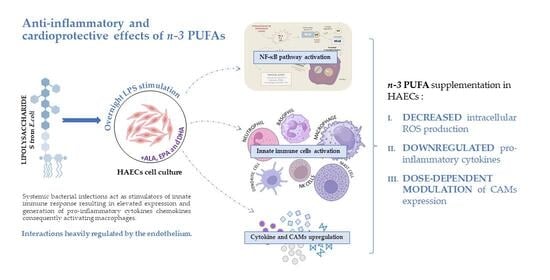
Polyunsaturated Fatty Acid (PUFA) Composition of Growth Medium Changes the Atherogenic Potential of Human Aortic Endothelial Cells (HAECs) Following Endotoxin Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. HAECs: Cell Culture Treatment
- Basal cell medium group (control);
- ALA-supplemented cell medium group (ALA);
- EPA-supplemented cell medium group (EPA);
- DHA-supplemented cell medium group (DHA);
- ALA/EPA/DHA-supplemented cell medium group (3PUFA).
- (a)
- No stimulation—baseline conditions;
- (b)
- Stimulation with 0.75 µg/mL of LPS (LPS75)—moderate inflammatory conditions;
- (c)
- Stimulation with 1 µg/mL of LPS (LPS100)—high inflammatory conditions.
2.3. Flow Cytometry
2.3.1. Intracellular ROS Production
2.3.2. CAM Expression
2.4. Luminex Assay
2.5. ELISA Assay
2.6. Statistical Analysis
3. Results
3.1. Flow Cytometry
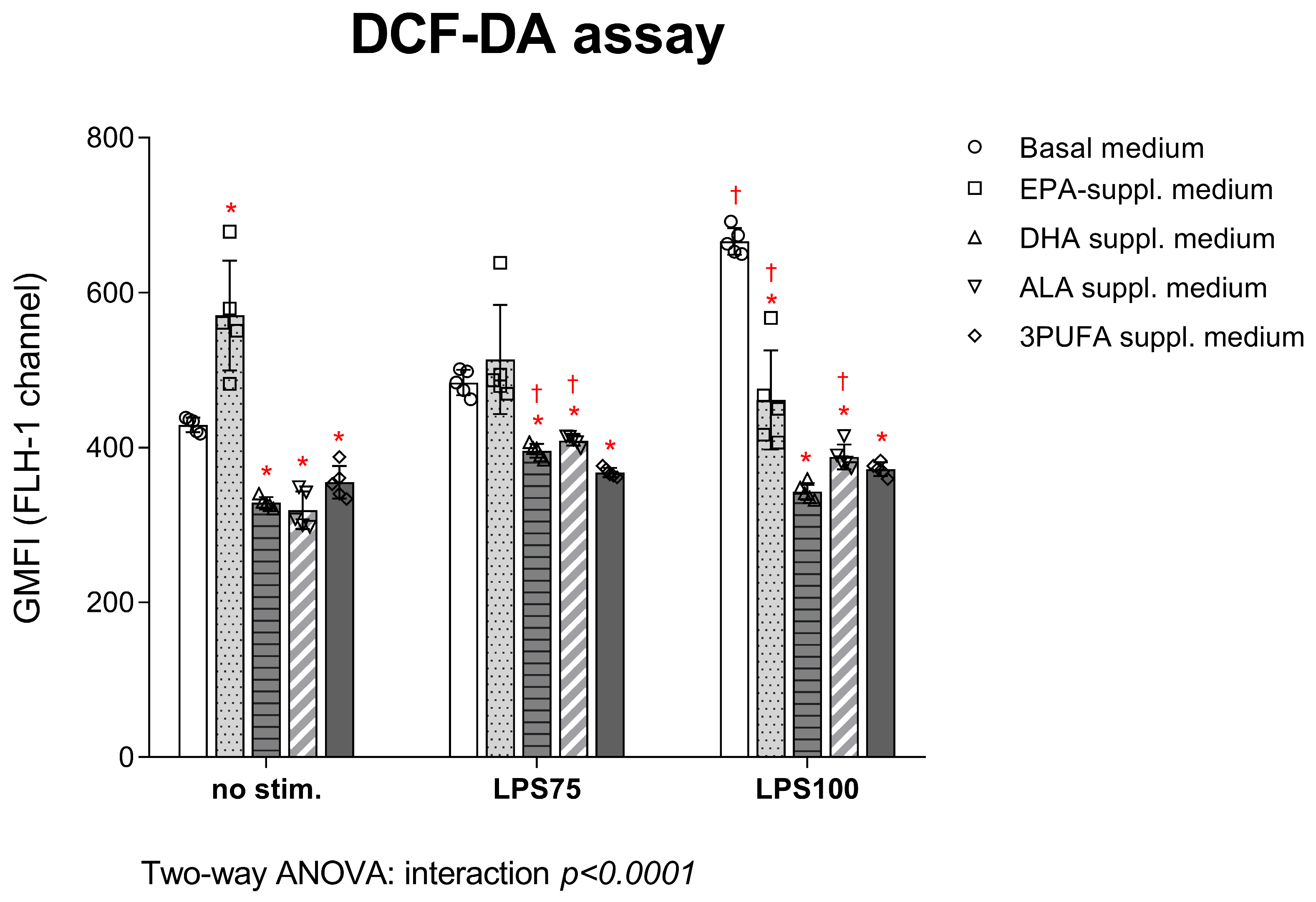
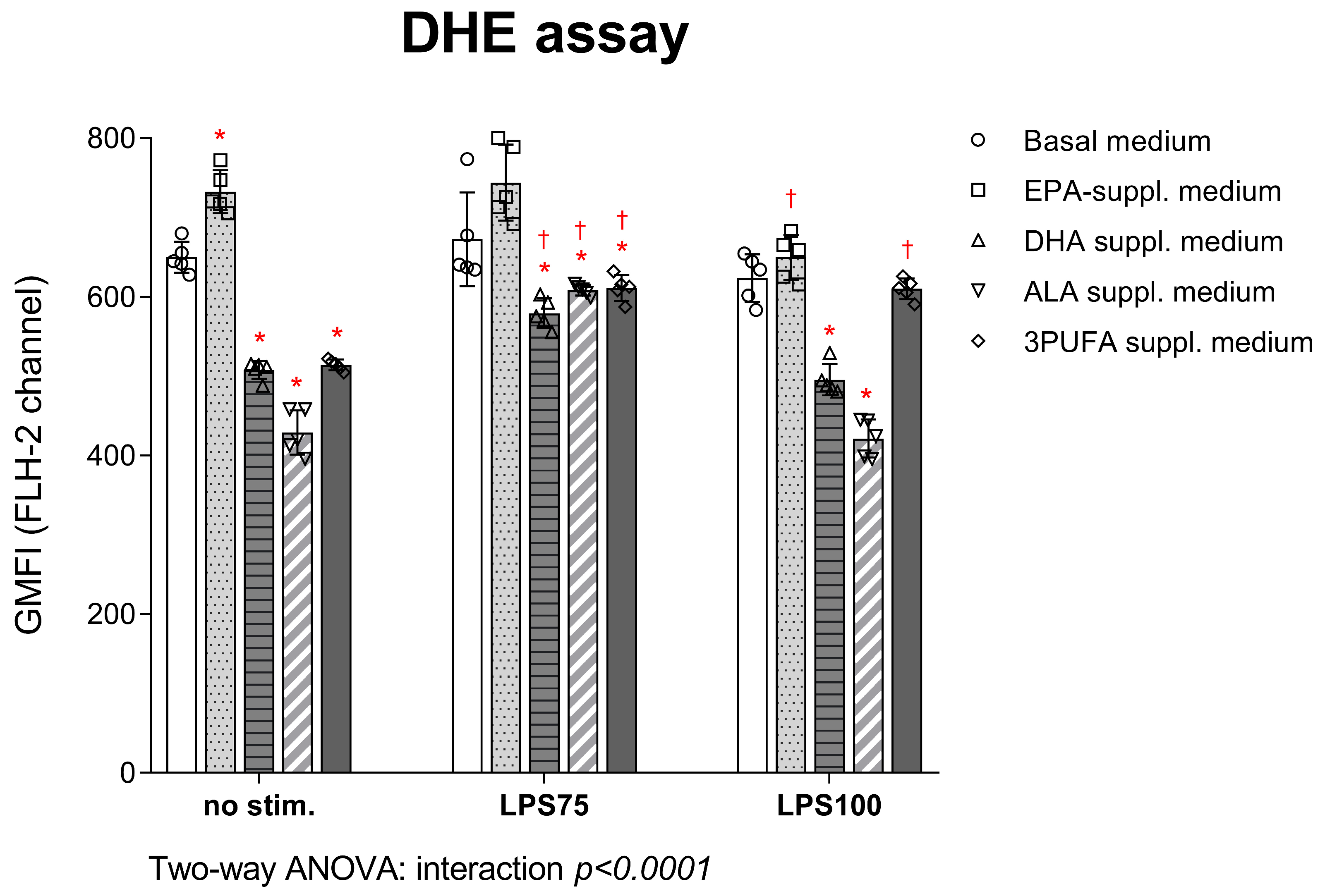
3.1.1. Intracellular ROS Production in HAECs: DCF-DA and DHE Assay
3.1.2. Expression of CAMs in HAECs
3.2. Endotoxin-Induced Cytokine Concentrations Following n-3 PUFA Treatment
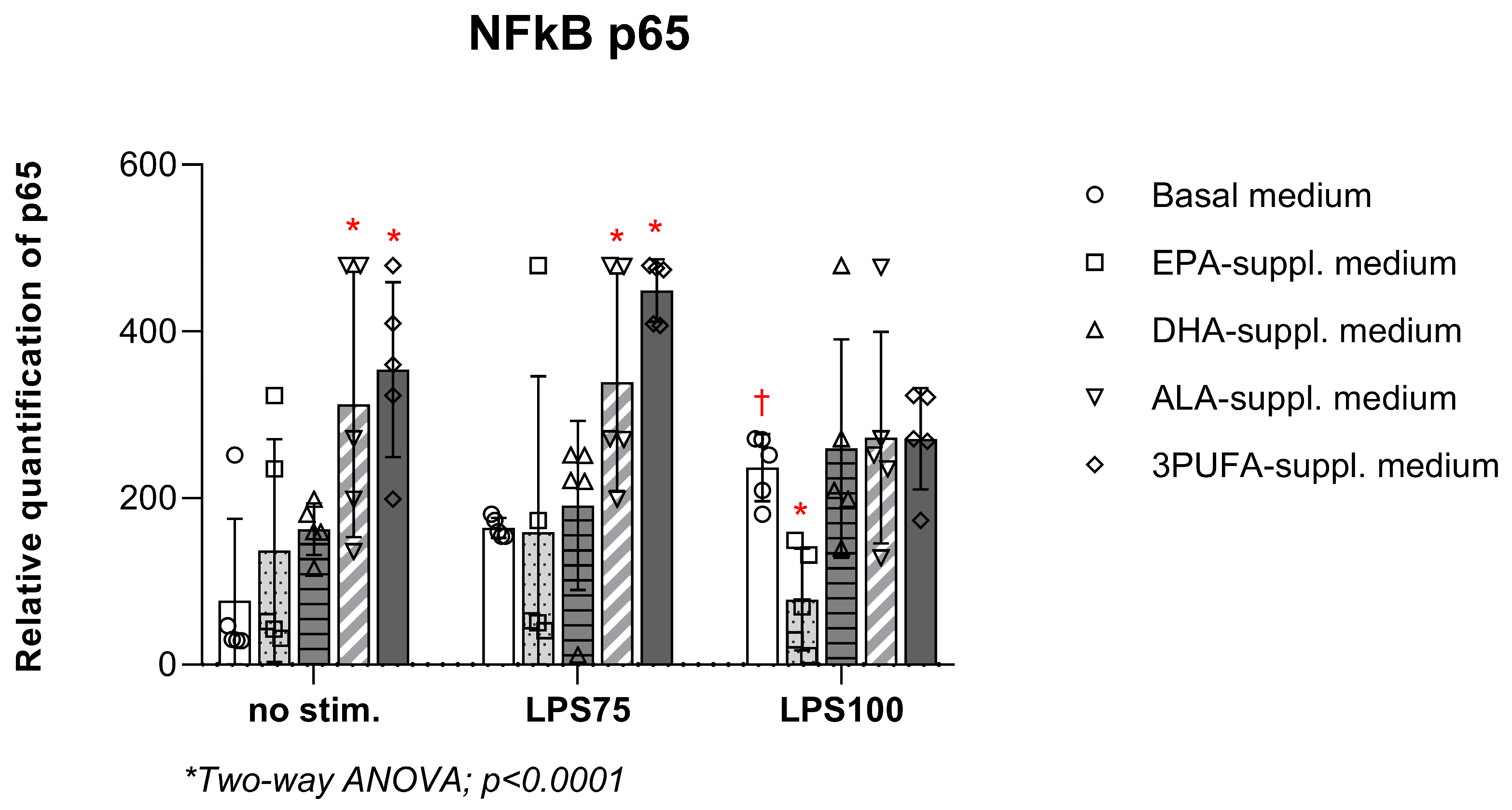
3.3. Semi-Quantitative Measurement of NF-κB p65 in HAEC Lysates Following n-3 PUFA Treatment and Endotoxin Stimulation
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| Ach | acetyl choline |
| ALA | alpha- linolenic acid |
| AMPK | AMP-activated protein kinase |
| ANOVA | analysis of variance |
| CAM | cell adhesion molecule |
| CRP | C reactive protein |
| CVD | cardiovascular disease |
| DCF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DHA | docosahexaenoic acid |
| DHE | dihydroethidium |
| EC | endothelial cell |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| eNOS | endothelial nitric oxide synthase |
| EPA | eicosapentaenoic acid |
| FA | fatty acid |
| FBS | fetal bovine serum |
| FVD | fixable viability dye |
| FMD | flow-mediated dilation |
| GMFI | geometric mean fluorescence intensity |
| H2O2 | hydrogen peroxide |
| HAEC | human aortic endothelial cell |
| HDL | high-density lipoprotein |
| HK-2 | human kidney-2 cells |
| HFD-STZ | high-fat diet streptozocin rats |
| HUVEC | human umbilical vein endothelial cells |
| ICAM-1 | intercellular cell adhesion molecule 1 |
| IFNγ | interferon gamma |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LA | linoleic acid |
| LDL | low-density lipoprotein |
| LPS | lipopolysaccharides |
| LSGS | low serum growth supplement |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| NOS2 | nitric oxide synthase 2 |
| NOX | nicotinamide adenine dinucleotide phosphate oxidase |
| NFκB | nuclear factor kappa B |
| O2− | superoxide anion |
| OD | optical density |
| ONOO− | peroxynitrite |
| PBS | phosphate-buffered saline |
| PPAR | peroxisome proliferator-activated receptor |
| PUFA | polyunsaturated fatty acid |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| ROS | reactive oxygen species |
| SHR | spontaneously hypertensive rat |
| SOD2 | superoxide dismutase 2 |
