Salivary Biomarkers for the Diagnosis of Sjögren’s Syndrome: A Review of the Last Decade
Abstract
1. Introduction
1.1. Definition
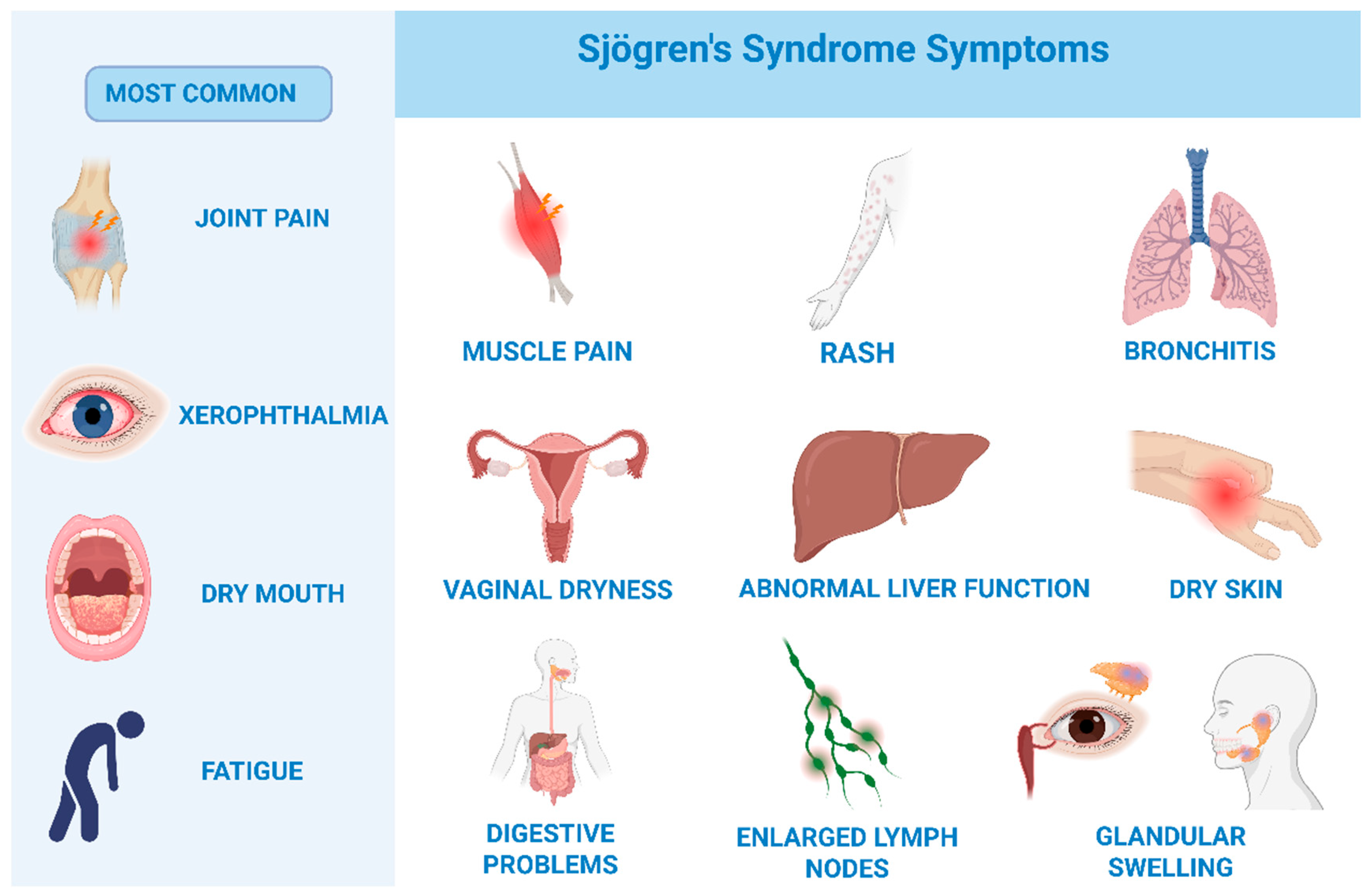
1.2. Symptoms
1.3. Epidemiology
1.4. Rationale for the Review
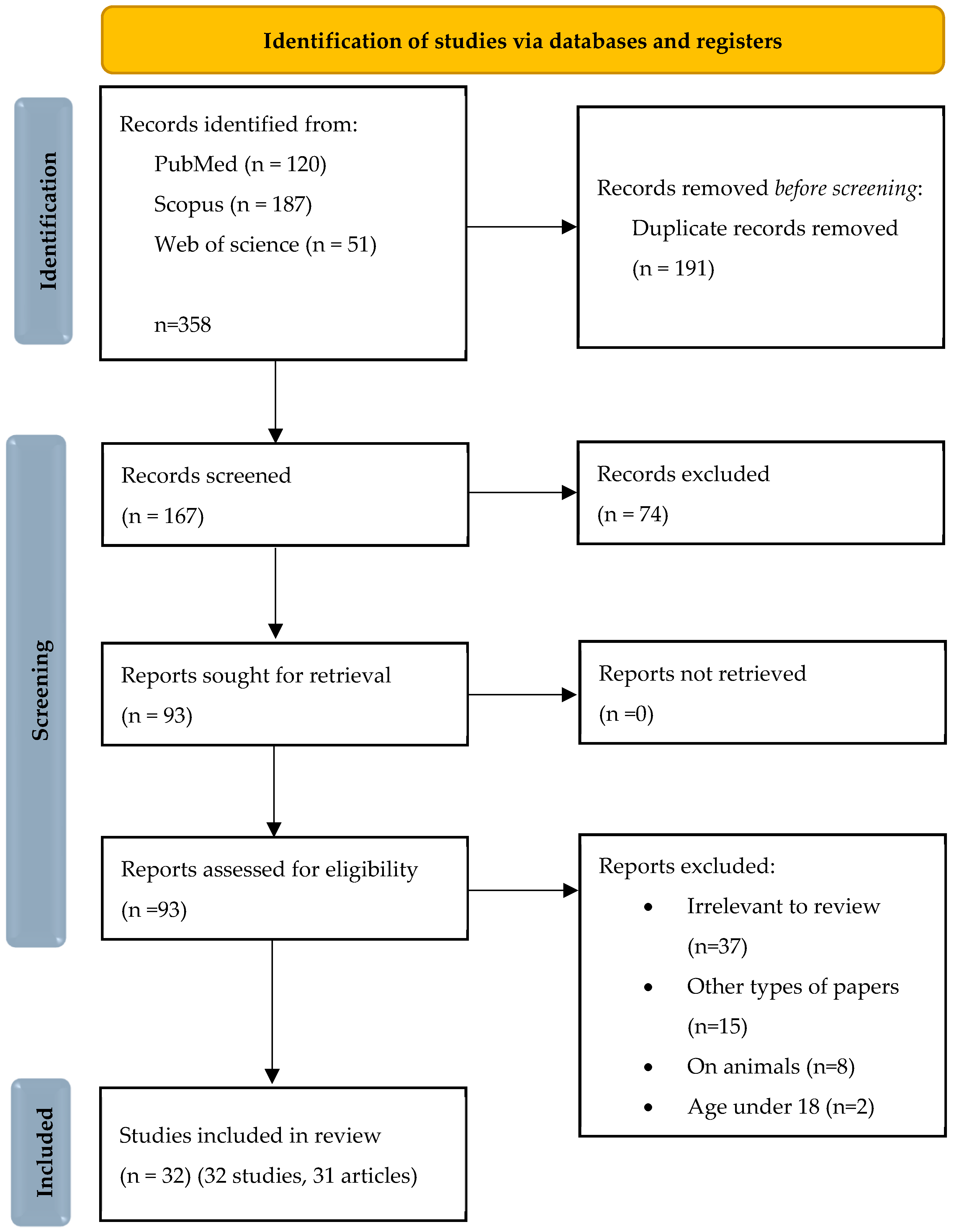
2. Materials and Methods
2.1. Search Strategy and Data Extraction
2.2. Registration
2.3. Quality Assessment and Critical Appraisal for the Systematic Review of Included Studies
3. Results
3.1. Metabolomic
3.1.1. Lactate, Alanine, and Malate
3.1.2. Leucine, Valine, and Isoleucine
3.1.3. Glucose, Glycerol, and Taurine
3.1.4. Metabolomic
3.1.5. Tryptophan, Tyrosine, and Aspartate
3.1.6. Salivary Adiponectin
3.2. Salivary Proteomics
3.2.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
3.2.2. S100 Protein Family, Annexin A2, and CD14
3.2.3. MUC5B, Prolactin-Inducible Protein (PIP)
3.2.4. β2-Microglobulin
3.2.5. Thrombospondin 1
3.3. Molecular Biomarkers
3.3.1. Non-Coding RNA
3.3.2. Imprinting Control Region (ICR) of the H19 Locus
3.3.3. microRNA
3.4. Autoimmune Biomarker Panel
3.4.1. Monomeric and Polymeric Anti-SSA/Ro52 Immunoglobulin A1 Isoforms
3.4.2. Inducible T Cell Co-Stimulator (ICOS)
3.4.3. Interleukin 6
3.4.4. Tissue-Specific Autoantibodies
3.4.5. Soluble Siglec-5
3.4.6. Free Light Chains (FLCs)
3.5. Enzymatic Markers in Saliva
3.5.1. Dipeptidyl Peptidase-4 (DPP4/CD26)
3.5.2. Matrix Metalloproteinase-9 (MMP9), Neutrophil Elastase (ELANE), Cathepsin G (CTSG), and Myeloblastin (PRTN3)
3.5.3. α-Enolase (ENO1)
4. Discussion
4.1. Metabolomic
4.2. Salivary Proteomics
Exosome-like Vesicles EVs
4.3. Molecular Biomarkers
4.4. Autoimmune Biomarker Panel
4.5. Enzymatic Markers in Saliva
