Clinical Characteristics, Management, and Prognostic Factors of Appendiceal Neuroendocrine Neoplasms: Insights from a Multicenter International Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| AJCC | American Joint Committee on Cancer |
| SPSS | Statistical Package for the Social Sciences |
| HPF | High Power Field |
| OS | Overall Survival |
| RFS | Recurrence-Free Survival |
| US | Ultrasonography |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| 5-HIAA | 5-Hydroxyindoleacetic Acid |
| ENETS | European Neuroendocrine Tumor Society |
| aNEN | Appendiceal Neuroendocrine Neoplasm |
| G1 | Grade 1 |
| G2 | Grade 2 |
| G3 | Grade 3 |
| R0 | Radical Resection with Negative Margins |
References
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef]
- Kaltsas, G.; Walter, T.; Knigge, U.; Toumpanakis, C.; Santos, A.P.; Begum, N.; Pape, U.F.; Volante, M.; Frilling, A.; Couvelard, A. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for appendiceal neuroendocrine tumours (aNET). J. Neuroendocrinol. 2023, 35, e13332. [Google Scholar] [CrossRef] [PubMed]
- Rault-Petit, B.; Do Cao, C.; Guyétant, S.; Guimbaud, R.; Rohmer, V.; Julié, C.; Baudin, E.; Goichot, B.; Coriat, R.; Tabarin, A.; et al. Current Management and Predictive Factors of Lymph Node Metastasis of Appendix Neuroendocrine Tumors: A National Study from the French Group of Endocrine Tumors (GTE). Ann. Surg. 2019, 270, 165–171. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Nagesh, V.K.; Aguilar, I.K.; Elias, D.; Mansour, C.; Tran, H.H.; Bhuju, R.; Sethi, T.; Sanjeeva, P.R.P.; Rivas, M.G.; Martinez, E.; et al. Factors Affecting Survival Outcomes in Neuroendocrine Tumor of the Appendix over the Past Two Decades. Diseases 2024, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Pape, U.F.; Niederle, B.; Costa, F.; Gross, D.; Kelestimur, F.; Kianmanesh, R.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; et al. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology 2016, 103, 144–152. [Google Scholar] [CrossRef]
