Preventive and Therapeutic Interventions in Solar Elastosis and Photoaging: A Comprehensive Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
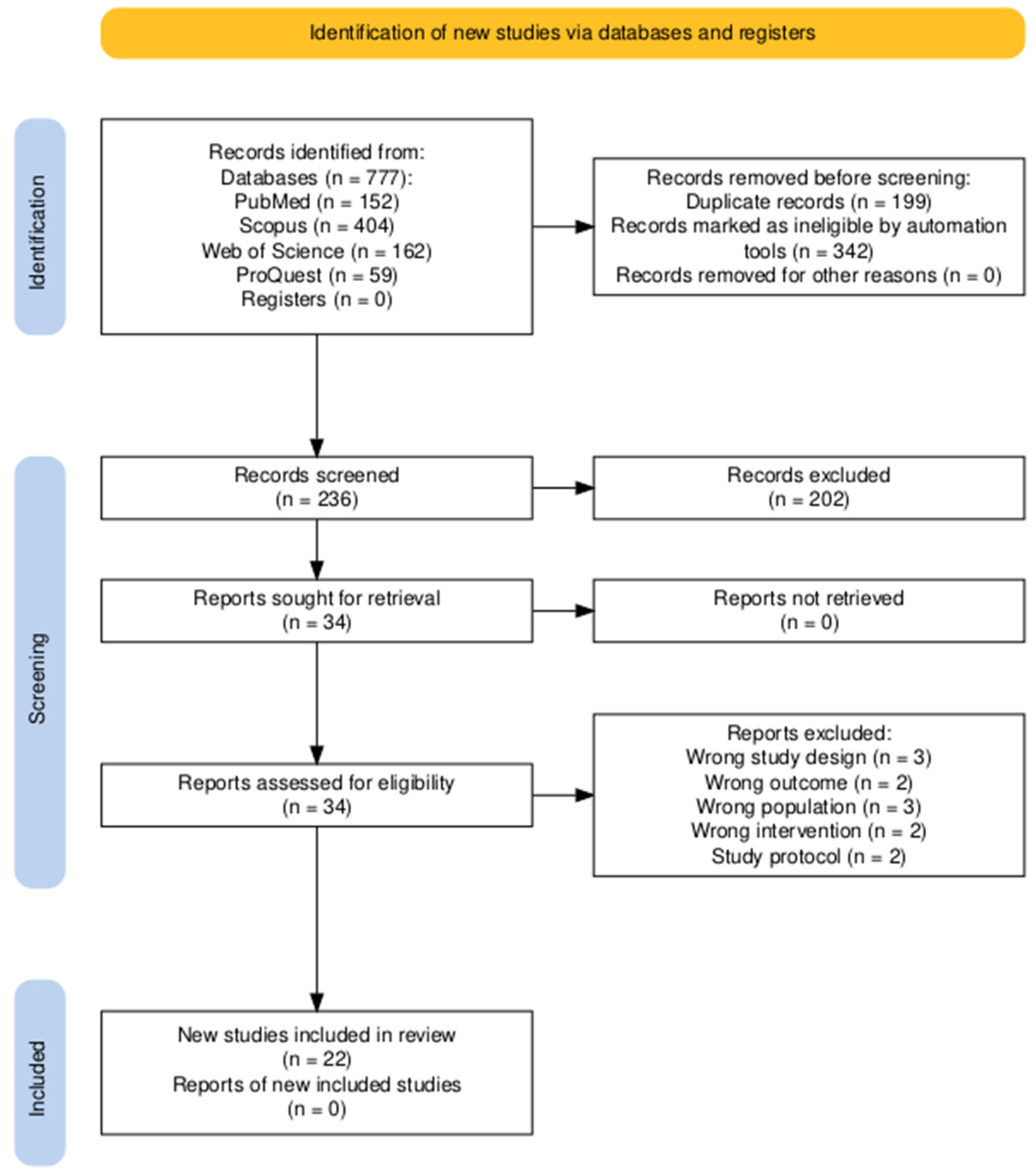
2.2. Search Strategy and Study Selection
2.3. Eligibility Criteria
- Population (P)
- Intervention (I)
- Comparator (C)
- Outcomes (O)
- Study Designs (S)
- Animal or in vitro studies.
- Reviews, editorials, commentaries, letters to the editor, study protocols without original data, abstracts, case reports, or case series.
- Studies not published in English.
2.4. Screening
2.5. Data Extraction and Synthesis
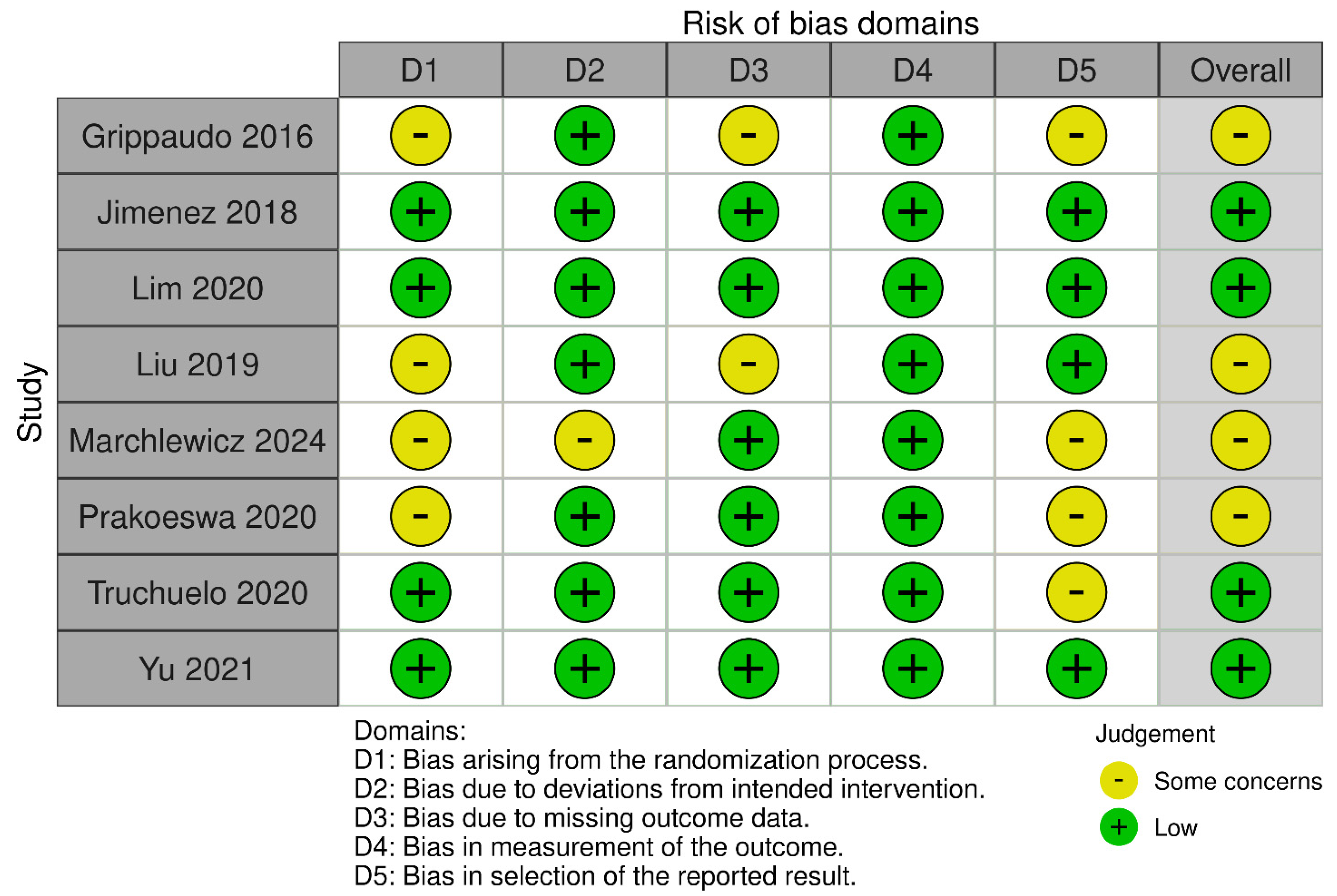
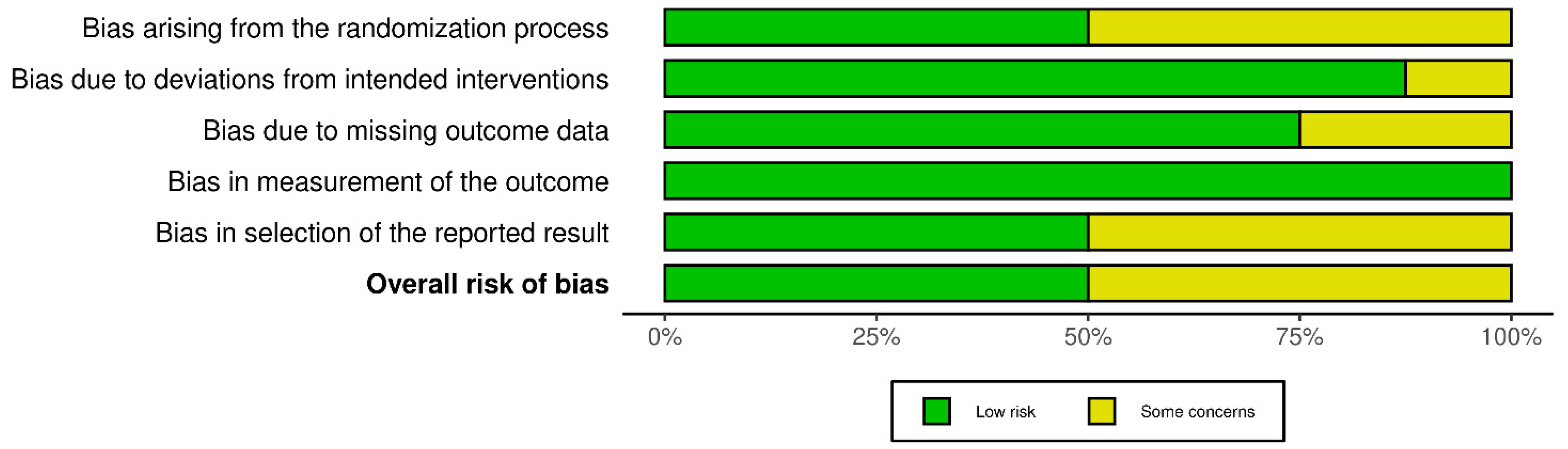
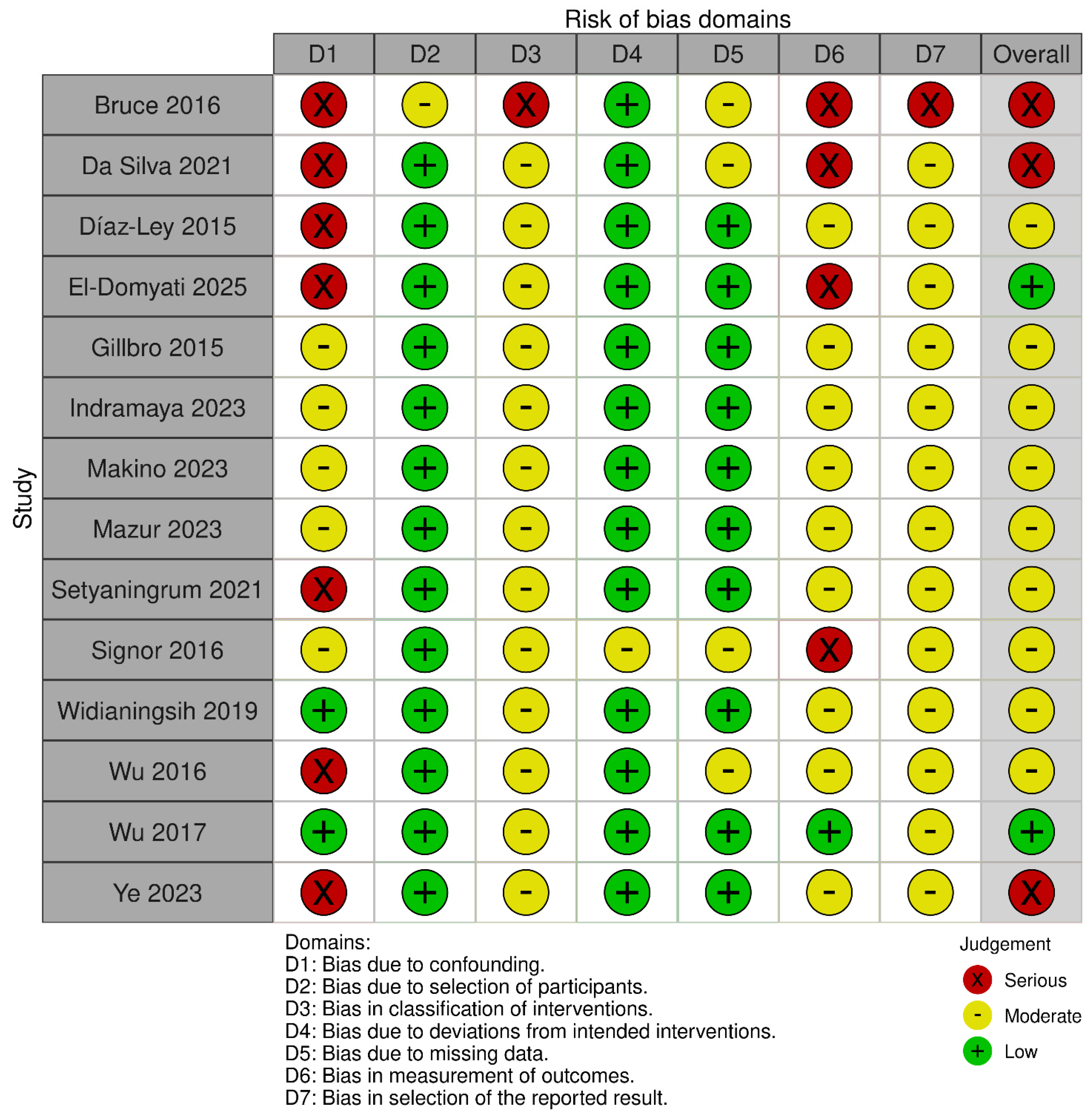
2.6. Risk of Bias Assessment
3. Results
3.1. Overview of Included Studies
3.2. Clinical and Histopathological Improvement in Photoaging and Solar Elastosis
3.3. Skin Elasticity and Functionality
3.4. Patient-Reported Outcomes
3.5. Safety and Adverse Events
3.6. Precancerous Lesions Onset
| Intervention Type | Specific Interventions | Reference | Description |
|---|---|---|---|
| Energy-based devices (lasers, light, ecc…) | Photodynamic therapy | [33] | Topical photosensitizers activated by specific light to generate reactive oxygen species, targeting dysplastic keratinocytes in AK and remodeling photodamaged tissue. |
| Fractional CO2 laser + topical conjugated linolenic acid | [39] | Ablative fractional resurfacing followed by omega-conjugated linolenic acid to modulate inflammation and dermal repair after laser injury. | |
| Fractional CO2 laser + ionic hydrogel ± clarithromycin | [26] | Post-laser wound care using an ionic hydrogel (with or without topical antibiotic) to support re-epithelialization and barrier recovery. | |
| 755 nm picosecond alexandrite laser with diffractive lens array | [40,42] | Picosecond photothermolysis using a DLA to create laser-induced optical breakdown microzones for pigment correction and dermal remodeling. | |
| Fractional non-ablative laser + SCA®-based cosmeceuticals | [37] | Non-ablative fractional photothermolysis combined with a snail-secretion—derived regimen to enhance post-laser regeneration. | |
| Fractional Er:YAG laser-assisted delivery of AMSC-MP | [38] | Ablative fractional Er:YAG microchannels used to enhance transdermal penetration of amniotic membrane stem cell metabolite product. | |
| Fractional CO2 laser-assisted delivery of AMSC-CM + vitamin C | [27] | CO2 laser microchannels facilitating delivery of stem cell conditioned medium with ascorbic acid to photodamaged skin. | |
