Uterine Contractility Changes in Adenomyosis: Evidence from a Systematic Review and Meta-Analysis
Abstract
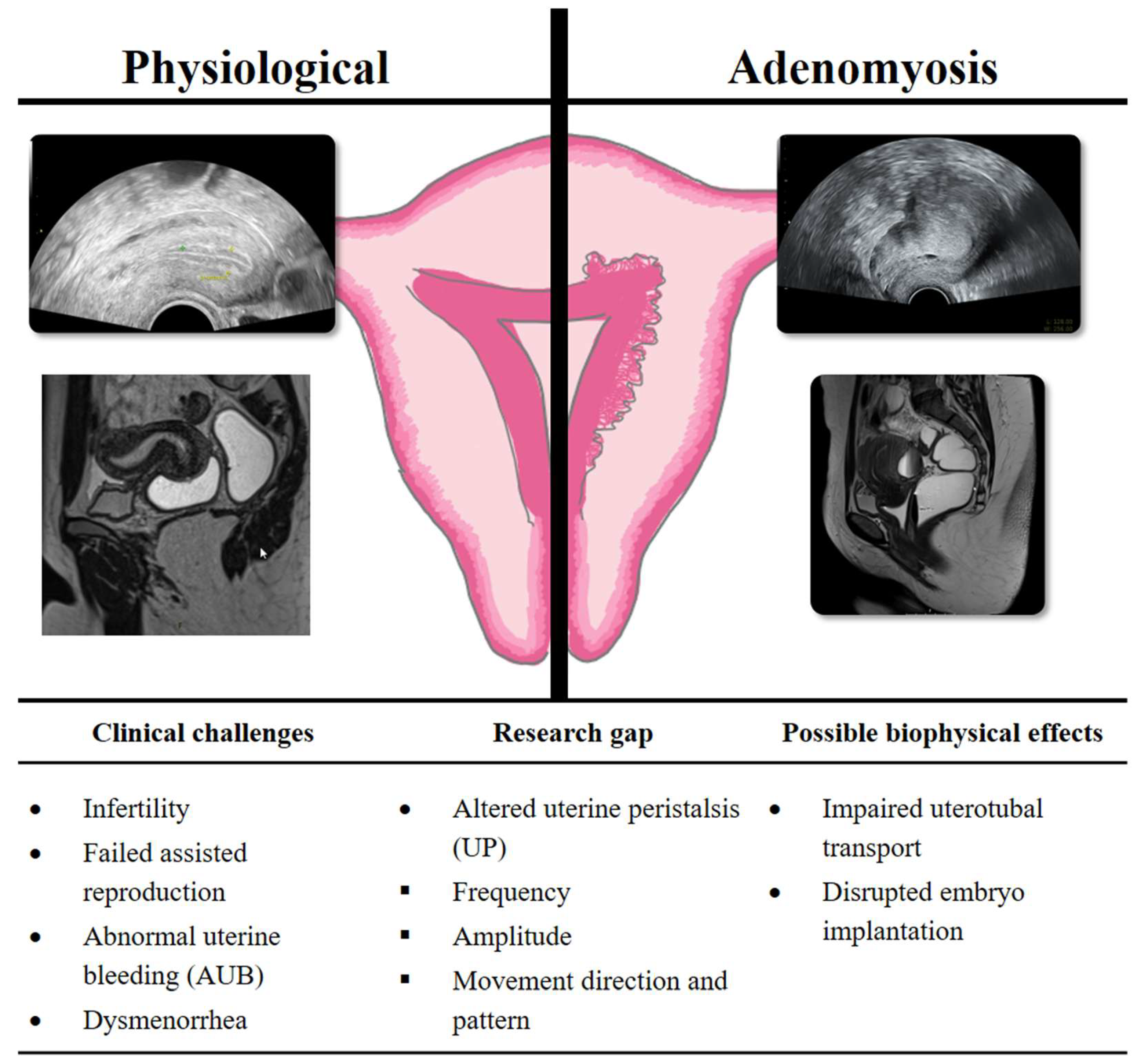
1. Introduction
2. Materials and Methods
2.1. Registration of Protocols
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
| First Author, Year of Publication | Study Design | Country | Number of Participants (Study Group) | Number of Participants (Control Group) | Total Number of Participants | Age (y), Mean or Median ± SD (Study Group) | Age (y), Mean or Median ± SD (Control Group) | Treatment Cycle | Moment of Contractility Measurement | UP Measurement Method | Uterus Dimensions, mm (Study Group) | Uterus Volume, cm3 (Study Group | Uterus Dimensions, mm (Control Group) | Uterus Volume, cm3 (Control Group) | Endometrium Thickness, mm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kissler et al., 2006 [25] | Prospective observational study | Germany | 35 (24 focal and 11 diffuse) | 6 (only endometriosis) | 41 | focal: 32.4 ± 2.6; diffuse: 35.2 ± 4.3 | 33.2 ± 2.9 | natural | NR | HSSG | NR | NR | NR | NR | ≥9 mm |
| Kissler et al., 2007 [26] | Observational cohort study | Germany | Review | NR | NR | NR | NR | NR | NR | NR | NR | ||||
| Soares et al., 2023 [27] | Prospective case–control study | Brazil | 4 | 11 | 15 | NA | NA | NR | NR | MRI | NR | NR | NR | NR | NR |