4.6. Integrative Perspective and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, I.W.; Chen, H.C.; Lin, Y.F.; Yang, J.-H.; Chang, C.-C.; Chou, C.-T.; Lee, M.-T.M.; Chou, Y.-C.; Chen, C.-H.; Chen, Y.-T.; et al. Identification of susceptibility gene associated with female primary Sjögren’s syndrome in Han Chinese by genome-wide association study. Hum. Genet. 2016, 135, 1287–1294. [Google Scholar] [CrossRef]
- Khatri, B.; Tessneer, K.L.; Rasmussen, A.; Aghakhanian, F.; Reksten, T.R.; Adler, A.; Alevizos, I.; Anaya, J.-M.; Aqrawi, L.A.; Baecklund, E.; et al. Genome-wide association study identifies Sjögren’s risk loci with functional implications in immune and glandular cells. Nat. Commun. 2022, 13, 4287, Erratum in Nat. Commun. 2022, 13, 6519. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.; Busson, M.; Loiseau, P.; Cohen-Solal, J.; Lepage, V.; Charron, D.; Sibilia, J.; Mariette, X. In primary Sjögren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 2003, 48, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Imgenberg-Kreuz, J.; Rasmussen, A.; Sivils, K.; Nordmark, G. Genetics and epigenetics in primary Sjögren’s syndrome. Rheumatology 2021, 60, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- McCoy, S.S.; Sampene, E.; Baer, A.N. Association of Sjögren’s Syndrome With Reduced Lifetime Sex Hormone Exposure: A Case–Control Study. Arthritis Care Res. 2019, 72, 1315–1322. [Google Scholar] [CrossRef]
- Valtysdóttir, S.T.; Wide, L.; Hällgren, R. Low Serum Dehydroepiandrosterone Sulfate in Women with Primary Sjögren’s Syndrome as an Isolated Sign of Impaired HPA Axis Function. J. Rheumatol. 2001, 28, 1259–1265. [Google Scholar]