| YAG laser + regenerative serum | [28] | Laser treatment followed by topical application of a bioactive, growth-factor–rich serum to stimulate extracellular matrix renewal. | |
| Radiofrequency and microneedling | Microneedling + needle-free mesotherapy with Helix aspersa mucus filtrate | [32] | Percutaneous collagen induction combined with transdermal delivery (needle-free) of snail-mucin filtrate rich in glycosaminoglycans and peptides. |
| Microneedling + sublative fractional radiofrequency | [24] | Mechanical microinjury paired with dermal RF heating via fractionated electrodes to induce neocollagenesis and elastin remodeling. | |
| Microneedling radiofrequency | [30] | RF energy delivered through insulated microneedles to create controlled thermal zones in the dermis for texture and laxity improvement. | |
| Plasma- and cell-based injectables | Plasma-rich in growth factors | [23] | Autologous plasma fraction enriched in platelet-derived growth factors injected to promote dermal remodeling. |
| Lyophilized platelet-rich plasma | [22] | Dehydrated, reconstituted platelet concentrate intended to deliver standardized growth factors for skin rejuvenation. | |
| Stromal vascular fraction injections | [36] | Autologous adipose-derived SVF (mesenchymal/stromal and perivascular cells) injected to enhance regenerative pathways and matrix deposition. | |
| Stem cell-derived topicals | AMSC-MP + vitamin C via microneedling (Dermapen®) | [34] | Topical amniotic membrane stem cell metabolite product with ascorbic acid driven through microchannels created by microneedling. |
| AMSC-CM + vitamin C (topical) | [35] | Topical conditioned medium from amniotic membrane stem cells combined with antioxidant therapy for photodamage. | |
| Topical cosmeceuticals and retinoids | SCA® + IFC-CAF® regimen (Cryptomphalus aspersa derivatives) | [29] | Combination of snail-secretion filtrate with FGF-like activity and egg extracts aimed at enhancing epidermal function and firmness. |
| Supramolecular 0.1% retinol | [41] | Stabilized, complexed retinol designed for improved cutaneous delivery and tolerability to stimulate epidermal turnover and dermal matrix | |
| 1% acetyl aspartic acid | [25] | Topical ECM-modulating amino-acid derivative intended to upregulate basement-membrane and elastic-fiber components | |
| Chemical resurfacing | AGE/MELA chemical resurfacing + daily Phytochromatic MD® Complex | [21] | Sequential medium-depth peels followed by a liposomal sodium-copper chlorophyllin complex as maintenance skincare |