| sVCAM | soluble vascular cell adhesion molecule |
| TGFβ | transforming growth factor beta |
| TLR4 | toll-like receptor 4 |
| TNFα | tumor necrosis factor alpha |
| UCP3 | uncoupling protein 3 |
| VEGF | vascular endothelial growth factor |
References
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar]
- Mori, T.A.; Beilin, L.J. Long-chain omega 3 fatty acids, blood lipids and cardiovascular risk reduction. Curr. Opin. Lipidol. 2001, 12, 11. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits–A review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. n-3 PUFA Sources (Precursor/Products): A Review of Current Knowledge on Rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; Telis, N.; German, J.B.; Hammock, B.D. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif. Agric. 2011, 65, 106–111. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. International Society for the Study of Fatty Acids and Lipids, ISSFAL alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yang, H.; Zhang, P.; Wu, F.; Li, Y.; Zhou, Y.; Zhang, X.; Ma, H.; Zhang, W.; et al. α-Linolenic acid but not linolenic acid protects against hypertension: Critical role of SIRT3 and autophagic flux. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fu, F.; Tie, R.; Liang, X.; Tian, F.; Xing, W.; Li, J.; Ji, L.; Xing, J.; Sun, X.; et al. Alpha-linolenic acid intake prevents endothelial dysfunction in high-fat diet-fed streptozotocin rats and underlying mechanisms. Vasa 2013, 42, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; Li, J.; Wang, W.; Tie, R.; Tian, F.; Liang, X.; Xing, W.; He, Y.; Yu, L.; et al. Alpha-Linolenic Acid Exerts an Endothelial Protective Effect against High Glucose Injury via PI3K/Akt Pathway. PLoS ONE 2013, 8, e68489. [Google Scholar] [CrossRef]
- Bork, C.S.; Baker, E.J.; Lundbye-Christensen, S.; Miles, E.A.; Calder, P.C. Lowering the linoleic acid to alpha-linoleic acid ratio decreases the production of inflammatory mediators by cultured human endothelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2019, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Dod, H.; Sandhu, M.S.; Bedi, R.; Dod, S.; Konat, G.; Chopra, H.K.; Sharma, R.; Jain, A.C.; Nanda, N. Acute effects of diets rich in almonds and walnuts on endothelial function. Indian. Heart J. 2018, 70, 497–501. [Google Scholar] [CrossRef]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, M.; Vessby, B.; Millgård, J.; Lind, L. Endothelium-dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis 2001, 156, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Steer, P.; Vessby, B.; Lind, L. Endothelial vasodilatory function is related to the proportions of saturated fatty acids and alpha-linolenic acid in young men, but not in women. Eur. J. Clin. Investig. 2003, 33, 390–396. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-γ–dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, I.-H.; Kim, Y. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Uncoupling Protein 3 Gene Expression in C2C12 Muscle Cells. Nutrients 2013, 5, 1660–1671. [Google Scholar] [CrossRef]