| Arena et al., 2024 [28] | Prospective observational study | Italy | 18 | 18 | 36 | 34.6 ± 6.1 | 33.3 ± 5.9 | natural | periovulatory phase (11–14 d) Graafian follicle >18 mm, trilaminar endometrium ≥7 mm | TVS (2D)—reconstruction of coronal plane using 3D software | (33.3 ± 5.9) × (46.9 ± 16.8) × (59.2 ± 15.1) | 137.5 ± 117.7 | (75.3 ± 9.5) × (37.2 ± 6.2) × (49.6 ± 7.6) | 74.5 ± 27.6 | ≥7 mm periovulatory phase |
| Rees et al., 2024 [29] | Multicenter prospective study | The Netherlands, Italy, Greece | 39 | 106 | 145 | 38.23 ± 7.46 | 29.4 ± 6.74 | natural | menses, midfollicular, late follicular, early luteal, late luteal | TVS | (81.1 ± 14.1) × (49.1 ± 9.9) × (55.0 ± 13.4) | 114.67 ± 36.89 | (59.1 ± 21.5) × (31.7 ± 10.3) × (40.9 ± 19.9) | 40.12 ± 24.34 | study group Menses 4.68 ± 2.68 Midfollicular 5.23 ± 2.54 Late follicular 6.65 ± 3.56 Early luteal 10.00 ± 0.00 Late luteal 10.35 ± 3.43 control group Menses 3.50 ± 0.71; Midfollicular 6.50 ± 0.71; Late follicular 5.67 ± 2.08; Early luteal 8.67 ± 0.58; Late luteal 7.33 ± 0.58 |
| Kido et al., 2025 [30] | Prospective observational study | Japan | 182 walls (Diffuse 84 and focal 98) | 96 walls | 139 | NR | NR | natural | proliferative, luteal, menstrual | MRI | NR | NR | NR | NR | NR |
| Latif et al., 2025 [31] | Inter- and intra-observer reproducibility study | UK | 26 | 40 | 66 | 39 (35–43) | 36 (29–40) | ART | baseline, ovarian stimulation, embryotransfer | TVS | NR | NR | NR | NR | NR |