| Mechanical resurfacing | Diamond-tip microdermabrasion + hyaluronic-acid serum | [31] | Controlled epidermal exfoliation coupled with immediate application of a hyaluronan-rich serum to rehydrate and smooth the skin |
| Pore Appearance | Spots and Dyschromia | Clinical Photoaging (PGA, IGA) | Trans-Epithelial Water Loss | Healing Time | Erythema | Rhytides | Skin Texture | Epidermal Thickness | Dermal Thickness | Collagen Fiber Deposition and Organization | Cellular Proliferation rate | Histopathological Signs of Solar Elastosis | Dermoscopic Pattern | Skin Elasticity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% acetyl aspartic acid | |||||||||||||||
| 755 nm picosecond alexandrite laser with diffractive lens array | |||||||||||||||
| AGE/MELA chemical resurfacing + daily Phytochromatic MD® Complex | |||||||||||||||
| AMSC-CM + vitamin C (topical) | |||||||||||||||
| AMSC-MP + vitamin C via microneedling (Dermapen®) | |||||||||||||||
| Diamond-tip microdermabrasion + hyaluronic-acid serum | |||||||||||||||
| Fractional CO2 laser + ionic hydrogel + clarithromycin | |||||||||||||||
| Fractional CO2 laser + topical conjugated linolenic acid | |||||||||||||||
| Fractional CO2 laser-assisted delivery of AMSC-CM + vitamin C | |||||||||||||||
| Fractional Er:YAG laser-assisted delivery of AMSC-MP | |||||||||||||||
| Fractional non-ablative laser + SCA®-based cosmeceuticals | |||||||||||||||
| Intradermal PRGF | |||||||||||||||
| Microneedling + needle-free mesotherapy with Helix aspersa mucus filtrate | |||||||||||||||
| Microneedling + sublative fractional radiofrequency | |||||||||||||||
| Photodynamic therapy | |||||||||||||||
| Plasma-rich in growth factors | |||||||||||||||
| SCA® + IFC-CAF® regimen (Cryptomphalus aspersa derivatives) | |||||||||||||||
| Stromal vascular fraction injections | |||||||||||||||
| Supramolecular 0.1% retinol | |||||||||||||||
| YAG laser + regenerative serum |
4. Discussion
4.1. Comparison of Intervention Mechanisms
- Energy-Based and Physical Approaches: Treatments such as radiofrequency microneedling have shown a normalization of type I collagen expression and a reduction in elastotic material, confirming the ability of these technologies to induce deep dermal remodeling [47]. Fractional lasers (both ablative and non-ablative) remain a cornerstone in the treatment of photoaging, with their effectiveness in promoting deep collagen remodeling being well-documented and serving as a benchmark [48]. Picosecond lasers offer an alternative with a potentially superior safety profile, inducing minimal thermal damage and leveraging photomechanical effects to stimulate skin regeneration [49]. The growing use of these technologies to enhance the delivery of topical drugs, a technique called laser-assisted drug delivery (LADD), represents a further frontier for maximizing therapeutic efficacy [50].