- Cavalcoli, F.; Castellaneta, M.; Garrahy, A.; Tamagno, G. Epidemiology of Neuroendocrine Tumours: By Site of Tumour and by Geographical Area. In Neuroendocrine Tumors in Real Life; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Priyadharshini, R.; Vishali, V.M.S.; Sonti, S. A Decade-Long Retrospective Clinicopathological Study of Appendiceal Neoplasms. Cureus 2024, 16, e70778. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, Z.; Yildiz, Y.A.; Akdeniz, Y.; Gonullu, E.; Altintoprak, F.; Mantoglu, B.; Capoglu, R.; Kahyaoglu Akkaya, Z. Appendix Neuroendocrine Tumor: Retrospective Analysis of 4026 Appendectomy Patients in a Single Center. Emerg. Med. Int. 2020, 2020, 4030527. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Ercegović, V.; Bašković, M.; Jukić, M.; Karaman, I.; Mrklić, I. Incidence and Management of Appendiceal Neuroendocrine Tumors in Pediatric Population: A Bicentric Experience with 6285 Appendectomies. Children 2023, 10, 1899. [Google Scholar] [CrossRef]
- Kourkoumelis, J.; Siag, H.; Loustalot, M.; Palmer, S.K. Incidental Appendiceal Neuroendocrine Tumor Post Appendectomy: Surgery Is Here to Stay. Cureus 2025, 17, e78700. [Google Scholar] [CrossRef]
- McCusker, M.E.; Coté, T.R.; Clegg, L.X.; Sobin, L.H. Primary malignant neoplasms of the appendix: A population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 2002, 94, 3307–3312. [Google Scholar] [CrossRef]
- Boström, L.; Jovic, V.; Dahlberg, M.; Holtenius, F.; Sandblom, G.; Järnbert-Pettersson, H. Survival among 148 patients with an incidentally detected appendiceal tumours at surgery for acute appendicitis: A population-based cohort follow-up study. Eur. J. Trauma. Emerg. Surg. 2024, 50, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.A.; Wu, V.S.; Kakish, H.; Elshami, M.; Ocuin, L.M.; Rothermel, L.D.; Mohamed, A.; Hoehn, R.S. Surgical management of 1- to 2-cm neuroendocrine tumors of the appendix: Appendectomy or right hemicolectomy? Surgery 2024, 175, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Troester, A.; Weaver, L.; Frebault, J.; Mott, S.L.; Welton, L.; Allievi, N.; Hassan, I.; Gaertner, W.; Goffredo, P. Risk of lymph node metastases and conditional survival in appendiceal neuroendocrine neoplasms. Surgery 2025, 180, 109039. [Google Scholar] [CrossRef]