| First Author, Year of Publication | Frequency (Contraction/min) | Direction (Study Group) | Direction (Control Group) | Adenomyosis Diagnostic | Classification | Chronic Pain/ Pelvic pain | Infertility Associated | AUB Associated | Endometriosis Associated |
|---|---|---|---|---|---|---|---|---|---|
| Kissler et al., 2006 [25] | NR | Focal: ipsi/bilateral 10/24 (42%), contralateral 8/24 (33%), failure 6/24 (25%); Diffuse: ipsi/bilateral 1/11 (9%), contralateral 2/11 (18%), failure 8/11 (73%) | ipsi/bilateral 4/6 (67%), contralateral 2/6 (33%), failure 0/6 (0%) | T2-MRI (JZ ≥ 9 mm) | NR | NR | NR | NR | 35/35 (100%) |
| Kissler et al., 2007 [26] | NR | Focal: physiologic 13/28 (46%), pathophysiologic 15/28 (54%); Diffuse: physiologic 3/14 (21.5%), pathophysiologic 11/14 (78.5%) | Physiologic 5/8 (62.5%), pathophysiologic 3/8 (37.5%) | T2-MRI | NR | NR | NR | NR | NR |
| Soares et al., 2023 [27] | Adenomyosis: 0.8/2 min; Without: 3.18/2 min | 1/4 (25%) antegrade; 3/5 (75%) retrograde; 11/11 (100%) control retrograde | NR | MRI | NR | NR | NR | NR | NR |
| Arena et al., 2024 [28] | NR | 2/18 (11.1) antegrade; 5/18 (27.8) retrograde; 7/18 (38.9) opposing; 4/18 (22.2) random; 0 absent | 3/18 (16.7) antegrade; 13/18 (72.2) retrograde; 1/18 (5.6) opposing; 1/18 (5.6) random; 0 absent | TVS (2D)—3D software reconstruction | MUSA | 2.6 ± 3.0 vs. 0.4 ± 1.0 | 6 (33.3%) vs. 2 (11.1%) | 9 (50%) vs. 1 (5.6%) | NR |