- Biological Therapies and Bioactive Compounds: The use of bioactive compounds, such as mesenchymal stem cell (MSC) derivatives or their secretomes, demonstrates a significant capacity to modulate fibroblast activity and reduce local inflammation [51]. An emblematic example is the role of hyaluronic acid, a key component of the ECM whose biological functions critically depend on its molecular weight. It has been shown that low molecular weight HA (LMWHA) fragments actively promote tissue regeneration and restore epithelial architecture in atrophic contexts, highlighting their potential as bioactive agents in dermatology [52]. This ability to modulate local inflammation is particularly relevant in the context of “inflammaging,” the chronic low-grade inflammation that characterizes the aging process and significantly contributes to tissue degradation, a phenomenon driven in part by the senescence-associated secretory phenotype (SASP) [53].
4.2. Ethnic Differences in Outcomes
4.3. Feasibility in Clinical Practice and Outcomes
4.4. Future Research Directions and RCT Design
- Head-to-head comparisons: Directly comparing the efficacy of different therapeutic modalities (e.g., picosecond vs. fractional ablative lasers) or different formulations of biological therapies (e.g., standardized PRP preparations) to identify superior approaches.
- Standardized outcome measures: Employing validated and objective assessment tools, such as the Cutometer®, alongside standardized histological and molecular analyses (e.g., quantification of type I collagen or SASP markers) to allow for reliable cross-study comparisons.
- Long-term follow-up: Assessing the durability of clinical and histological improvements to determine the stability of results over time and the potential need for maintenance treatments.
4.5. Limitations
5. Conclusions
- Larger sample sizes to enhance the generalizability of the findings.
- Head-to-head comparisons to directly evaluate the relative efficacy of different therapeutic modalities, such as picosecond versus fractional ablative lasers or different standardized PRP preparations.
- The use of standardized and objective measures, including instrumental assessments like Cutometer® for elasticity and quantitative histological analyses for neocollagenesis, to allow for reliable cross-study comparisons.
- Long-term follow-up to assess the durability of clinical and histological improvements over time.