- Yamagata, K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017, 16, 118. [Google Scholar] [CrossRef]
- Véricel, E.; Colas, R.; Calzada, C.; Lê, Q.H.; Feugier, N.; Cugnet, C.; Vidal, H.; Laville, M.; Moulin, P.; Lagarde, M. Moderate oral supplementation with docosahexaenoic acid improves platelet function and oxidative stress in type 2 diabetic patients. Thromb. Haemost. 2015, 114, 289–296. [Google Scholar] [CrossRef]
- Peña-de-la-Sancha, P.; Muñoz-García, A.; Espínola-Zavaleta, N.; Bautista-Pérez, R.; Mejía, A.M.; Luna-Luna, M.; López-Olmos, V.; Rodríguez-Pérez, J.-M.; Fragoso, J.-M.; Carreón-Torres, E.; et al. Eicosapentaenoic and Docosahexaenoic Acid Supplementation Increases HDL Content in n-3 Fatty Acids and Improves Endothelial Function in Hypertriglyceridemic Patients. Int. J. Mol. Sci. 2023, 24, 5390. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Kolobarić, N.; Drenjančević, I.; Matić, A.; Šušnjara, P.; Mihaljević, Z.; Mihalj, M. Dietary Intake of n-3 PUFA-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults—A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Stupin, A.; Mihalj, M.; Kolobarić, N.; Šušnjara, P.; Kolar, L.; Mihaljević, Z.; Matić, A.; Stupin, M.; Jukić, I.; Kralik, Z.; et al. Anti-Inflammatory Potential of n-3 Polyunsaturated Fatty Acids Enriched Hen Eggs Consumption in Improving Microvascular Endothelial Function of Healthy Individuals—Clinical Trial. Int. J. Mol. Sci. 2020, 21, 4149. [Google Scholar] [CrossRef]
- Ćurić, Ž.B.; Masle, A.M.; Kibel, A.; Selthofer-Relatić, K.; Stupin, A.; Mihaljević, Z.; Jukić, I.; Stupin, M.; Matić, A.; Kozina, N.; et al. Effects of n-3 Polyunsaturated Fatty Acid-Enriched Hen Egg Consumption on the Inflammatory Biomarkers and Microvascular Function in Patients with Acute and Chronic Coronary Syndrome—A Randomized Study. Biology 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Kolar, L.; Stupin, M.; Stupin, A.; Šušnjara, P.; Mihaljević, Z.; Matić, A.; Jukić, I.; Kolobarić, N.; Drenjančević, I. Does the Endothelium of Competitive Athletes Benefit from Consumption of n-3 Polyunsaturated Fatty Acid-Enriched Hen Eggs? Prev. Nutr. Food Sci. 2021, 26, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Gaine, S.P.; Michos, E.D. Eicosapentaenoic acid vs. docosahexaenoic acid for the prevention of cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 87–93. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Huo, S.; Chen, P. Unraveling the discrepancies between REDUCE-IT and STRENGTH trials with omega-3 fatty acids: New analytical approaches. Front. Nutr. 2024, 11, 1490953. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Menden, H.; Tate, E.; Hogg, N.; Sampath, V. LPS-mediated endothelial activation in pulmonary endothelial cells: Role of Nox2-dependent IKK-β phosphorylation. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L445–L455. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, J.; Zhou, D.; Wang, J. Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: A new predictive and therapeutic paradigm for sepsis. Eur. J. Med. Res. 2023, 28, 339. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identifications. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Dauphinee, S.M.; Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab. Investig. 2006, 86, 9–22. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chen, L.-Y.; Papadimos, T.J.; Huang, S.; Zuraw, B.L.; Pan, Z.K. Lipopolysaccharide-driven Th2 Cytokine Production in Macrophages Is Regulated by Both MyD88 and TRAM. J. Biol. Chem. 2009, 284, 29391–29398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Chaiwut, R.; Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef]
- Sul, O.-J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Li, M.-Y.; Gao, C.-S.; Du, X.-Y.; Zhao, L.; Niu, X.-T.; Wang, G.-Q.; Zhang, D.-M. Amelioration of LPS-induced inflammatory response and oxidative stress by astaxanthin in Channa argus lymphocyte via activating glucocorticoid receptor signalling pathways. Aquac. Res. 2020, 51, 2687–2697. [Google Scholar] [CrossRef]
- Hsueh, T.-Y.; Baum, J.I.; Huang, Y. Effect of Eicosapentaenoic Acid and Docosahexaenoic Acid on Myogenesis and Mitochondrial Biosynthesis during Murine Skeletal Muscle Cell Differentiation. Front. Nutr. 2018, 5, 15. [Google Scholar] [CrossRef]
- Kwon, M.J.; Jung, H.S.; Kang, S.M.; Lee, S.H.; Park, J.H. The Protective Effects of Eicosapentaenoic Acid for Stress-induced Accelerated Senescence in Vascular Endothelial Cells. Int. J. Med. Sci. 2023, 20, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Park, G.; McAllister, H.; Leahey, W.; Devine, A.; McVeigh, G.E.; Gardiner, T.A.; McDonald, D.M. Docosahexaenoic Acid Improves the Nitroso-Redox Balance and Reduces VEGF-Mediated Angiogenic Signaling in Microvascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6815–6825. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Fan, J.; Liu, C.; Xie, W.; Kong, H. DPP-4 exacerbates LPS-induced endothelial cells inflammation via integrin-α5β1/FAK/AKT signaling. Exp. Cell Res. 2024, 435, 113909. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Nanchal, R.; Husnain, F.; Audi, S.; Konduri, G.G.; Densmore, J.C.; Medhora, M.; Jacobs, E.R. Hypoxia preconditioning increases survival and decreases expression of Toll-like receptor 4 in pulmonary artery endothelial cells exposed to lipopolysaccharide. Pulm. Circ. 2013, 3, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kolobarić, N.; Kozina, N.; Mihaljević, Z.; Drenjančević, I. Angiotensin II Exposure In Vitro Reduces High Salt-Induced Reactive Oxygen Species Production and Modulates Cell Adhesion Molecules’ Expression in Human Aortic Endothelial Cell Line. Biomedicines 2024, 12, 2741. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Komatsu, W.; Ishihara, K.; Murata, M.; Saito, H.; Shinohara, K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 2003, 34, 1006–1016. [Google Scholar] [CrossRef]
- Zúñiga, J.; Cancino, M.; Medina, F.; Varela, P.; Vargas, R.; Tapia, G.; Videla, L.A.; Fernández, V. N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: Anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS ONE 2011, 6, e28502. [Google Scholar] [CrossRef]