| Rees et al., 2024 [29] | Late follicular Adenomyose: 1.54 ± 0.26 Control group: 1.70 ± 0.26 | Direction not explicitly reported. Velocity was measured for both F2C and C2F propagation, and differences can be interpreted, but no cut-off values were defined | Direction not explicitly reported. Velocity was measured for both F2C and C2F propagation, and differences can be interpreted, but no cut-off values were defined | TVS/MRI | MUSA | NR | NR | NR | NR |
| Kido et al., 2025 [30] | proliferative phase focal AM: 8.8/3 min diffuse AM: 8.06/3 min healthy: 9.52/3 min | cervix—fundus: focal AM: 11/22 diffuse AM: 19/27 fundus—cervix: focal AM: 4/22 diffuse AM: 2/27 opposing: focal AM: 5/22 diffuse AM: 3/27 | cervix—fundus: 11/24 fundus—cervix: 8/24 opposing: 4/24 | TVS/MRI | NR | NR | NR | NR | NR |
| Latif et al., 2025 [31] | Ovarian stimulation 3.03 | 5/26 antegrade vs. 0/40 control 7/26 opposing vs. 6/40 control 6/26 random | 0 retrograde; others NR | TVS | NR | NR | NR | NR | NR |
| First Author, Year | Representativeness of Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcome Not Present at Study Start | Comparability of Cohorts (Confounders Controlled) | Assessment of Outcome | Sufficient Length of Follow-Up | Adequacy of Follow-Up of Cohorts | Total | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Kissler, 2006 [25] | ★ | - | ★ | - | – | ★ | – | ★ | 4 | poor |
| Kissler, 2007 [26] | – | – | – | – | – | – | – | – | - | - |
| Soares, 2023 [27] | – | ★ | ★ | - | – | ★ | – | ★ | 4 | poor |