- Greater demographic inclusivity by enrolling participants across a wider range of Fitzpatrick phototypes, particularly types V and VI, to validate efficacy and safety in currently underrepresented populations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
References
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet Radiation, Aging and the Skin: Prevention of Damage by Topical CAMP Manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Yu, Z.W.; Zheng, M.; Fan, H.Y.; Liang, X.H.; Tang, Y.L. Ultraviolet (UV) Radiation: A Double-Edged Sword in Cancer Development and Therapy. Mol. Biomed. 2024, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Lucas, R.M.; Byrne, S.N.; Hollestein, L.; Rhodes, L.E.; Yazar, S.; Young, A.R.; Berwick, M.; Ireland, R.A.; Olsen, C.M. The Effects of Exposure to Solar Radiation on Human Health. Photochem. Photobiol. Sci. 2023, 22, 1011–1047. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin Structure and Its Involvement in Skin Photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular Basis of Sun-Induced Premature Skin Ageing and Retinoid Antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Ayer, J.; Griffiths, T.W.; Rashdan, E.; Naidoo, K.; Caley, M.P.; Birch-Machin, M.A.; O’Toole, E.A.; Watson, R.E.B.; Griffiths, C.E.M. Distinctive Clinical and Histological Characteristics of Atrophic and Hypertrophic Facial Photoageing. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Su, W.; Wang, F. Skin Ageing: A Progressive, Multi-Factorial Condition Demanding an Integrated, Multilayer-Targeted Remedy. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1215–1229. [Google Scholar] [CrossRef]
- Mambwe, B.; Mellody, K.T.; Kiss, O.; O’Connor, C.; Bell, M.; Watson, R.E.B.; Langton, A.K. Cosmetic Retinoid Use in Photoaged Skin: A Review of the Compounds, Their Use and Mechanisms of Action. Int. J. Cosmet. Sci. 2025, 47, 45–57. [Google Scholar] [CrossRef]
- Zhou, Y.; Hamblin, M.R.; Wen, X. An Update on Fractional Picosecond Laser Treatment: Histology and Clinical Applications. Lasers Med. Sci. 2023, 38, 45. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Cho, M.; Kim, H.S.; Lee, S.J.; Song, K.Y.; Kim, H.S. Clinical and Histological Evaluation of Microneedle Fractional Radiofrequency Treatment on Facial Fine Lines and Skin Laxity in Koreans. J. Cosmet. Dermatol. 2023, 22, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Concise Review: Adipose-Derived Stem Cells (ASCs) and Adipocyte-Secreted Exosomal Microrna (A-SE-MiR) Modulate Cancer Growth and Promote Wound Repair. J. Clin. Med. 2019, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Hesseler, M.J.; Shyam, N. Platelet-Rich Plasma and Its Utility in Medical Dermatology: A Systematic Review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Bruce, S.; Roberts, W.; Teller, C.; Colvan Suzanne Bruce, L.B. The Effects of a Daily Skincare Regimen on Maintaining the Benefits Obtained from Previous Chemical Resurfacing Treatments. J. Drugs Dermatol. JDD 2016, 15, 1145–1150. [Google Scholar]
- da Silva, L.Q.; Cancela, R.B.B.; de Lima Montalvão, S.A.; Huber, S.C.; Vieira-Damiani, G.; Triglia, R.M.; Annichino-Bizzacchi, J.M. The Effect of Lyophilized Platelet Rich-Plasma on Skin Aging: A Non-Randomized, Controlled, Pilot Trial. Arch. Dermatol. Res. 2021, 313, 863–871. [Google Scholar] [CrossRef]
- Díaz-Ley, B.; Cuevast, J.; Alonso-Castro, L.; Calvo, M.I.; Ríos-Buceta, L.; Orive, G.; Anitua, E.; Jaén, P. Benefits of Plasma Rich in Growth Factors (PRGF) in Skin Photodamage: Clinical Response and Histological Assessment. Dermatol. Ther. 2015, 28, 258–263. [Google Scholar] [CrossRef]
- El-Domyati, M.; Moawad, O.; Abdel-Wahab, H.; Behairy, E.F.; Rezk, A.F. A New Approach with Combined Microneedle and Sublative Fractional Radiofrequency for Photoaging Management: A Clinical, Histometric, and Immunohistochemical Study. Aesthetic Plast. Surg. 2025, 49, 1435–1443. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Merinville, E.; Cattley, K.; Al-Bader, T.; Hagforsen, E.; Nilsson, M.; Mavon, A. In Vivo Topical Application of Acetyl Aspartic Acid Increases Fibrillin-1 and Collagen IV Deposition Leading to a Significant Improvement of Skin Firmness. Int. J. Cosmet. Sci. 2015, 37, 41–46. [Google Scholar] [CrossRef]
- Grippaudo, F.R. Ionic Hydrogel Monotherapy and in Combination with Antiviral-Antibiotic Prophylaxis, in the Post-Procedure Management of Fractional Laser-Treated Patients. J. Clin. Aesthetic Dermatol. 2016, 9, 23. [Google Scholar]
- Indramaya, D.M.; Listiawan, M.Y.; Citrashanty, I.; Sari, M.; Umborowati, M.A.; Adiningtyas, V.; Pitasari, D.A.; Ayu, A.A.; Rantam, F.A.; Prakoeswa, C.R.S. The Comparison between Microneedling and Fractional CO2 Laser for Amniotic Membrane Stem Cell-Conditioned Medium and Vitamin C in Photoaging Treatment. Indian J. Dermatol. 2023, 68, 430–436. [Google Scholar] [CrossRef]
- Jiménez, N.; Hermosa, A.; de Miguel, L.; Sánchez-Neila, N.; Truchuelo, M.T.; Eraña, I.; Cuevas, J. Assessment of the Efficacy and Tolerance of an Innovative Regenerative Serum on Cutaneous Regeneration, Following Fractional Laser Procedure Using Erbium:YAG. J. Cosmet. Dermatol. 2018, 17, 1115–1121. [Google Scholar] [CrossRef]
- Lim, V.Z.; Yong, A.A.; Tan, W.P.M.; Zhao, X.; Vitale, M.; Goh, C.L. Efficacy and Safety of a New Cosmeceutical Regimen Based on the Combination of Snail Secretion Filtrate and Snail Egg Extract to Improve Signs of Skin Aging. J. Clin. Aesthetic Dermatol. 2020, 13, 31. [Google Scholar]