- Wang-Renault, S.-F.; Boudaoud, S.; Nocturne, G.; Roche, E.; Sigrist, N.; Daviaud, C.; Tinggaard, A.B.; Renault, V.; Deleuze, J.-F.; Mariette, X.; et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2018, 77, 133–140. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, H.; Liu, N.; Li, Y.; Chen, J. Advances in Pathogenesis of Sjögren’s Syndrome. J. Immunol. Res. 2021, 2021, 5928232. [Google Scholar] [CrossRef]
- Parisis, D.; Chivasso, C.; Perret, J.; Soyfoo, M.S.; Delporte, C. Current state of knowledge on primary sjögren’s syndrome, an autoimmune exocrinopathy. J. Clin. Med. 2020, 9, 2299. [Google Scholar] [CrossRef]
- Del Papa, N.; Minniti, A.; Lorini, M.; Carbonelli, V.; Maglione, W.; Pignataro, F.; Montano, N.; Caporali, R.; Vitali, C. The role of interferons in the pathogenesis of Sjögren’s syndrome and future therapeutic perspectives. Biomolecules 2021, 11, 251. [Google Scholar] [CrossRef]
- Meng, F.; Ren, S.; Meng, Y.; Tao, N.; Zhang, J. Association Between Stressful Life Events and Female Primary Sjogren’s Syndrome and Their Role in Disease Activity: A Retrospective Case–Control Study in China. Neuropsychiatr. Dis. Treat. 2021, 17, 213–220. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjögren’s Syndrome. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 273–285. [Google Scholar] [CrossRef]
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Manoussakis, M.N.; Boiu, S.; Korkolopoulou, P.; Kapsogeorgou, E.K.; Kavantzas, N.; Ziakas, P.; Patsouris, E.; Moutsopoulos, H.M. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007, 56, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Hillen, M.R.; A Ververs, F.; A Kruize, A.; A Van Roon, J. Dendritic cells, T-cells and epithelial cells: A crucial interplay in immunopathology of primary Sjögren’s syndrome. Expert Rev. Clin. Immunol. 2014, 10, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2021, 22, 9–25. [Google Scholar] [CrossRef]