- Toumpanakis, C.; Fazio, N.; Tiensuu Janson, E.; Hörsch, D.; Pascher, A.; Reed, N.; O Apos Toole, D.; Nieveen van Dijkum, E.; Partelli, S.; Rinke, A.; et al. Unmet Needs in Appendiceal Neuroendocrine Neoplasms. Neuroendocrinology 2019, 108, 37–44. [Google Scholar] [CrossRef]
- Ritter, A.S.; Poppinga, J.; Steinkraus, K.C.; Hackert, T.; Nießen, A. Novel Surgical Initiatives in Gastroenteropancreatic Neuroendocrine Tumours. Curr. Oncol. Rep. 2025, 27, 157–167. [Google Scholar] [CrossRef]
- Raikot, S.R.; Potter, D.D.; Day, C.N.; Gudmundsdottir, H.; Allen-Rhoades, W.A.; Habermann, E.B.; Polites, S.F. Appendectomy vs. Right Hemicolectomy for Pediatric Neuroendocrine Tumor of the Appendix in a National Cohort: A Call to Further Decrease Colectomy. J. Am. Coll. Surg. 2025, 241, 620–625. [Google Scholar] [CrossRef]
- Nesti, C.; Bräutigam, K.; Benavent, M.; Bernal, L.; Boharoon, H.; Botling, J.; Bouroumeau, A.; Brcic, I.; Brunner, M.; Cadiot, G.; et al. Hemicolectomy versus appendectomy for patients with appendiceal neuroendocrine tumours 1–2 cm in size: A retrospective, Europe-wide, pooled cohort study. Lancet Oncol. 2023, 24, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Soltani, H.; Ahmadinejad, M.; Shafiee, A.; Afshar Rezaee, F.; Beik Mohamadi, M.; Bahrambeigi, A.; Hajialigol, A.H.; Fattan, S.; Zebarjadi Bagherpour, J. Expression rate and comparison of immunohistochemistry biomarkers in appendiceal neuroendocrine and other epithelial cell neoplasms: Systematic review and meta-analysis. Rare Tumors 2025, 17, 20363613251330179. [Google Scholar] [CrossRef]