| Arena, 2024 [28] | ★ | ★ | ★ | - | – | ★ | – | ★ | 5 | fair |
| Rees, 2024 [29] | ★ | ★ | ★ | - | – | ★ | – | ★ | 5 | fair |
| Kido, 2025 [30] | ★ | ★ | ★ | - | – | ★ | – | ★ | 5 | fair |
| Latif, 2025 [31] | ★ | ★ | ★ | - | – | ★ | – | ★ | 5 | fair |
2.6. Data Synthesis
2.7. Meta-Analysis Visualisation
3. Results
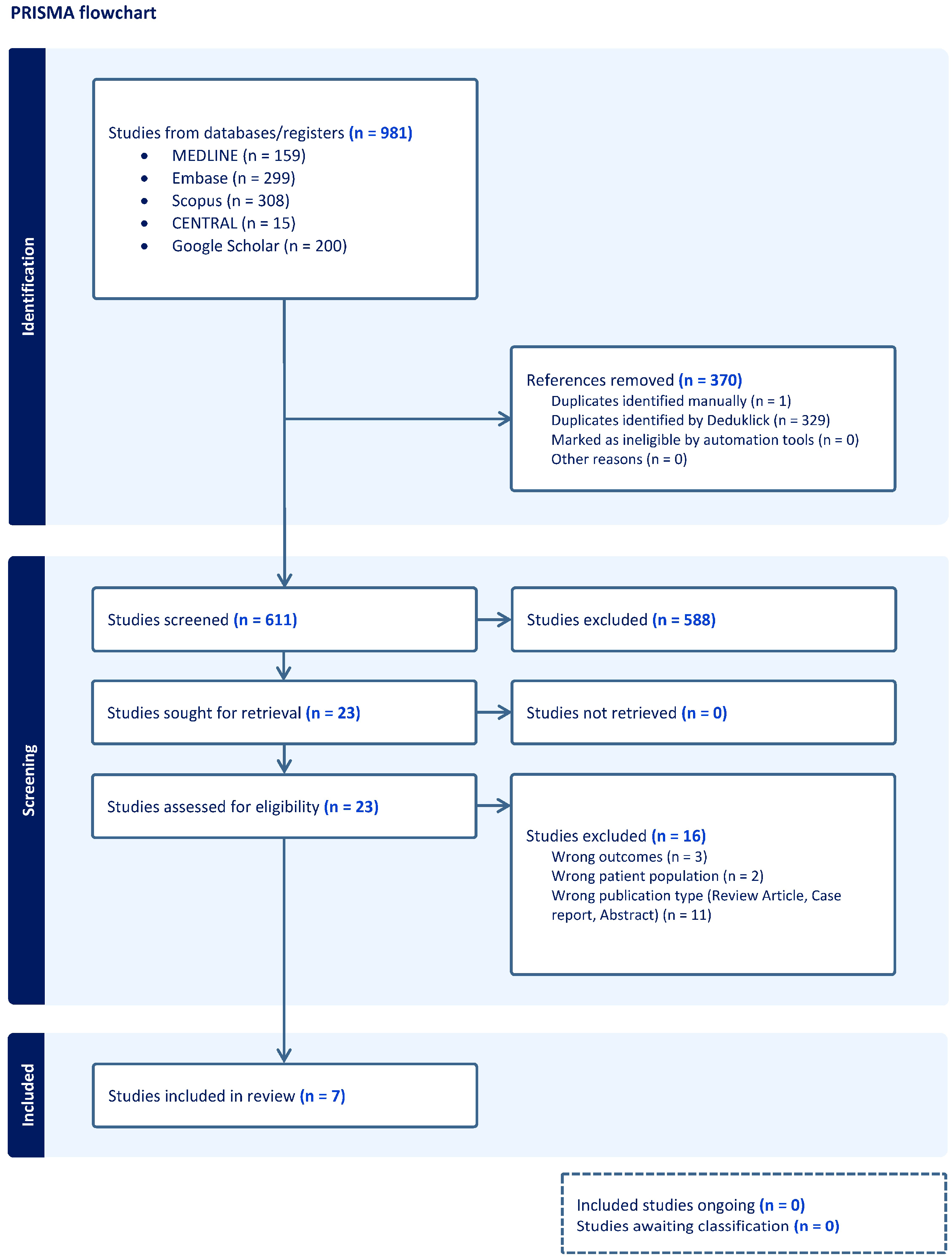
3.1. Results of the Systematic Review
3.2. Study Characteristics
3.3. Results of Individual Studies
3.3.1. Frequency of Uterine Contractions
3.3.2. Amplitude of Uterine Contractions
3.3.3. Direction of Uterine Contractions
3.3.4. Association with Symptoms of Adenomyosis
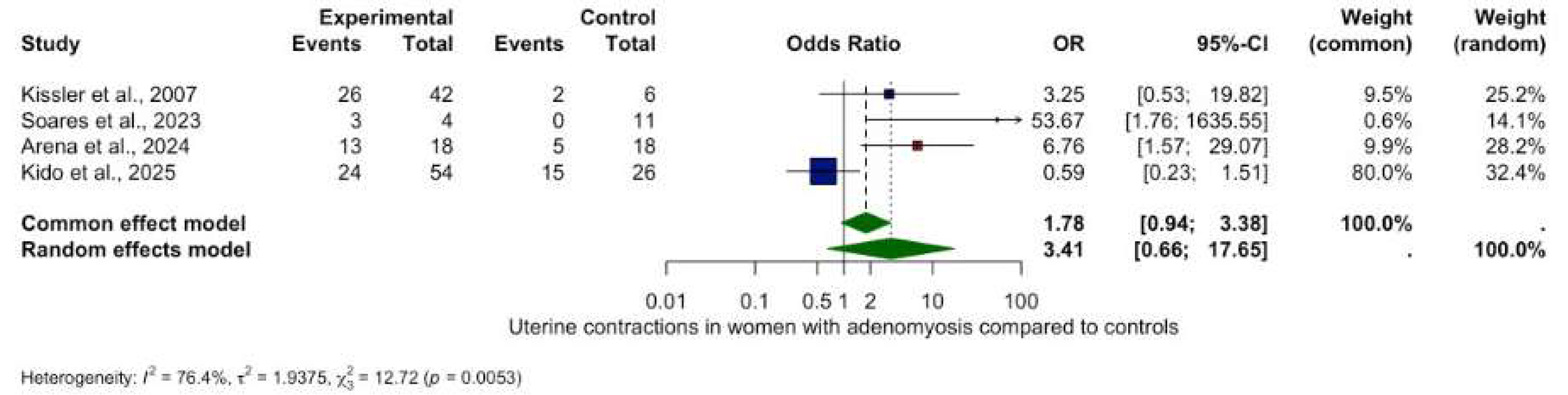
3.4. Results of the Meta-Analysis
Uterine Contraction Frequency
3.5. Directional Patterns of Uterine Contractility
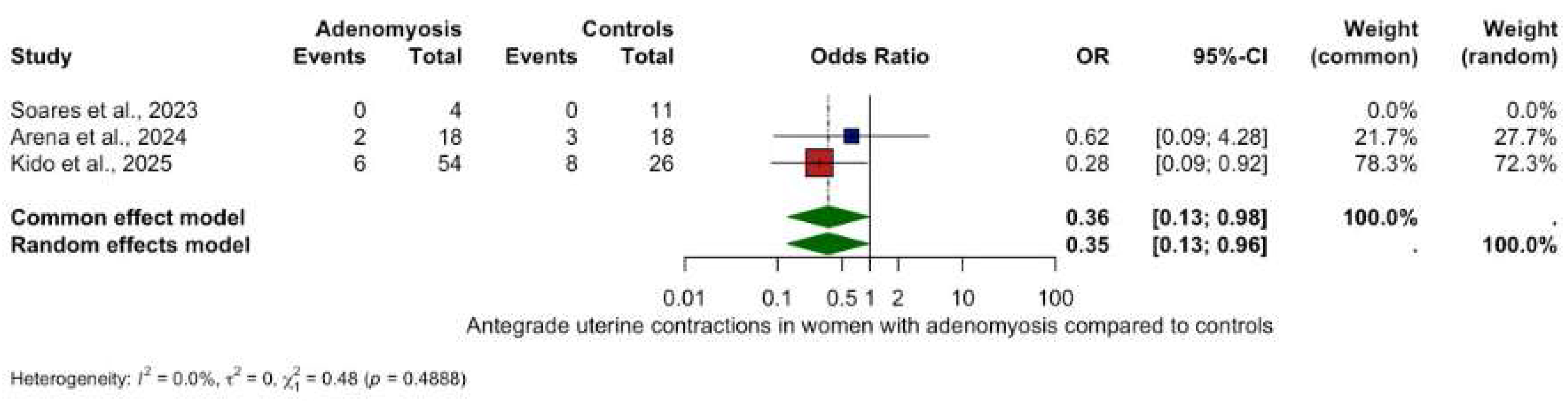
3.5.1. Antegrade Uterine Contractions
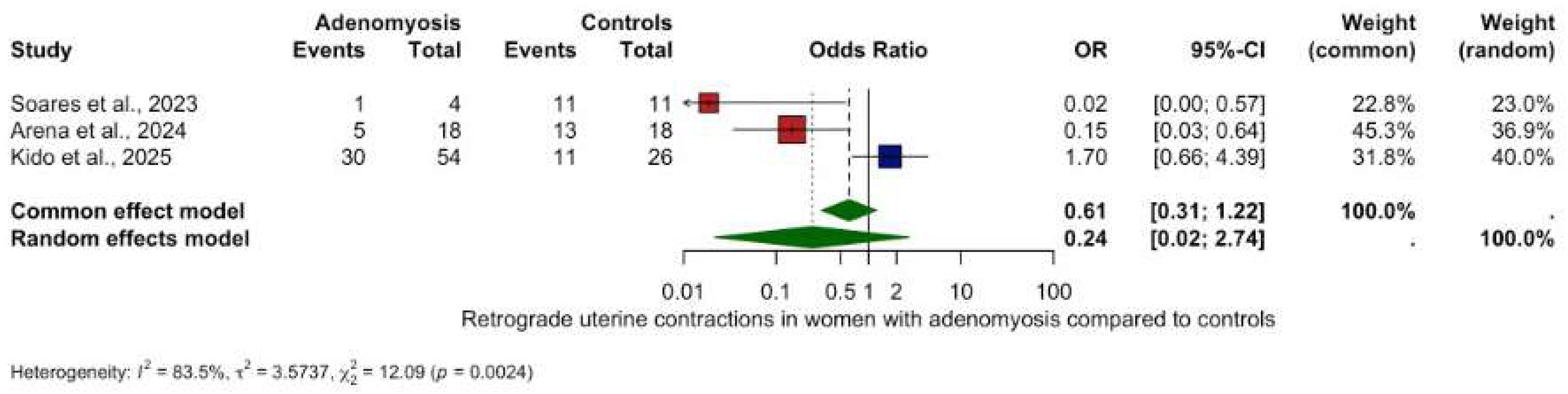
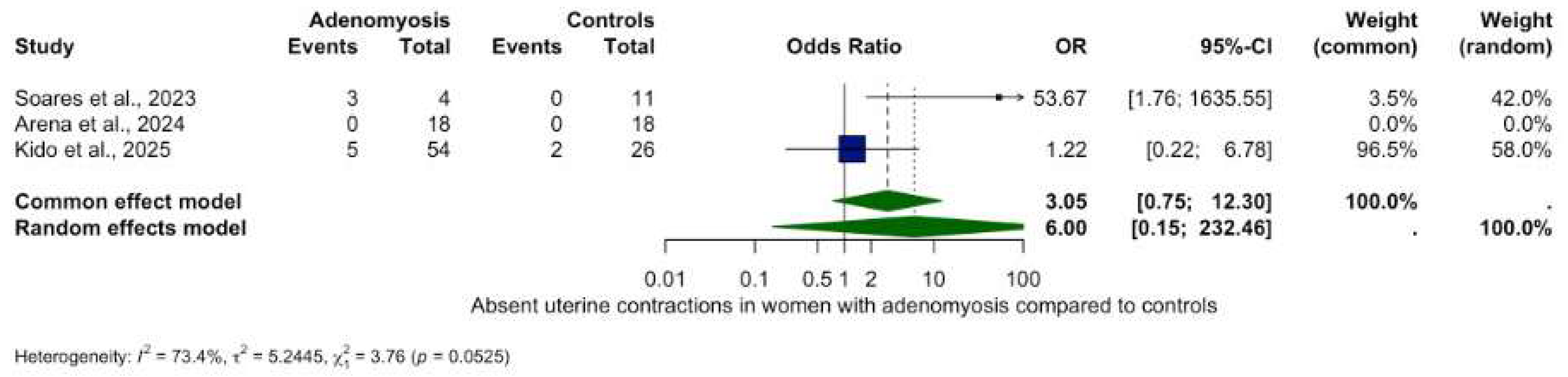
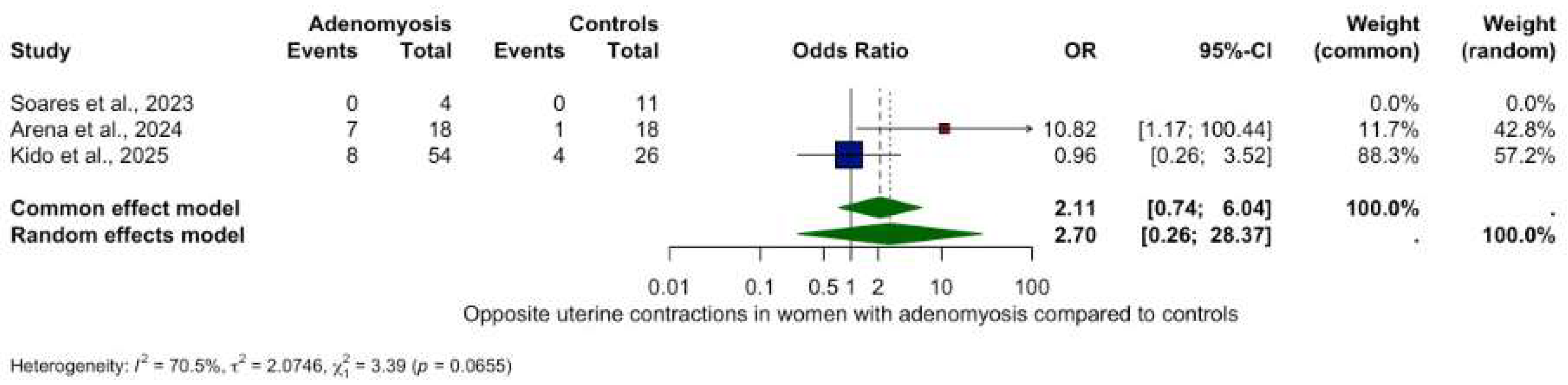
3.5.2. Other Contractility Patterns
- Retrograde contractions: OR 0.24 (95% CI: 0.02–2.74, p = 0.2534), (Figure 3).
- Absent contractions: OR 6.00 (95% CI: 0.15–232.46, p = 0.3370), (Figure 4).
- Antegrade contractions: OR 3.50 (95% CI: 0.15–0.96, p = 0.048), (Figure 5).
- Opposite contractions: OR 2.70 (95% CI: 0.26–28.37, p = 0.4079), (Figure 6).
- Random contractions: OR 4.32 (95% CI: 0.73–25.46, p = 0.1063).