- Mihalj, M.; Stupin, A.; Kolobarić, N.; Bujak, I.T.; Matić, A.; Kralik, Z.; Jukić, I.; Stupin, M.; Drenjančević, I. Leukocyte Activation and Antioxidative Defense Are Interrelated and Moderately Modified by n-3 Polyunsaturated Fatty Acid-Enriched Eggs Consumption-Double-Blind Controlled Randomized Clinical Study. Nutrients 2020, 12, 3122. [Google Scholar] [CrossRef] [PubMed]
- Nađ, T.; Kolobarić, N.; Mihaljević, Z.; Drenjančević, I.; Šušnjara, P.; Stupin, A.; Kardum, D.; Kralik, Z.; Kralik, G.; Košević, M.; et al. Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study. Antioxidants 2025, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Kolar, L.; Šušnjara, P.; Stupin, M.; Stupin, A.; Jukić, I.; Mihaljević, Z.; Kolobarić, N.; Bebek, I.; Nejašmić, D.; Lovrić, M.; et al. Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants-Randomized Clinical Trial. Life 2023, 13, 2140. [Google Scholar] [CrossRef] [PubMed]
- Šušnjara, P.; Kolobarić, N.; Matić, A.; Mihaljević, Z.; Stupin, A.; Marczi, S.; Drenjančević, I. Consumption of Hen Eggs Enriched with n-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E and Lutein Incites Anti-Inflammatory Conditions in Young, Healthy Participants—A Randomized Study. Front. Biosci. 2022, 27, 332. [Google Scholar] [CrossRef]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Vanden Heuvel, J.P.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005, 336, 909–917. [Google Scholar] [CrossRef]
- Takić, M.; Ranković, S.; Girek, Z.; Pavlović, S.; Jovanović, P.; Jovanović, V.; Šarac, I. Current Insights into the Effects of Dietary α-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 4909. [Google Scholar] [CrossRef]
- Wieczorek-Szukala, K.; Markiewicz, M.; Walczewska, A.; Zgorzynska, E. Docosahexaenoic Acid (DHA) Reduces LPS-Induced Inflammatory Response Via ATF3 Transcription Factor and Stimulates Src/Syk Signaling-Dependent Phagocytosis in Microglia. Cell Physiol. Biochem. 2023, 57, 411–425. [Google Scholar] [CrossRef]
- De Smedt-Peyrusse, V.; Sargueil, F.; Moranis, A.; Harizi, H.; Mongrand, S.; Layé, S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008, 105, 296–307. [Google Scholar] [CrossRef]
- AlAbduljader, H.; AlSaeed, H.; Alrabeea, A.; Sulaiman, A.; Haider, M.J.A.; Al-Mulla, F.; Ahmad, R.; Al-Rashed, F. Eicosapentaenoic Acid (EPA) Alleviates LPS-Induced Oxidative Stress via the PPARα–NF-κB Axis. bioRxiv 2025. bioRxiv:3509596. [Google Scholar] [CrossRef]
- Goraca, A.; Piechota, A.; Huk-Kolega, H. Effect of alpha-lipoic acid on LPS-induced oxidative stress in the heart. J. Physiol. Pharmacol. 2009, 60, 61–68. [Google Scholar]
- Skibska, B.; Goraca, A.; Skibska, A.; Stanczak, A. Effect of Alpha-Lipoic Acid on Rat Ventricles and Atria under LPS-Induced Oxidative Stress. Antioxidants 2022, 11, 734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Wei, H.; Hagen, T.; Frei, B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef]