- Murray, S.E.; Lloyd, R.V.; Sippel, R.S.; Chen, H.; Oltmann, S.C. Postoperative surveillance of small appendiceal carcinoid tumors. Am. J. Surg. 2014, 207, 342–345, discussion 345. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Grozinsky-Glasberg, S.; Chatzellis, E.; Grossman, A.B. Appendiceal neuroendocrine neoplasms: Diagnosis and management. Endocr. Relat. Cancer 2016, 23, R27–R41. [Google Scholar] [CrossRef]
- Watt, I.; Henry, L.; Gandhi, J.; Woodhouse, B.; Lawrence, B. Appendiceal neuroendocrine tumours, when to operate? Color. Dis. 2025, 27, e70239. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; American Joint Committee on Cancer. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- de Lambert, G.; Lardy, H.; Martelli, H.; Orbach, D.; Gauthier, F.; Guérin, F. Surgical Management of Neuroendocrine Tumors of the Appendix in Children and Adolescents: A Retrospective French Multicenter Study of 114 Cases. Pediatr. Blood Cancer 2016, 63, 598–603. [Google Scholar] [CrossRef]
- Henderson, L.; Fehily, C.; Folaranmi, S.; Kelsey, A.; McPartland, J.; Jawaid, W.B.; Craigie, R.; Losty, P.D. Management and outcome of neuroendocrine tumours of the appendix-a two centre UK experience. J. Pediatr. Surg. 2014, 49, 1513–1517. [Google Scholar] [CrossRef]
- Jensen, S.L.T.; Giovannoni, S.; Ankerstjerne, M.P.; Christensen, L.G.; Pedersen, A.K.; Möller, S.; Holmager, P.; Knigge, U.; Rathe, M.; Ellebaek, M.B. Surgical management of paediatric appendiceal neuroendocrine tumors: A 26-year Danish nationwide retrospective cohort study. J. Pediatr. Surg. 2025, 60, 162506. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Kunstreich, M.; Pape, U.F.; Seitz, G.; Lessel, L.; Vokuhl, C.; Frühwald, M.C.; Vorwerk, P.; Redlich, A. Lymph node metastases are more frequent in paediatric appendiceal NET ≥1.5 cm but without impact on outcome—Data from the German MET studies. Eur. J. Surg. Oncol. 2024, 50, 108051. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Partelli, S.; Campana, D.; Rinzivillo, M.; Alberici, L.; Andrini, E.; Menin, S.; D’Ambra, V.; Battistella, A.; Andreasi, V.; et al. Safety and cost-effectiveness of immediate right hemicolectomy versus active surveillance for well-differentiated appendiceal neuroendocrine tumors 1–2 cm in size: A Markov decision analysis. Endocrine 2025, 90, 347–355. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C.; et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef]