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ART | Assisted Reproductive Technology |
| Ca2+ | Calcium ion |
| CE | Chronic Endometritis |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CI | Confidence Interval |
| CPR | Clinical Pregnancy Rate |
| EHG | Electrohysterography |
| IVF | In Vitro Fertilization |
| JZ | Junctional Zone |
| MD | Mean Difference |
| MRI | Magnetic Resonance Imaging |
| MUSA | Morphological Uterus Sonographic Assessment |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds Ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| SD | Standard Deviation |
| TVS | Transvaginal Sonography |
| UP | Uterine Peristalsis |
| 2D/3D/4D | Two-/Three-/Four-Dimensional (Ultrasound) |
References
- Munro, M.G. Uterine Polyps, Adenomyosis, Leiomyomas, and Endometrial Receptivity. Fertil. Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.-J. Adenomyosis Pathogenesis: Insights from next-Generation Sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing Adenomyosis: An Integrated Clinical and Imaging Approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Chakravarti, D.; Parker, J.B.; Milad, M.; Yang, L.; Chaudhari, A.; Tsai, S.; Wei, J.J.; et al. Endometriosis and Adenomyosis: Shared Pathophysiology. Fertil. Steril. 2023, 119, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Di Giovanni, A.; Exacoustos, C.; Tosti, C.; Pinzauti, S.; Malzoni, M.; Petraglia, F.; Zupi, E. Preoperative and Postoperative Clinical and Transvaginal Ultrasound Findings of Adenomyosis in Patients with Deep Infiltrating Endometriosis. Reprod. Sci. 2014, 21, 1027–1033. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Kalaitzopoulos, D.R.; Schwartz, A.S.K.; Spaanderman, M.; Kramer, B.W.; Mueller, M.D.; Mueller, M. Fertility, Pregnancy and Neonatal Outcomes of Patients with Adenomyosis: A Systematic Review and Meta-Analysis. Reprod. BioMed. Online 2021, 42, 185–206. [Google Scholar] [CrossRef]
- Steinmann, M.; Anthon, C.; Schwartz, A.S.K.; Mackens, S.; Blockeel, C.; Kalaitzopoulos, D.R.; Vidal, A. GnRH Agonist Pretreatment Prior to Frozen Embryo Transfer in Women with Adenomyosis: A Systematic Review and Meta-Analysis. Reprod. BioMed. Online 2025, 51, 105075. [Google Scholar] [CrossRef]
- Arrowsmith, S.; Robinson, H.; Noble, K.; Wray, S. What Do We Know About What Happens to Myometrial Function as Women Age? J. Muscle Res. Cell Motil. 2012, 33, 209–217. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Herbertz, M.; Beil, D.; Huppert, P.; Mall, G.; Kissler, S.; Noe, M.; Wildt, L. Uterine Peristaltic Activity and the Development of Endometriosis. Ann. N. Y. Acad. Sci. 2004, 1034, 338–355. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Wildt, L.; Beil, D.; Deininger, H. Uterine Hyperperistalsis and Dysperistalsis as Dysfunctions of the Mechanism of Rapid Sperm Transport in Patients with Endometriosis and Infertility. Hum. Reprod. 1996, 11, 1542–1551. [Google Scholar] [CrossRef]
- Vidal, A.; Trejos, V.; Pape, J.; Karrer, T.; Yilmaz, G.; Von Wolff, M. Lower Pregnancy Rate in Women with High Uterine Peristalsis Before Embryo Transfer: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2025, 23, 49. [Google Scholar] [CrossRef]
- Kuijsters, N.P.M.; Methorst, W.G.; Kortenhorst, M.S.Q.; Rabotti, C.; Mischi, M.; Schoot, B.C. Uterine Peristalsis and Fertility: Current Knowledge and Future Perspectives: A Review and Meta-Analysis. Reprod. Biomed. Online 2017, 35, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Bell, S.C.; Pringle, J.H.; Habiba, M.A. Uterine Adenomyosis Is Associated with Ultrastructural Features of Altered Contractility in the Inner Myometrium. Fertil. Steril. 2010, 93, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- De Boer, A.; Rees, C.O.; Blank, C.; Huang, Y.; Wessels, B.; Wagenaar, L.; Van Vliet, H.; Huppelschoten, A.G.; Zizolfi, B.; Foreste, V.; et al. The influence of hormonal stimulation during IVF/ICSI treatment on uterine peristalsis measured by ultrasound speckle tracking. Fertil. Steril. 2022, 118, e56. [Google Scholar] [CrossRef]
- Moawad, G.; Fruscalzo, A.; Youssef, Y.; Kheil, M.; Tawil, T.; Nehme, J.; Pirtea, P.; Guani, B.; Afaneh, H.; Ayoubi, J.M.; et al. Adenomyosis: An Updated Review on Diagnosis and Classification. J. Clin. Med. 2023, 12, 4828. [Google Scholar] [CrossRef]
- Kido, A.; Togashi, K.; Nishino, M.; Miyake, K.; Koyama, T.; Fujimoto, R.; Iwasaku, K.; Fujii, S.; Hayakawa, K. Cine MR Imaging of Uterine Peristalsis in Patients with Endometriosis. Eur. Radiol. 2007, 17, 1813–1819. [Google Scholar] [CrossRef]