- Liu, T.M.; Sun, Y.M.; Tang, Z.Y.; Li, Y.H. Microneedle Fractional Radiofrequency Treatment of Facial Photoageing as Assessed in a Split-Face Model. Clin. Exp. Dermatol. 2019, 44, e96–e102. [Google Scholar] [CrossRef]
- Makino, E.T.; Huang, P.C.; Emmerich, T.; Jiang, L.I.; Mehta, R.C. Efficacy and Tolerability of Cosmetic Serums Enriched with Five Forms of Hyaluronic Acid as Part of Biweekly Diamond Tip Microdermabrasion Treatments for Facial Skin Dryness and Age-Associated Features. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1123–1134. [Google Scholar] [CrossRef]
- Marchlewicz, M.; Wojnarowicz, J.; Wilk, A.; Misiakiewicz-Has, K.; Wiszniewska, B.; Szumilas, K.; Duchnik, E. Effect of Helix Aspersa Mucus on the Regeneration of Skin with Photoaging Features in Different Methods of Application. Appl. Sci. 2024, 14, 7394. [Google Scholar] [CrossRef]
- Mazur, E.; Kwiatkowska, D.; Reich, A. Reflectance Confocal Microscopy and Dermoscopy of Facial Pigmented and Non-Pigmented Actinic Keratosis Features before and after Photodynamic Therapy Treatment. Cancers 2023, 15, 5598. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Effendy, Z.F.; Herwanto, N.; Ervianty, E.; Rantam, F.A. Efficacy of Topical Application of a Mixture of Amniotic Membrane Stem Cell Metabolic Products and Vitamin C after Microneedling Treatment in Patients with Photoaging. J. Pak. Assoc. Dermatol. 2020, 30, 485–489. [Google Scholar]
- Setyaningrum, T.; Adiningtyas, V.; Noor Oktaviyanti, R.; Ayu Umborowati, M.; Yulianto Listiawan, M.; Santoso, B.; Prakoeswa, C.R.S. Efficacy of Amniotic Membrane Stem Cell Conditioned Medium (AMSC-CM) and Vitamin C Following CO2 Fractional Laser for Photoaging Therapy. Malays. J. Med. Health Sci. 2021, 17, 30. [Google Scholar]
- Signor, K.C.; Steiner, D.; Roth, D.; Júnior, M.L.B.; de Souza, L.G.; dos Santos, K.B.N.H. Stromal Vascular Fraction, a New Therapy in Photoaging: A Comparative Controlled Study. Surg. Cosmet. Dermatol. 2016, 8, 104–108. [Google Scholar] [CrossRef]
- Truchuelo, M.T.; Vitale, M. A Cosmetic Treatment Based on the Secretion of Cryptomphalus Aspersa 40% Improves the Clinical Results after the Use of Nonablative Fractional Laser in Skin Aging. J. Cosmet. Dermatol. 2020, 19, 622–628. [Google Scholar] [CrossRef]
- Widianingsih, N.P.S.; Setyaningrum, T.; Prakoeswa, C.R.S. The Efficacy and Safety of Fractional Erbium Yag Laser Combined with Topical Amniotic Membrane Stem Cell (AMSC) Metabolite Product for Facial Rejuvenation: A Controlled, Split-Face Study. Dermatol. Rep. 2019, 11. [Google Scholar] [CrossRef]
- Wu, D.C.; Goldman, M.P. A Topical Anti-Inflammatory Healing Regimen Utilizing Conjugated Linolenic Acid for Use Post-Ablative Laser Resurfacing of the Face: A Randomized, Controlled Trial. J. Clin. Aesthetic Dermatol. 2017, 10, 12. [Google Scholar]
- Wu, D.C.; Fletcher, L.; Guiha, I.; Goldman, M.P. Evaluation of the Safety and Efficacy of the Picosecond Alexandrite Laser with Specialized Lens Array for Treatment of the Photoaging Décolletage. Lasers Surg. Med. 2016, 48, 188–192. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Y.; Xu, C.; Wei, X. Improvement of Mild Photoaged Facial Skin in Middle-Aged Chinese Females by a Supramolecular Retinol plus Acetyl Hexapeptide-1 Containing Essence. Ski. Health Dis. 2023, 3, ski2.239. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, J.; Yu, W.; Shang, Y.; Lyu, D.; Lin, X.; Xu, H.; Zhang, Z. Three-Year Results of Facial Photoaging in Asian Patients After Alexandrite 755 Nm Picosecond Laser with Diffractive Lens Array: A Split-Face, Single-Blinded, Randomized Controlled Comparison. Lasers Surg. Med. 2021, 53, 1065–1072. [Google Scholar] [CrossRef]
- Wu, D.C.; Goldman, M.P. A Prospective, Randomized, Double-Blind, Split-Face Clinical Trial Comparing the Efficacy of Two Topical Human Growth Factors for the Rejuvenation of the Aging Face. J. Clin. Aesthetic Dermatol. 2017, 10, 31. [Google Scholar]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Alexiades, M. Radiofrequency Microneedling. Facial Plast. Surg. Clin. N. Am. 2023, 31, 495–502. [Google Scholar] [CrossRef]
- Cunha, J.L.S.; de Carvalho, F.M.d.A.; Filho, R.N.P.; Ribeiro, M.A.G.; de Albuquerque-Júnior, R.L.C. Effects of Different Protocols of Low-Level Laser Therapy on Collagen Deposition in Wound Healing. Braz. Dent. J. 2019, 30, 317–324. [Google Scholar] [CrossRef]
- Torbeck, R.L.; Schilling, L.; Khorasani, H.; Dover, J.S.; Arndt, K.A.; Saedi, N. Evolution of the Picosecond Laser: A Review of Literature. Dermatol. Surg. 2019, 45, 183–194. [Google Scholar] [CrossRef]
- Sklar, L.R.; Burnett, C.T.; Waibel, J.S.; Moy, R.L.; Ozog, D.M. Laser Assisted Drug Delivery: A Review of an Evolving Technology. Lasers Surg. Med. 2014, 46, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Porcaro, G.; Mappa, I.; Leonforte, F.; Baldini, G.M.; Guarneri, M.F.; La Verde, M.; Sorrentino, F.; Laganà, A.S. Hyaluronic Acid in Female Reproductive Health: Tailoring Molecular Weight to Clinical Needs in Obstetric and Gynecological Fields. Pharmaceutics 2025, 17, 991. [Google Scholar] [CrossRef]
- Amato, M.; Polizzi, A.; Viglianisi, G.; Leonforte, F.; Mascitti, M.; Isola, G. Impact of Periodontitis and Oral Dysbiosis Metabolites in the Modulation of Accelerating Ageing and Human Senescence. Metabolites 2025, 15, 35. [Google Scholar] [CrossRef]