- Washington, M.K.; Tang, L.H.; Berlin, J.; Branton, P.A.; Burgart, L.J.; Carter, D.K.; Compton, C.C.; Fitzgibbons, P.L.; Frankel, W.L.; Jessup, J.M.; et al. Protocol for the examination of specimens from patients with neuroendocrine tumors (carcinoid tumors) of the small intestine and ampulla. Arch. Pathol. Lab. Med. 2010, 134, 181–186. [Google Scholar] [CrossRef]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef]
- Volante, M.; Daniele, L.; Asioli, S.; Cassoni, P.; Comino, A.; Coverlizza, S.; De Giuli, P.; Fava, C.; Manini, C.; Berruti, A.; et al. Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: A retrospective clinical pathologic analysis of 138 cases. Am. J. Surg. Pathol. 2013, 37, 606–612. [Google Scholar] [CrossRef]
- Brighi, N.; La Rosa, S.; Rossi, G.; Grillo, F.; Pusceddu, S.; Rinzivillo, M.; Spada, F.; Tafuto, S.; Massironi, S.; Faggiano, A.; et al. Morphological Factors Related to Nodal Metastases in Neuroendocrine Tumors of the Appendix: A Multicentric Retrospective Study. Ann. Surg. 2020, 271, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Patil, S.; Roomi, S.; Shiwani, M.H. Appendicular Neuroendocrine Neoplasm is Associated with Acute Appendicitis—Don’t Miss the Boat. Chirurgia 2019, 114, 461–466. [Google Scholar] [CrossRef]
- AlAli, M.N.; AlWadani, S.T.; Amer, S.M.; Essa, M.S.; AlAmodi, M.; AlHassan, H.M.; Alrasheed, A.F.; Alsulaiman, A.A.; Aldeghaither, S.K. Appendiceal neuroendocrine tumors: A case series and literature review. J. Surg. Case Rep. 2025, 2025, rjaf237. [Google Scholar] [CrossRef]