- Makvandi, A.; Kowsar, R.; Hajian, M.; Mahdavi, A.H.; Tanhaei Vash, N.; Nasr-Esfahani, M.H. Alpha lipoic acid reverses the negative effect of LPS on mouse spermatozoa and developmental competence of resultant embryos in vitro. Andrology 2019, 7, 350–356. [Google Scholar] [CrossRef]
- Bo, Q.; Xie, Y.; Lin, Q.; Fu, L.; Hu, C.; Zhang, Z.; Meng, Q.; Xu, F.; Wang, G.; Miao, Z.; et al. Docosahexaenoic acid protects against lipopolysaccharide-induced fetal growth restriction via inducing the ubiquitination and degradation of NF-κB p65 in placental trophoblasts. J. Nutr. Biochem. 2023, 118, 109359. [Google Scholar] [CrossRef]
- Hsu, Y.-M.; Yin, M.-C. EPA or DHA enhanced oxidative stress and aging protein expression in brain of d-galactose treated mice. Biomedicine 2016, 6, 17. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Nenseter, M.S.; Drevon, C.A. Dietary polyunsaturates and peroxidation of low density lipoprotein. Curr. Opin. Lipidol. 1996, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, K.; Jiang, Y.; Huang, Q.; Wang, J.; Kan, W.; Wang, S. Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock 2002, 17, 433–438. [Google Scholar] [CrossRef]
- Huang, K.; Fishwild, D.M.; Wu, H.M.; Dedrick, R.L. Lipopolysaccharide-induced E-selectin expression requires continuous presence of LPS and is inhibited by bactericidal/permeability-increasing protein. Inflammation 1995, 19, 389–404. [Google Scholar] [CrossRef]
- Huang, K.; Fishwild, D.M.; Wu, H.-M.; Dedrick, R.L. Lipopolysaccharide (LPS)-Induced E-Selectin Expression and NF-κB Activation in Endothelial Cells Require Continuous Presence of LPS. In Vascular Endothelium: Responses to Injury; Catravas, J.D., Callow, A.D., Ryan, U.S., Eds.; Springer: Boston, MA, USA, 1996; pp. 283–284. ISBN 978-1-4613-0355-8. [Google Scholar]
- Wong, D.; Dorovini-Zis, K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J. Neuropathol. Exp. Neurol. 1996, 55, 225–235. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Sheu, W.H.-H.; Chiang, A.-N. Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms. Mol. Nutr. Food Res. 2015, 59, 751–762. [Google Scholar] [CrossRef]
- Yates, C.M.; Tull, S.P.; Madden, J.; Calder, P.C.; Grimble, R.F.; Nash, G.B.; Rainger, G.E. Docosahexaenoic Acid Inhibits the Adhesion of Flowing Neutrophils to Cytokine Stimulated Human Umbilical Vein Endothelial Cells12. J. Nutr. 2011, 141, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- ten Dijke, P.; Goumans, M.-J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lebrin, F.; Goumans, M.-J.; Jonker, L.; Carvalho, R.L.C.; Valdimarsdottir, G.; Thorikay, M.; Mummery, C.; Arthur, H.M.; ten Dijke, P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004, 23, 4018–4028. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Remolina, L.; Ollauri-Ibáñez, C.; Pérez-Roque, L.; Núñez-Gómez, E.; Pérez-Barriocanal, F.; López-Novoa, J.M.; Pericacho, M.; Rodríguez-Barbero, A. Circulating soluble endoglin modifies the inflammatory response in mice. PLoS ONE 2017, 12, e0188204. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, S.; Yao, D.C.; Savarraj, J.P.J.; Choi, H.A.; Chen, P.R.; Kim, E. Soluble Endoglin Stimulates Inflammatory and Angiogenic Responses in Microglia That Are Associated with Endothelial Dysfunction. Int. J. Mol. Sci. 2022, 23, 1225. [Google Scholar] [CrossRef]