- Preissig, J.; Hamilton, K.; Markus, R. Current Laser Resurfacing Technologies: A Review That Delves Beneath the Surface. Semin. Plast. Surg. 2012, 26, 109. [Google Scholar] [CrossRef]
- Sowash, M.; Alster, T. Review of Laser Treatments for Post-Inflammatory Hyperpigmentation in Skin of Color. Am. J. Clin. Dermatol. 2023, 24, 381–396. [Google Scholar] [CrossRef]
- Manjaly, P.; Xia, E.; Allan, A.; Vinjamuri, S.; La Garza, H.D.; Manjaly, C.; Szeto, M.D.; Eichstadt, S.; Maymone, M.; Vashi, N. Skin Phototype of Participants in Laser and Light Treatments of Cosmetic Dermatologic Conditions: A Systematic Review. J. Cosmet. Dermatol. 2023, 22, 2434–2439. [Google Scholar] [CrossRef]
- Silpa-archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory Hyperpigmentation: A Comprehensive Overview: Epidemiology, Pathogenesis, Clinical Presentation, and Noninvasive Assessment Technique. J. Am. Acad. Dermatol. 2017, 77, 591–605. [Google Scholar] [CrossRef]
- Chang, Y.F.; Chen, L.C.; Kim, D.H.; Hsu, S.H.; Chung, H.J. Racial Differences in Tolerability of Topical Retinoids: A 15-Year Single-Center Retrospective Cohort Study. JAAD Int. 2024, 16, 122. [Google Scholar] [CrossRef]
- Podgórna, K.; Kołodziejczak, A.; Rotsztejn, H. Cutometric Assessment of Elasticity of Skin with Striae Distensae Following Carboxytherapy. J. Cosmet. Dermatol. 2018, 17, 1170–1174. [Google Scholar] [CrossRef]
- Griffiths, T.W.; Watson, R.E.B.; Langton, A.K. Skin Ageing and Topical Rejuvenation Strategies. Br. J. Dermatol. 2023, 189, I17–I23. [Google Scholar] [CrossRef]
- Seirafianpour, F.; Pour Mohammad, A.; Moradi, Y.; Dehghanbanadaki, H.; Panahi, P.; Goodarzi, A.; Mozafarpoor, S. Systematic Review and Meta-Analysis of Randomized Clinical Trials Comparing Efficacy, Safety, and Satisfaction between Ablative and Non-Ablative Lasers in Facial and Hand Rejuvenation/Resurfacing. Lasers Med. Sci. 2022, 37, 2111–2122. [Google Scholar] [CrossRef]
- Akhter, M.F.; Sirmans, S.; Hernandez, M.; Chishom, T.; Woltjen, N.; Turner, E.; Bieber, S.; Nazerali, R.S.; Durkin, A.J. A Clinical Safety Assessment of Hybrid Fractional Laser Use at Increased Depths for Facial Skin Rejuvenation. Plast. Reconstr. Surg. Glob. Open 2024, 12, 1. [Google Scholar] [CrossRef]
- Rezapour, A.; Arabloo, J.; Moradi, N.; Ehsanzadeh, S.J.; Hourzad, M.; Alipour, V. Safety and Effectiveness of Endodermal Radiofrequency for Skin Rejuvenation: A Systematic Review. Aesthetic Plast. Surg. 2023, 47, 378–386. [Google Scholar] [CrossRef]
- Huang, A.; Austin, E.; Jagdeo, J. Patient-Reported Outcomes in Lasers and Light Therapy. G. Ital. Dermatol. Venereol. 2019, 154, 120–126. [Google Scholar] [CrossRef]
- Lai-Kwon, J.; Thorner, E.; Rutherford, C.; Crossnohere, N.; Brundage, M. Integrating Patient-Reported Outcomes into the Care of People with Advanced Cancer—A Practical Guide. Am. Soc. Clin. Oncol. Educ. 2024, 44, e438512. [Google Scholar] [CrossRef]
- Worley, B.; Harikumar, V.; Reynolds, K.; Dirr, M.K.A.; Christensen, R.E.; Anvery, N.; Yi, M.D.; Poon, E.; Alam, M. Treatment of Actinic Keratosis: A Systematic Review. Arch. Dermatol. Res. 2023, 315, 1099–1108. [Google Scholar] [CrossRef]
- Morton, C.A.; Braathen, L.R. Daylight Photodynamic Therapy for Actinic Keratoses. Am. J. Clin. Dermatol. 2018, 19, 647–656. [Google Scholar] [CrossRef]

| Author, Year | Country | Study Design | Sample Size and Characteristics | Intervention Details | Key Findings |
|---|---|---|---|---|---|
| Bruce et al., 2016 [21] | USA | Non-randomized controlled trial | 13 subjects, Fitzpatrick I–IV post chemical resurfacing | 12-week continuation skin care post AGE/MELA peel | Maintenance/enhancement of resurfacing benefits; no adverse events. |
| Da Silva et al., 2021 [22] | Brazil | Non-randomized controlled trial with split-face design | 19 women, mean age 54, Fitzpatrick I-IV, photoaging types II/III | Lyophilized PRP (2 months) | No significant improvement in skin aging with PRP |
| Díaz-Ley et al., 2015 [23] | Spain | Non-randomized, non-controlled (pre-post) experimental study | 10 healthy volunteers (7F/3M), aged 34–59 years | Intradermal PRGF | Increased dermal/epidermal thickness; reduced solar elastosis; high satisfaction. |
| El-Domyati et al., 2025 [24] | Egypt | Non-randomized, non-controlled (pre-post) experimental study | 12 subjects, Fitzpatrick III–IV, moderate photoaging | Microneedle + sublative fractional RF (3 sessions) | Biopsy-based analysis: increased collagen I and normalized elastic fibers; improved skin texture and wrinkles |
| Gillbro et al., 2015 [25] | Sweden | Non-randomized controlled trial | 16 females (for each of two different trials), >55 years | Topical AAA 1% (12 days) | Increased COL-IV and fibrillin-1; improved skin firmness vs. vehicle; comparable to retinol. |
| Grippaudo et al., 2016 [26] | Italy | Randomized controlled trial | 50 patients, Fitzpatrick II–V, photo/chronoaging | Fractional laser CO2 + ionic hydrogel (±antibiotics) | No difference between groups in healing time, post-operative complications and satisfaction; good adherence, no infections. |
| Indramaya et al., 2023 [27] | Indonesia | Non-randomized controlled trial | 60 women | Microneedling vs. fractional CO2 laser + AMSC-CM + Vitamin C (3 sessions) | Fractional CO2 group superior on wrinkles, UV spots, pores |
| Jiménez et al., 2018 [28] | Spain | Randomized controlled trial with split-face design | 10 female subjects, moderate-severe photoaging | YAG laser + regenerative serum vs. placebo | Superior epidermal/dermal regeneration and wrinkle reduction on the treated side; high satisfaction. |