- Shitano, F.; Kido, A.; Kataoka, M.; Fujimoto, K.; Kiguchi, K.; Fushimi, Y.; Togashi, K. Evaluation of Uterine Peristalsis Using Cine MRI on the Coronal Plane in Comparison with the Sagittal Plane. Acta Radiol. 2016, 57, 122–127. [Google Scholar] [CrossRef]
- Togashi, K.; Kawakami, S.; Kimura, I.; Asato, R.; Takakura, K.; Mori, T.; Konishi, J. Sustained Uterine Contractions: A Cause of Hypointense Myometrial Bulging. Radiology 1993, 187, 707–710. [Google Scholar] [CrossRef]
- Vidal, A.; Bora, C.; Von Holzen, J.; Gulz, M.; Obmann, V.C.; Pape, J.; Karrer, T.; Yilmaz, G.; Von Wolff, M. Cine-MRI for Quantifying Uterine Peristalsis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1021. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-Duplication of Database Search Results for Systematic Reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243, Erratum in J. Med. Libr. Assoc. 2017, 105, 111. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, S.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2009. [Google Scholar]
- Kissler, S.; Hamscho, N.; Zangos, S.; Wiegratz, I.; Schlichter, S.; Menzel, C.; Doebert, N.; Gruenwald, F.; Vogl, T.; Gaetje, R.; et al. Uterotubal Transport Disorder in Adenomyosis and Endometriosis—A Cause for Infertility. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 902–908. [Google Scholar] [CrossRef]
- Kissler, S.; Zangos, S.; Wiegratz, I.; Kohl, J.; Rody, A.; Gaetje, R.; Doebert, N.; Wildt, L.; Kunz, G.; Leyendecker, G.; et al. Utero-Tubal Sperm Transport and Its Impairment in Endometriosis and Adenomyosis. Ann. N. Y. Acad. Sci. 2007, 1101, 38–48. [Google Scholar] [CrossRef]
- Soares, D.M.; Bittencourt, L.K.; Lopes, F.P.P.L.; Oliveira, M.A.P.D. Deep Infiltrating Endometriosis: Cine Magnetic Resonance Imaging in the Evaluation of Uterine Contractility. Radiol. Bras. 2023, 56, 119–124. [Google Scholar] [CrossRef]
- Arena, A.; Zanello, M.; Orsini, B.; Degli Esposti, E.; Iodice, R.; Altieri, M.; Borgia, A.; Moro, E.; Seracchioli, R.; Casadio, P. Uterine Peristalsis in Women Affected by Adenomyosis: A Step towards Functional Assessment. Int. J. Gynecol. Obstet. 2024, 165, 666–671. [Google Scholar] [CrossRef]
- Rees, C.O.; Thomas, S.; De Boer, A.; Huang, Y.; Zizolfi, B.; Foreste, V.; Di Spiezio Di Sardo, A.; Christoforidis, N.; Van Vliet, H.A.A.M.; Mischi, M.; et al. Quantitative Ultrasound Measurement of Uterine Contractility in Adenomyotic vs. Normal Uteri: A Multicenter Prospective Study. Fertil. Steril. 2024, 121, 864–872. [Google Scholar] [CrossRef]
- Kido, A.; Ohta, I.; Himoto, Y.; Fujii, S.; Noguchi, K.; Nakamoto, Y.; Ogawa, T. Uterine Peristalsis of Adenomyosis Patients on Cine Magnetic Resonance Imaging. Int. J. Gynecol. Obstet. 2025, 170, 242–249. [Google Scholar] [CrossRef]
- Latif, S.; Saeed, S.U.; Hu, Y.; De Braud, L.; Yasmin, E.; Saridogan, E.; Mavrelos, D. Quantitative Analysis of Uterine Peristalsis on Transvaginal Sonography in Women with Adenomyosis Using Optical Flow for Motion Quantification: Inter- and Intra-Observer Reproducibility Study. Reprod. BioMed. Online 2025, 105073. [Google Scholar] [CrossRef]
- Pirtea, P.; De Ziegler, D.; Ayoubi, J.M. Endometrial Receptivity in Adenomyosis and/or Endometriosis. Fertil. Steril. 2023, 119, 741–745. [Google Scholar] [CrossRef]
- Pirtea, P.; Cicinelli, E.; De Nola, R.; De Ziegler, D.; Ayoubi, J.M. Endometrial Causes of Recurrent Pregnancy Losses: Endometriosis, Adenomyosis, and Chronic Endometritis. Fertil. Steril. 2021, 115, 546–560. [Google Scholar] [CrossRef]
- Campo, S.; Campo, V.; Benagiano, G. Adenomyosis and Infertility. Reprod. BioMed. Online 2012, 24, 35–46. [Google Scholar] [CrossRef]
- van Gestel, I.; IJland, M.M.; Hoogland, H.J.; Evers, J.L.H. Endometrial Wave-like Activity in the Non-Pregnant Uterus. Hum. Reprod. Update 2003, 9, 131–138. [Google Scholar] [CrossRef]
- Blank, C.; Sammali, F.; Kuijsters, N.; Huang, Y.; Rabotti, C.; de Sutter, P.; Mischi, M.; Schoot, B. Assessment of Uterine Activity during IVF by Quantitative Ultrasound Imaging: A Pilot Study. Reprod. BioMed. Online 2020, 41, 1045–1053. [Google Scholar] [CrossRef]
- De Boer, A.; Rees, C.; Blank, C.; Huang, Y.; Wessels, B.; Wagenaar, L.; Van Vliet, H.; Huppelschoten, D.; Zizolfi, B.; Foreste, V.; et al. The Influence of Hormonal Stimulation on Uterine Peristalsis Measured by Ultrasound Speckle Tracking in Women with IVF/ICSI Treatment Compared to Normal Ovulating Women. Hum. Reprod. 2022, 37, i348–i349. [Google Scholar] [CrossRef]
- De Boer, A.; Rees, C.O.; Mischi, M.; Van Vliet, H.; Huirne, J.; Schoot, B.C. The Influence of Uterine Abnormalities on Uterine Peristalsis in the Non-Pregnant Uterus: A Systematic Review. J. Endometr. Uterine Disord. 2023, 3, 100038. [Google Scholar] [CrossRef]