- Maleki-Fischbach, M.; Kastsianok, L.; Koslow, M.; Chan, E.D. Manifestations and management of Sjögren’s disease. Arthritis Res. Ther. 2024, 26, 43. [Google Scholar] [CrossRef]
- Jonsson, R.; Brokstad, K.A.; Jonsson, M.V.; Delaleu, N.; Skarstein, K. Current concepts on Sjögren’s syndrome—Classification criteria and biomarkers. Eur. J. Oral Sci. 2018, 126, 37–48. [Google Scholar] [CrossRef]
- André, F.; Böckle, B.C. Sjögren’s syndrome. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 980–1002. [Google Scholar] [CrossRef]
- Chalifoux, S.L.; Konyn, P.G.; Choi, G.; Saab, S. Extrahepatic manifestations of primary biliary cholangitis. Gut Liver 2017, 11, 771–780. [Google Scholar] [CrossRef]
- Scofield, R.H.; Bruner, G.R.; Harley, J.B.; Namjou, B. Autoimmune thyroid disease is associated with a diagnosis of secondary Sjögren’s syndrome in familial systemic lupus. Ann. Rheum. Dis. 2007, 66, 410–413. [Google Scholar] [CrossRef]
- Björk, A.; Mofors, J.; Wahren-Herlenius, M. Environmental factors in the pathogenesis of primary Sjögren’s syndrome. J. Intern. Med. 2020, 287, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.N.; Walitt, B. Update on Sjögren Syndrome and Other Causes of Sicca in Older Adults. Rheum. Dis. Clin. N. Am. 2018, 44, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Stefanski, A.-L.; Tomiak, C.; Pleyer, U.; Dietrich, T.; Burmester, G.R.; Dörner, T. The diagnosis and treatment of Sjögren’s syndrome. Dtsch. Aerzteblatt Online 2017, 114, 354–361. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Systematic Review Registration OSF. Available online: https://doi.org/10.17605/OSF.IO/FZX4T (accessed on 18 September 2025).