- Bautch, V.L. Endoglin moves and shapes endothelial cells. Nat. Cell Biol. 2017, 19, 593–595. [Google Scholar] [CrossRef]
- Jin, Y.; Muhl, L.; Burmakin, M.; Wang, Y.; Duchez, A.-C.; Betsholtz, C.; Arthur, H.M.; Jakobsson, L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. 2017, 19, 639–652. [Google Scholar] [CrossRef]
- Conley, B.A.; Koleva, R.; Smith, J.D.; Kacer, D.; Zhang, D.; Bernabéu, C.; Vary, C.P.H. Endoglin controls cell migration and composition of focal adhesions: Function of the cytosolic domain. J. Biol. Chem. 2004, 279, 27440–27449. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, M.J.A.; Goumans, M.-J.T.H.; Hawinkels, L.J.A.C. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, W.; Chen, J.; Wang, X.; Wang, Y. AMP-activated protein kinase is required for the anti-adipogenic effects of alpha-linolenic acid. Nutr. Metab. 2015, 12, 10. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, S.; Yang, L.; Jiang, M.; Xin, Y.; Liao, X.; Li, Y.; Lu, J. The Antitumor Effects of α-Linolenic Acid. J. Pers. Med. 2024, 14, 260. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Li, S.; Wang, K.; Ke, R.; Shi, W.; Wang, J.; Yan, X.; Zhang, Q.; Wang, Q.; et al. Activation of AMPK inhibits TGF-β1-induced airway smooth muscle cells proliferation and its potential mechanisms. Sci. Rep. 2018, 8, 3624. [Google Scholar] [CrossRef]
- Thakur, S.; Viswanadhapalli, S.; Kopp, J.B.; Shi, Q.; Barnes, J.L.; Block, K.; Gorin, Y.; Abboud, H.E. Activation of AMP-Activated Protein Kinase Prevents TGF-β1–Induced Epithelial-Mesenchymal Transition and Myofibroblast Activation. Am. J. Pathol. 2015, 185, 2168–2180. [Google Scholar] [CrossRef]
- Gao, J.; Ye, J.; Ying, Y.; Lin, H.; Luo, Z. Negative regulation of TGF-β by AMPK and implications in the treatment of associated disorders. Acta Biochim. Biophys. Sin. 2018, 50, 523–531. [Google Scholar] [CrossRef]
- Rossi, E.; Bernabeu, C.; Smadja, D.M. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-β. Front. Med. 2019, 6, 10. [Google Scholar] [CrossRef]
- Li, N.-S.; Zou, J.-R.; Lin, H.; Ke, R.; He, X.-L.; Xiao, L.; Huang, D.; Luo, L.; Lv, N.; Luo, Z. LKB1/AMPK inhibits TGF-β1 production and the TGF-β signaling pathway in breast cancer cells. Tumor Biol. 2016, 37, 8249–8258. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.; Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Otsuki, T.; Kozu, K.; Numano, K.; Suzuki, K.; et al. Protective Roles of Endothelial AMP-Activated Protein Kinase Against Hypoxia-Induced Pulmonary Hypertension in Mice. Circ. Res. 2016, 119, 197–209. [Google Scholar] [CrossRef]
- Cheng, Y.-F.; Young, G.-H.; Lin, J.-T.; Jang, H.-H.; Chen, C.-C.; Nong, J.-Y.; Chen, P.-K.; Kuo, C.-Y.; Kao, S.-H.; Liang, Y.-J.; et al. Activation of AMP-Activated Protein Kinase by Adenine Alleviates TNF-Alpha-Induced Inflammation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2015, 10, e0142283. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Batirel, S.; Mantzoros, C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism 2014, 63, 1447–1454. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef]