- Alabraba, E.; Pritchard, D.M.; Griffin, R.; Diaz-Nieto, R.; Banks, M.; Cuthbertson, D.J.; Fenwick, S. The impact of lymph node metastases and right hemicolectomy on outcomes in appendiceal neuroendocrine tumours (aNETs). Eur. J. Surg. Oncol. 2021, 47, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Galanopoulos, M.; McFadyen, R.; Drami, I.; Naik, R.; Evans, N.; Luong, T.V.; Watkins, J.; Caplin, M.; Toumpanakis, C. Challenging the Current Risk Factors of Appendiceal Neuroendocrine Neoplasms: Can They Accurately Predict Local Lymph Nodal Invasion? Results from a Large Case Series. Neuroendocrinology 2019, 109, 179–186. [Google Scholar] [CrossRef]
- Holmager, P.; Willemoe, G.L.; Nielsen, K.; Grøndahl, V.; Klose, M.; Andreassen, M.; Langer, S.W.; Hansen, C.P.; Kjær, A.; Federspiel, B.H.; et al. Neuroendocrine neoplasms of the appendix: Characterization of 335 patients referred to the Copenhagen NET Center of Excellence. Eur. J. Surg. Oncol. 2021, 47, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Kaltsas, G.; Grozinsky-Glasberg, S.; Oleinikov, K.; Kos-Kudła, B.; Kogut, A.; Srirajaskanthan, R.; Pizanias, M.; Poulia, K.A.; Ferreira, C.; et al. The effect of prophylactic surgery in survival and HRQoL in appendiceal NEN. Endocrine 2020, 70, 178–186. [Google Scholar] [CrossRef]
- Pawa, N.; Clift, A.K.; Osmani, H.; Drymousis, P.; Cichocki, A.; Flora, R.; Goldin, R.; Patsouras, D.; Baird, A.; Malczewska, A.; et al. Surgical Management of Patients with Neuroendocrine Neoplasms of the Appendix: Appendectomy or More. Neuroendocrinology 2018, 106, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Canbak, T.; Erdem, O.; Acar, A.; Başak, F. Evaluating the necessity of right hemicolectomy in high-risk appendiceal neuroendocrine tumors: A retrospective analysis. BMC Surg. 2025, 25, 320. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Haider, M.; Pelle, E.; Maratta, M.G.; Strosberg, J. Do Appendiceal Neuroendocrine Tumors Metastasize Post Appendectomy or Right Hemicolectomy? J. Natl. Compr. Cancer Netw. 2024, 23, e247069. [Google Scholar] [CrossRef]
- Moris, D.; Tsilimigras, D.I.; Vagios, S.; Ntanasis-Stathopoulos, I.; Karachaliou, G.S.; Papalampros, A.; Alexandrou, A.; Blazer, D.G.; Felekouras, E. Neuroendocrine Neoplasms of the Appendix: A Review of the Literature. Anticancer. Res. 2018, 38, 601–611. [Google Scholar] [CrossRef]
