small cell lung cancer. Cancer Cell 2023, 41, 837–852.e836. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Pham, Q.M.; Lee, Y.T.; Cauley, L.S.; Puddington, L.; Lefrancois, L. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 2014, 40, 747–757. [Google Scholar] [CrossRef]
- Wu, J.; Madi, A.; Mieg, A.; Hotz-Wagenblatt, A.; Weisshaar, N.; Ma, S.; Mohr, K.; Schlimbach, T.; Hering, M.; Borgers, H.; et al. T Cell Factor 1 Suppresses CD103+ Lung Tissue-Resident Memory T Cell Development. Cell Rep. 2020, 31, 107484. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martinez-Cano, S.; Mejias-Perez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef]
- Beltran, E.; Gerdes, L.A.; Hansen, J.; Flierl-Hecht, A.; Krebs, S.; Blum, H.; Ertl-Wagner, B.; Barkhof, F.; Kumpfel, T.; Hohlfeld, R.; et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J. Clin. Investig. 2019, 129, 4758–4768. [Google Scholar] [CrossRef]
- Osborn, J.F.; Hobbs, S.J.; Mooster, J.L.; Khan, T.N.; Kilgore, A.M.; Harbour, J.C.; Nolz, J.C. Central memory CD8+ T cells become CD69+ tissue-residents during viral skin infection independent of CD62L-mediated lymph node surveillance. PLoS Pathog. 2019, 15, e1007633. [Google Scholar] [CrossRef]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279ra239. [Google Scholar] [CrossRef]
- Pallett, L.J.; Davies, J.; Colbeck, E.J.; Robertson, F.; Hansi, N.; Easom, N.J.W.; Burton, A.R.; Stegmann, K.A.; Schurich, A.; Swadling, L.; et al. IL-2(high) tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J. Exp. Med. 2017, 214, 1567–1580. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Chen, T.; Yi, T.; Zheng, Z.; Fan, H.; Chen, Z. Berberine Attenuates Inflammation Associated with Delayed-Type Hypersensitivity via Suppressing Th1 Response and Inhibiting Apoptosis. Inflammation 2017, 40, 221–231. [Google Scholar] [CrossRef]
- Gao, H.; Liu, X.; Sun, W.; Kang, N.; Liu, Y.; Yang, S.; Xu, Q.M.; Wang, C.; Chen, X. Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death Dis. 2017, 8, e3004. [Google Scholar] [CrossRef]
- Takamura, S.; Yagi, H.; Hakata, Y.; Motozono, C.; McMaster, S.R.; Masumoto, T.; Fujisawa, M.; Chikaishi, T.; Komeda, J.; Itoh, J.; et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 2016, 213, 3057–3073. [Google Scholar] [CrossRef]
- Chen, Y.E.; Bousbaine, D.; Veinbachs, A.; Atabakhsh, K.; Dimas, A.; Yu, V.K.; Zhao, A.; Enright, N.J.; Nagashima, K.; Belkaid, Y.; et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. Science 2023, 380, 203–210. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat. Med. 2010, 16, 224–227. [Google Scholar] [CrossRef]
- Gopinath, S.; Lu, P.; Iwasaki, A. Cutting Edge: The Use of Topical Aminoglycosides as an Effective Pull in “Prime and Pull” Vaccine Strategy. J. Immunol. 2020, 204, 1703–1707. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, H.; Wang, Y.; Gao, C.; Fan, L.; Li, J.; Zhu, Q. Tanshinone I Enhances the Pulmonary Immune Response of CD8+ T Cells by Promoting Memory Differentiation. Biomedicines 2025, 13, 2805. https://doi.org/10.3390/biomedicines13112805
Wang M, Wang H, Wang Y, Gao C, Fan L, Li J, Zhu Q. Tanshinone I Enhances the Pulmonary Immune Response of CD8+ T Cells by Promoting Memory Differentiation. Biomedicines. 2025; 13(11):2805. https://doi.org/10.3390/biomedicines13112805
Chicago/Turabian StyleWang, Manqiu, Honglei Wang, Yaling Wang, Changxing Gao, Leqi Fan, Jing Li, and Qing Zhu. 2025. "Tanshinone I Enhances the Pulmonary Immune Response of CD8+ T Cells by Promoting Memory Differentiation" Biomedicines 13, no. 11: 2805. https://doi.org/10.3390/biomedicines13112805
APA StyleWang, M., Wang, H., Wang, Y., Gao, C., Fan, L., Li, J., & Zhu, Q. (2025). Tanshinone I Enhances the Pulmonary Immune Response of CD8+ T Cells by Promoting Memory Differentiation. Biomedicines, 13(11), 2805. https://doi.org/10.3390/biomedicines13112805