| Lim et al., 2020 [29] | South Korea | Randomized controlled trial | 50 women, 45–65 years, Fitzpatrick II–V, photoaging signs | SCA® + IFC®-CAF vs. vehicle | Reduced TEWL, improved firmness, elasticity; superior PGA/IGA vs. placebo; similar wrinkle reduction in both groups |
| Liu et al., 2019 [30] | China | Randomized controlled trial with split-face design | 22 participants, Fitzpatrick III-IV, photoaged facial skin | Microneedle fractional radiofrequency | Improved wrinkles, texture, and roughness score (Sa); minimal adverse events. |
| Makino et al., 2023 [31] | USA | Non-randomized, non-controlled (pre-post) experimental study | 23 females, mean age 42.7, Fitzpatrick I–VI | Diamond-tip microdermabrasion + HA5-DG serum (5 different forms of HA) | Immediate and long-term improvements in hydration, fine lines, texture, and radiance |
| Marchlewicz et al., 2024 [32] | Poland | Randomized controlled trial | 30 women, Fitzpatrick II-III | Microneedling or needle-free mesotherapy with 98.2% snail mucus | Histological improvements: increased collagen bundle thickness, dermal regeneration markers (Ki67, PCNA, MMP-2) improved |
| Mazur et al., 2023 [33] | Poland | Non-randomized, non-controlled (pre-post) experimental study | 52 patients (34 women, 18 men) and 300 AK lesions, facial grade II AK, 275 non-pigmented, 25 pigmented | PDT | High remission rates of dermoscopic and RCM features, solar elastosis reduced |
| Prakoeswa et al., 2020 [34] | Indonesia | Randomized controlled trial | 60 women, split group | AMSC-MP + Vitamin C vs. AMSC-MP alone, Dermapen®-assisted delivery (3 sessions) | Significant improvements in wrinkles, UV spots, pores in the combined group |
| Setyaningrum et al., 2021 [35] | Indonesia | Non-randomized, non-controlled (pre-post) experimental study | 30 women, Glogau II–III | CO2 fractional laser + AMSC-CM + Vitamin C (3 sessions) | Significant wrinkle, pore, spot improvement; minor effect on skin tone |
| Signor et al., 2016 [36] | Brazil | Non-randomized controlled trial | 10 patients (split group), Fitzpatrick I–V | SVF vs. calcium hydroxyapatite filler | Slight collagen increase in SVF group; no statistical differences; moderate satisfaction. |
| Truchuelo et al., 2020 [37] | Spain | Randomized controlled trial with split-face design | 20 patients, split-face, moderate photoaging | Non-ablative fractional Laser (2 session) + SCA® secretion 40% (daily for 28-days) vs. vehicle | SCA® improved skin recovery post-laser, enhanced elasticity, reduced wrinkles and adverse effects |
| Widianingsih et al., 2019 [38] | Indonesia | Non-randomized controlled trial with split-face design | 9 female participants, Glogau II–III | Fractional erbium laser + topical AMSC-MP | Improved pores, pigmentation; mixed wrinkle results; no serious adverse events |
| Wu et al., 2017 [39] | USA | Non-randomized, non-controlled (pre-post) experimental study | 34 healthy subjects, 18–75 years, Fitzpatrick I–IV | Topical conjugated linolenic acid derived from pomegranate seed extract vs. dimethicone after fractionated ablative CO2-laser resurfacing | Faster resolution of itching, reduced edema; improved wrinkling and elastosis at day 14 |
| Wu et al., 2016 [40] | USA | Non-randomized, non-controlled (pre-post) experimental study | 20 subjects, Fitzpatrick I–IV | PSAL with DLA | Improved dyspigmentation, keratosis, texture; moderate satisfaction |
| Ye et al., 2023 [41] | China | Non-randomized, non-controlled (pre-post) experimental study | 32 Chinese women, Fitzpatrick I–IV, mild photoaging | Supramolecular 0.1% retinol (twice-daily use) | Improved wrinkles, texture, elasticity, hydration, high tolerance |
| Yu et al., 2021 [42] | China | Randomized controlled trial with split-face design | 10 women, 45–55 years, Fitzpatrick III–IV | PSAL with DLA | Sustained improvement in dyschromia, texture, and wrinkles up to 36 months, minimal adverse effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonforte, F.; Pergolizzi, T.; Nicosia, V.; Nicoli, F.; Genovese, G.; Genovese, C.; Kiranantawat, K.; Perrotta, R.; Mistretta, A. Preventive and Therapeutic Interventions in Solar Elastosis and Photoaging: A Comprehensive Systematic Review. Biomedicines 2025, 13, 2758. https://doi.org/10.3390/biomedicines13112758
Leonforte F, Pergolizzi T, Nicosia V, Nicoli F, Genovese G, Genovese C, Kiranantawat K, Perrotta R, Mistretta A. Preventive and Therapeutic Interventions in Solar Elastosis and Photoaging: A Comprehensive Systematic Review. Biomedicines. 2025; 13(11):2758. https://doi.org/10.3390/biomedicines13112758
Chicago/Turabian StyleLeonforte, Francesco, Tiziano Pergolizzi, Vito Nicosia, Fabio Nicoli, Giovanni Genovese, Cristina Genovese, Kidakorn Kiranantawat, Rosario Perrotta, and Antonio Mistretta. 2025. "Preventive and Therapeutic Interventions in Solar Elastosis and Photoaging: A Comprehensive Systematic Review" Biomedicines 13, no. 11: 2758. https://doi.org/10.3390/biomedicines13112758
APA StyleLeonforte, F., Pergolizzi, T., Nicosia, V., Nicoli, F., Genovese, G., Genovese, C., Kiranantawat, K., Perrotta, R., & Mistretta, A. (2025). Preventive and Therapeutic Interventions in Solar Elastosis and Photoaging: A Comprehensive Systematic Review. Biomedicines, 13(11), 2758. https://doi.org/10.3390/biomedicines13112758