- Morais, C.; Silva, E.; Brandão, P.N.; Correia, R.; Foreid, S.; Valente, V. Neuroendocrine tumor of the appendix-a case report and review of the literature. J. Surg. Case Rep. 2019, 2019, rjz086. [Google Scholar] [CrossRef]
- Tamagno, G.; Bennett, A.; Ivanovski, I. Lights and darks of neuroendocrine tumors of the appendix. Minerva Endocrinol. 2020, 45, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, G.; Sheahan, K.; Skehan, S.J.; Geoghegan, J.G.; Fennelly, D.; Collins, C.D.; Maguire, D.; Traynor, O.; Brophy, D.P.; Cantwell, C.; et al. Initial impact of a systematic multidisciplinary approach on the management of patients with gastroenteropancreatic neuroendocrine tumor. Endocrine 2013, 44, 504–509. [Google Scholar] [CrossRef] [PubMed]
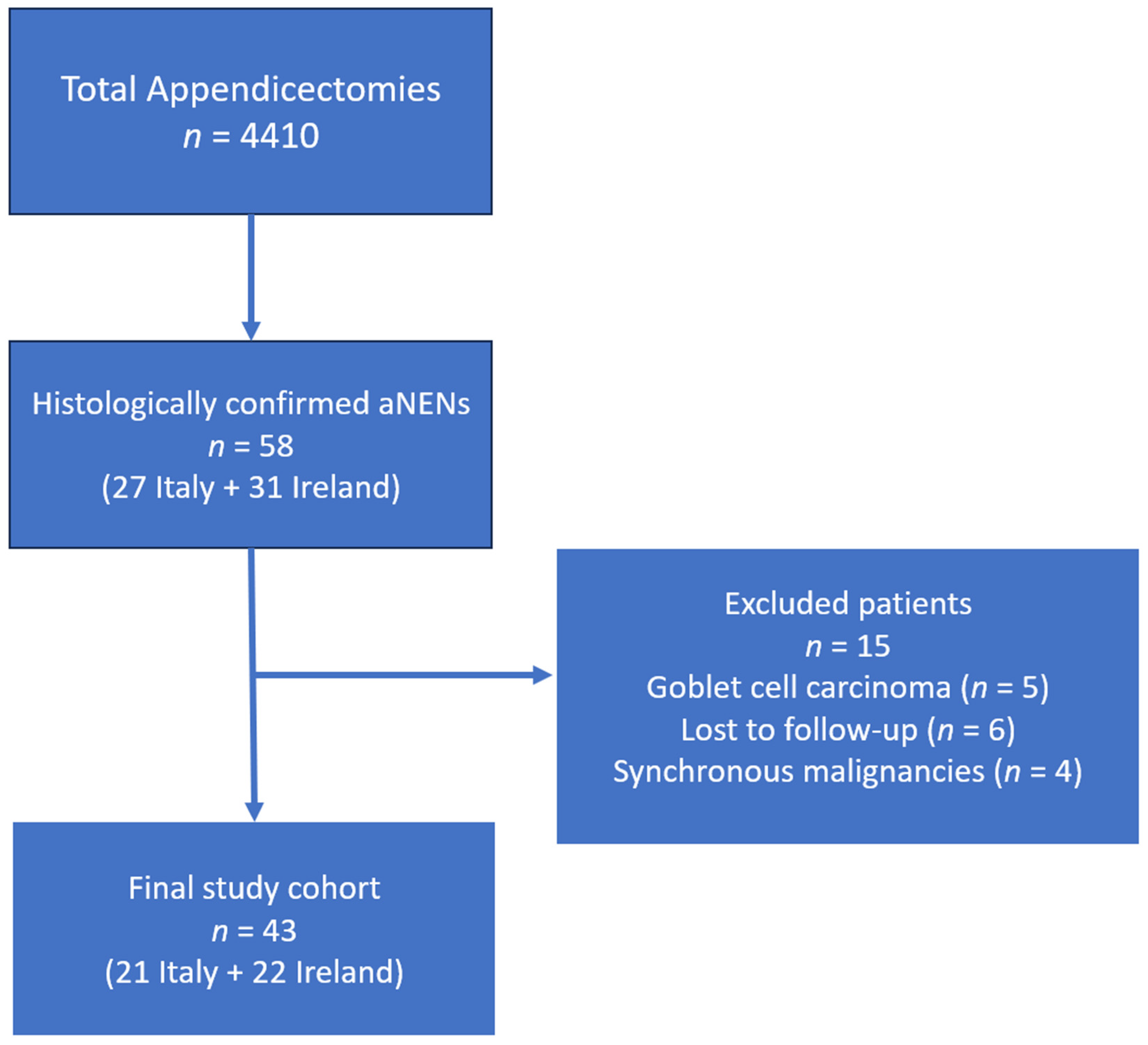
| Ireland (n = 22) | Italy (n = 21) | |
|---|---|---|
| Median age (range) | 28 years (14–63 years) | 31 years (9–75 years) |
| Female/Male | 12/10 | 13/8 |
| Median tumor size (range) | 10 mm (3–25 mm) | 5 mm (0.6–40 mm) |
| aNEN stage [23] I II III | 12 9 1 | 14 5 2 |
| aNEN grade G1 G2 G3 | 13 3 0 | 12 1 0 |
| Tumor location | Apex: 9 (40.9%) Base: 2 (9.1%) Not specified: 11 (50.0%) | Apex: 12 (57.1%) Base: 1 (4.8%) Not specified: 8 (38.1%) |
| Mesoappendiceal invasion | Present: 7 (31.8%) Absent: 8 (36.4%) Not reported: 7 (31.8%) | Present: 4 (19.0%) Absent: 11 (52.4%) Not reported: 6 (28.6%) |
| Initial surgery | Appendectomy: 20 (90.9%) Ileocecal resection: 1 (4.5%) Right hemicolectomy: 1 (4.5%) | Appendectomy: 19 (90.5%) Total colectomy: 1 (4.8%) Right hemicolectomy: 1 (4.8%) |
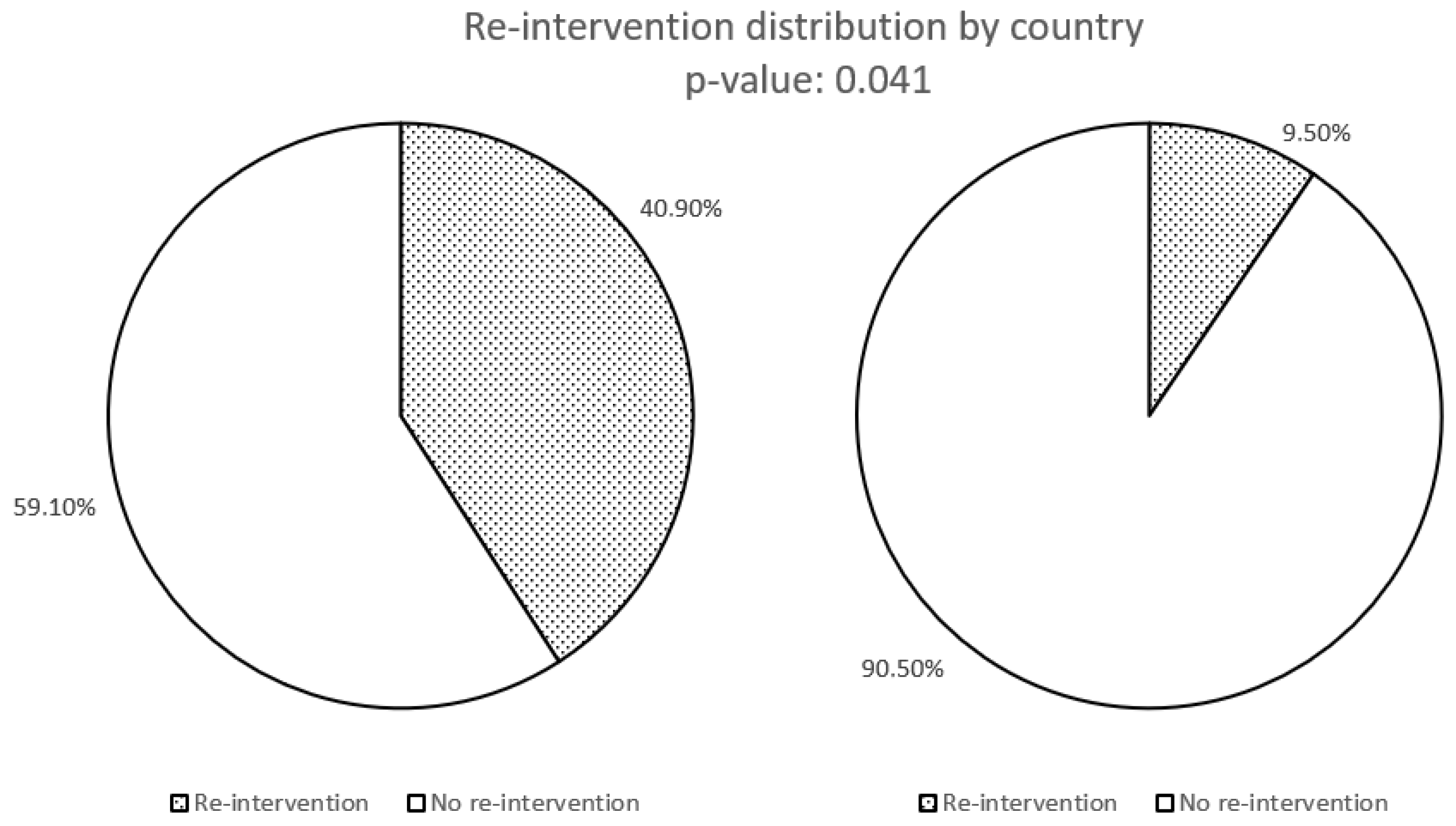
| Re-intervention with right hemicolectomy | 9 * | 2 * |
| Ireland (n = 22) | Italy (n = 21) | |
|---|---|---|
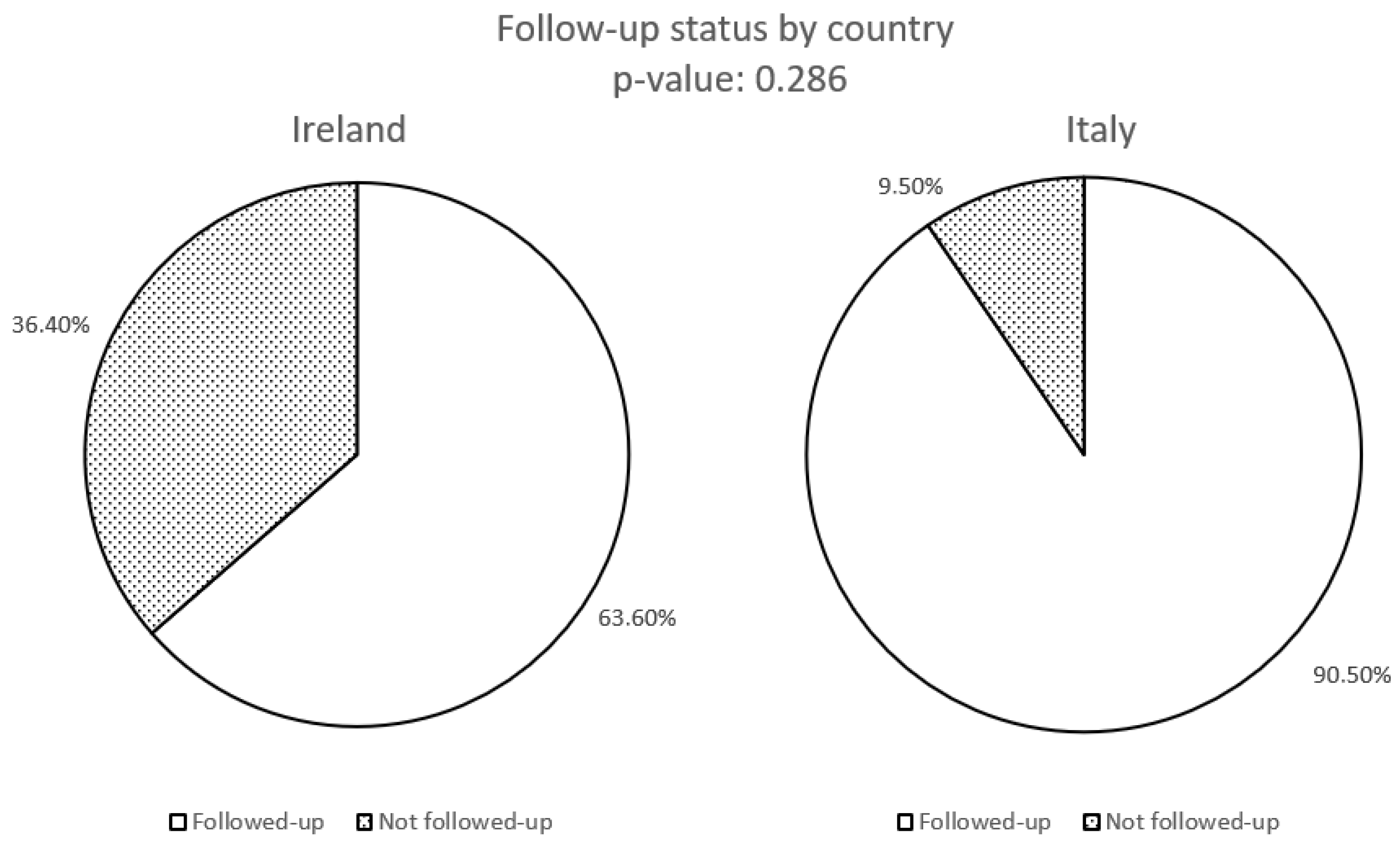
| Followed-up patients N (%) | 14 (63.6%) | 19 (90.5%) |
| Duration of the follow-up months (range) | 8 (6–60 months) | 24 (6–140 months) |