- Reifen, R.; Karlinsky, A.; Stark, A.H.; Berkovich, Z.; Nyska, A. α-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J. Nutr. Biochem. 2015, 26, 1632–1640. [Google Scholar] [CrossRef]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-Inflammatory Effects of Alpha Linolenic Acid on Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef]
- Schunk, S.J.; Triem, S.; Schmit, D.; Zewinger, S.; Sarakpi, T.; Becker, E.; Hütter, G.; Wrublewsky, S.; Küting, F.; Hohl, M.; et al. Interleukin-1α Is a Central Regulator of Leukocyte-Endothelial Adhesion in Myocardial Infarction and in Chronic Kidney Disease. Circulation 2021, 144, 893–908. [Google Scholar] [CrossRef]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Saadi, S.; Platt, J.L. Constitutive Repression of Interleukin-1α in Endothelial Cells. Circ. Res. 2008, 102, 823–830. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.-D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Dawoud, H.; Bhatt, D.L.; Mason, R.P. Eicosapentaenoic Acid Improves Endothelial Nitric Oxide Bioavailability Via Changes in Protein Expression During Inflammation. J. Am. Heart Assoc. 2024, 13, e034076. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.-C.; Patrikios, I.; Stephanou, A. Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations. Nutrients 2023, 15, 4830. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolobarić, N.; Mihaljević, Z.; Suver Stević, M.; Marinčić Žagar, A.; Vari, S.G.; Drenjančević, I. Polyunsaturated Fatty Acid (PUFA) Composition of Growth Medium Changes the Atherogenic Potential of Human Aortic Endothelial Cells (HAECs) Following Endotoxin Stimulation. Biomedicines 2025, 13, 2706. https://doi.org/10.3390/biomedicines13112706
Kolobarić N, Mihaljević Z, Suver Stević M, Marinčić Žagar A, Vari SG, Drenjančević I. Polyunsaturated Fatty Acid (PUFA) Composition of Growth Medium Changes the Atherogenic Potential of Human Aortic Endothelial Cells (HAECs) Following Endotoxin Stimulation. Biomedicines. 2025; 13(11):2706. https://doi.org/10.3390/biomedicines13112706
Chicago/Turabian StyleKolobarić, Nikolina, Zrinka Mihaljević, Mirjana Suver Stević, Ana Marinčić Žagar, Sandor G. Vari, and Ines Drenjančević. 2025. "Polyunsaturated Fatty Acid (PUFA) Composition of Growth Medium Changes the Atherogenic Potential of Human Aortic Endothelial Cells (HAECs) Following Endotoxin Stimulation" Biomedicines 13, no. 11: 2706. https://doi.org/10.3390/biomedicines13112706
APA StyleKolobarić, N., Mihaljević, Z., Suver Stević, M., Marinčić Žagar, A., Vari, S. G., & Drenjančević, I. (2025). Polyunsaturated Fatty Acid (PUFA) Composition of Growth Medium Changes the Atherogenic Potential of Human Aortic Endothelial Cells (HAECs) Following Endotoxin Stimulation. Biomedicines, 13(11), 2706. https://doi.org/10.3390/biomedicines13112706