- Bulletti, C.; De Ziegler, D.; Polli, V.; Del Ferro, E.; Palini, S.; Flamigni, C. Characteristics of Uterine Contractility during Menses in Women with Mild to Moderate Endometriosis. Fertil. Steril. 2002, 77, 1156–1161. [Google Scholar] [CrossRef]
- Wang, S.; Duan, H.; Li, B. Rapid Effects of Oestrogen on Intracellular Ca2+ in the Uterine Junctional Myometrium of Patients With and Without Adenomyosis in Different Phases of the Menstrual Cycle. Reprod. Sci. 2020, 27, 1992–2001. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.-W.; Petraglia, F. Pathogenesis of Adenomyosis: An Update on Molecular Mechanisms. Reprod. BioMed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lu, P.; Bellve, K.; Lifshitz, L.M.; ZhuGe, R. Mode Switch of Ca2+ Oscillation-Mediated Uterine Peristalsis and Associated Embryo Implantation Impairments in Mouse Adenomyosis. Front. Physiol. 2021, 12, 744745. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Ersöz, C.C.; Şükür, Y.E.; Özmen, B.; Sönmezer, M.; Berker, B.; Aytaç, R.; Atabekoğlu, C.S. The Role of Chronic Endometritis in the Etiopathogenesis of Adenomyosis. arXiv 2024. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, R.; Luo, Y.; Luo, C. Analysis of the Diagnostic Value of CD138 for Chronic Endometritis, the Risk Factors for the Pathogenesis of Chronic Endometritis and the Effect of Chronic Endometritis on Pregnancy: A Cohort Study. BMC Women’s Health 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Catena, U.; Schwartz, A.K.; Schoretsanitis, G.; Leeners, B.; Drakopoulos, P.; Samartzis, N. Chronic Endometritis and Endometriosis: Two Sides of the Same Coin? Reprod. Sci. 2025, 32, 474–487. [Google Scholar] [CrossRef]
- Kimura, F.; Takebayashi, A.; Ishida, M.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hirata, K.; Takahashi, A.; Tsuji, S.; Takashima, A.; et al. Review: Chronic Endometritis and Its Effect on Reproduction. J. Obstet. Gynaecol. 2019, 45, 951–960. [Google Scholar] [CrossRef]
- Geysenbergh, B.; Boes, A.-S.; Bafort, C.; Van Rompuy, A.-S.; Neyens, S.; Lie-Fong, S.; Debrock, S.; Vriens, J.; De Loecker, P.; Dancet, E.; et al. The Impact of Chronic Endometritis on Infertility: Prevalence, Reproductive Outcomes, and the Role of Hysteroscopy as a Screening Tool. Gynecol. Obstet. Investig. 2023, 88, 108–115. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Ogawa, K.; Koshiba, A.; Mori, T.; Itoh, K.; Nakashima, M.; Kitawaki, J. Occurrence of Chronic Endometritis in Different Types of Human Adenomyosis. Reprod. Med. Biol. 2022, 21, e12421. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Hu, Q.; Zhang, W.; Xie, Y.; Wei, W. The Alteration of Intrauterine Microbiota in Chronic Endometritis Patients Based on 16S rRNA Sequencing Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 4. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Van Den Bosch, T.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.J.K.; Groenman, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on Revised Definitions of Morphological Uterus Sonographic Assessment (MUSA) Features of Adenomyosis: Results of Modified Delphi Procedure. Ultrasound Obstet. Gynecol. 2022, 60, 118–131. [Google Scholar] [CrossRef]
- Van Den Bosch, T.; De Bruijn, A.M.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic Classification and Reporting System for Diagnosing Adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, A.; Tepasse, P.; Vinayahalingam, V.; Cottagnoud, S.; Gulz, M.; Karrer, T.; Yilmaz, G.; Pape, J.; von Wolff, M. Uterine Contractility Changes in Adenomyosis: Evidence from a Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 2728. https://doi.org/10.3390/biomedicines13112728
Vidal A, Tepasse P, Vinayahalingam V, Cottagnoud S, Gulz M, Karrer T, Yilmaz G, Pape J, von Wolff M. Uterine Contractility Changes in Adenomyosis: Evidence from a Systematic Review and Meta-Analysis. Biomedicines. 2025; 13(11):2728. https://doi.org/10.3390/biomedicines13112728
Chicago/Turabian StyleVidal, Angela, Paula Tepasse, Vithusha Vinayahalingam, Sophie Cottagnoud, Marietta Gulz, Tanya Karrer, Gürkan Yilmaz, Janna Pape, and Michael von Wolff. 2025. "Uterine Contractility Changes in Adenomyosis: Evidence from a Systematic Review and Meta-Analysis" Biomedicines 13, no. 11: 2728. https://doi.org/10.3390/biomedicines13112728
APA StyleVidal, A., Tepasse, P., Vinayahalingam, V., Cottagnoud, S., Gulz, M., Karrer, T., Yilmaz, G., Pape, J., & von Wolff, M. (2025). Uterine Contractility Changes in Adenomyosis: Evidence from a Systematic Review and Meta-Analysis. Biomedicines, 13(11), 2728. https://doi.org/10.3390/biomedicines13112728