| Outpatient clinics visit N (%) | 13 (59.1%) | 18 (85.7%) |
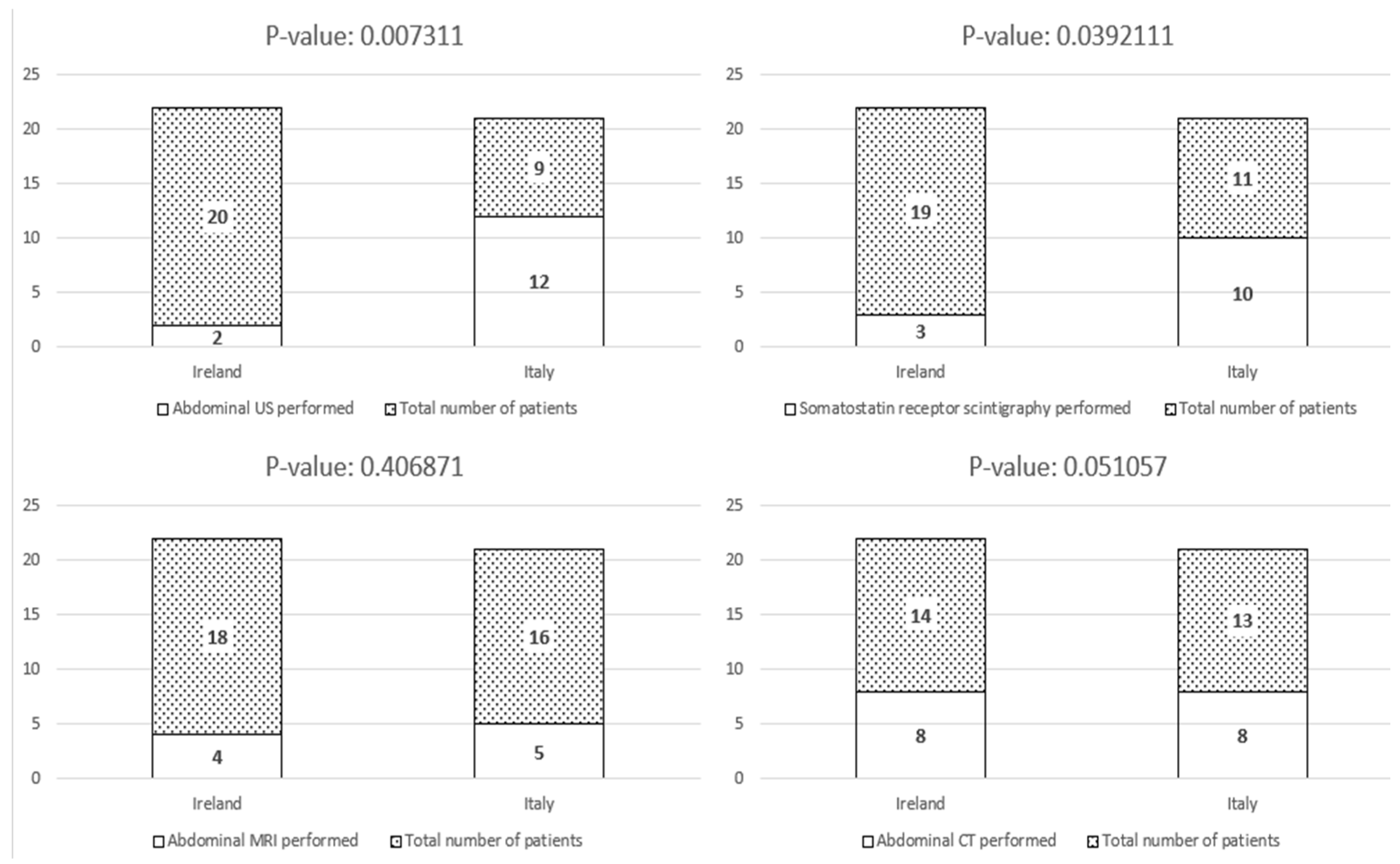
| Abdominal US N (%) | 2 (9.1%) * | 12 (57.1%) * |
| Abdominal CT N (%) | 8 (36.4%) | 8 (38.1%) |
| Abdominal MRI N (%) | 4 (18.2%) | 5 (23.8%) |
| Somatostatin receptor scintigraphy N (%) | 3 (13.6%) * | 10 (47.6%) * |
| 68Gallium PET/CT N (%) | 0 * | 2 (9.5%) * |
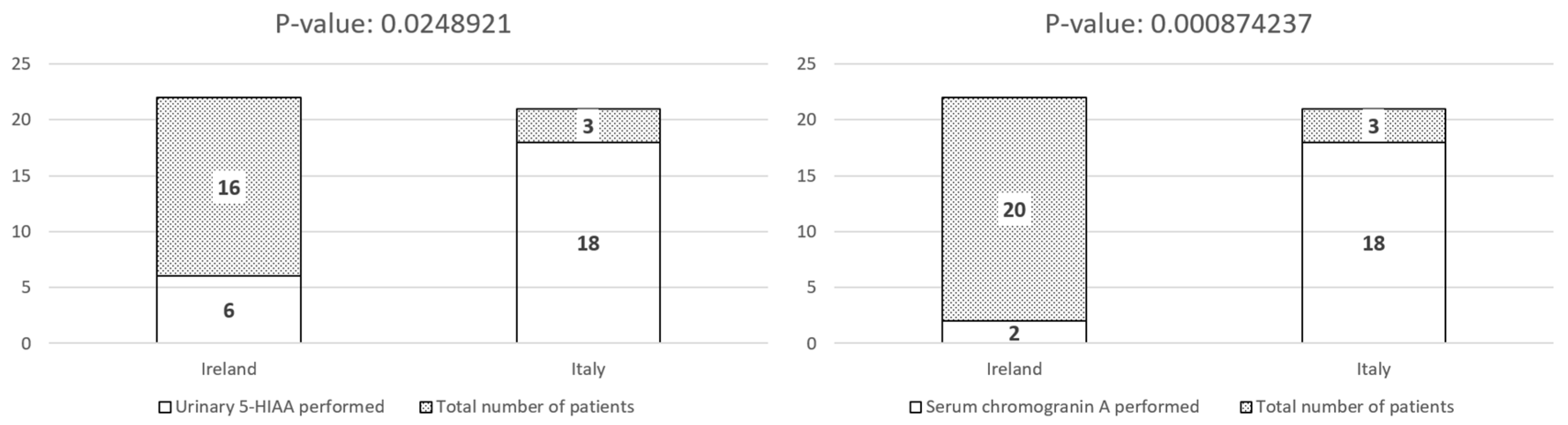
| Urinary 5-HIAA N (%) | 6 (27.3%) * | 18 (85.7%) * |
| Serum chromogranin A N (%) | 2 (9.1%) * | 18 (85.7%) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcoli, F.; Samarasinghe, K.; Del Gobbo, A.; Mulligan, N.; Rausa, E.; Caimo, A.; Cantù, P.; Tamagno, G.; Massironi, S. Clinical Characteristics, Management, and Prognostic Factors of Appendiceal Neuroendocrine Neoplasms: Insights from a Multicenter International Study. Biomedicines 2025, 13, 2724. https://doi.org/10.3390/biomedicines13112724
Cavalcoli F, Samarasinghe K, Del Gobbo A, Mulligan N, Rausa E, Caimo A, Cantù P, Tamagno G, Massironi S. Clinical Characteristics, Management, and Prognostic Factors of Appendiceal Neuroendocrine Neoplasms: Insights from a Multicenter International Study. Biomedicines. 2025; 13(11):2724. https://doi.org/10.3390/biomedicines13112724
Chicago/Turabian StyleCavalcoli, Federica, Kasun Samarasinghe, Alessandro Del Gobbo, Niall Mulligan, Emanuele Rausa, Alberto Caimo, Paolo Cantù, Gianluca Tamagno, and Sara Massironi. 2025. "Clinical Characteristics, Management, and Prognostic Factors of Appendiceal Neuroendocrine Neoplasms: Insights from a Multicenter International Study" Biomedicines 13, no. 11: 2724. https://doi.org/10.3390/biomedicines13112724
APA StyleCavalcoli, F., Samarasinghe, K., Del Gobbo, A., Mulligan, N., Rausa, E., Caimo, A., Cantù, P., Tamagno, G., & Massironi, S. (2025). Clinical Characteristics, Management, and Prognostic Factors of Appendiceal Neuroendocrine Neoplasms: Insights from a Multicenter International Study. Biomedicines, 13(11), 2724. https://doi.org/10.3390/biomedicines13112724