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 August 2020).
- OCEBM. OCEBM Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 22 April 2020).
- Florezi, G.P.; Barone, F.P.; Izidoro, M.A.; Soares, J.M., Jr.; Coutinho-Camillo, C.M.; Lourenço, S.V. Targeted saliva metabolomics in Sjögren’s syndrome. Clinics 2024, 79, 100459. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305, Erratum in Signal Transduct Target Ther. 2022, 7, 372. [Google Scholar] [CrossRef]
- Gemelli, T.; de Andrade, R.B.; Rojas, D.B.; Zanatta, Â.; Schirmbeck, G.H.; Funchal, C.; Wajner, M.; Dutra-Filho, C.S.; Wannmacher, C.M.D. Chronic Exposure to β-Alanine Generates Oxidative Stress and Alters Energy Metabolism in Cerebral Cortex and Cerebellum of Wistar Rats. Mol. Neurobiol. 2017, 55, 5101–5110. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Alt-Holland, A.; Huang, X.; Mendez, T.; Singh, M.L.; Papas, A.S.; Cimmino, J.; Bairos, T.; Tzavaras, E.; Foley, E.; Pagni, S.E.; et al. Identification of Salivary Metabolic Signatures Associated with Primary Sjögren’s Disease. Molecules 2023, 28, 5891. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2012, 46, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Bosman, P.; Pichon, V.; Acevedo, A.C.; Modesto, F.M.B.; Paula, L.M.; Le Pottier, L.; Pers, J.O.; Chardin, H.; Combès, A. Identification of potential salivary biomarkers for Sjögren’s syndrome with an untargeted metabolomic approach. Metabolomics 2023, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Setti, G.; Righi, V.; Mucci, A.; Panari, L.; Bernardelli, G.; Tarentini, E.; Gambini, A.; Consolo, U.; Generali, L.; Magnoni, C.; et al. Metabolic Profile of Whole Unstimulated Saliva in Patients with Sjögren’s Syndrome. Metabolites 2023, 13, 348. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Med. Rep. 2021, 24, 605. [Google Scholar] [CrossRef]
- Li, Z.; Mu, Y.; Guo, C.; You, X.; Liu, X.; Li, Q.; Sun, W. Analysis of the saliva metabolic signature in patients with primary Sjögren’s syndrome. PLoS ONE 2022, 17, e0269275. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2018, 23, 1407–1414. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Jensen, J.L.; Fromreide, S.; Galtung, H.K.; Skarstein, K. Expression of NGAL-specific cells and mRNA levels correlate with inflammation in the salivary gland, and its overexpression in the saliva, of patients with primary Sjögren’s syndrome. Autoimmunity 2020, 53, 333–343. [Google Scholar] [CrossRef]
- Pawar, R.D.; Goilav, B.; Xia, Y.; Zhuang, H.; Herlitz, L.; Reeves, W.H.; Putterman, C. Serum autoantibodies in pristane induced lupus are regulated by neutrophil gelatinase associated lipocalin. Clin. Immunol. 2014, 154, 49–65. [Google Scholar] [CrossRef]
- Finamore, F.; Cecchettini, A.; Ceccherini, E.; Signore, G.; Ferro, F.; Rocchiccioli, S.; Baldini, C. Characterization of extracellular vesicle cargo in Sjögren’s syndrome through a swath-ms proteomics approach. Int. J. Mol. Sci. 2021, 22, 4864. [Google Scholar] [CrossRef]
- Kozlyuk, N.; Monteith, A.J.; Garcia, V.; Damo, S.M.; Skaar, E.P.; Chazin, W.J. S100 Proteins in the Innate Immune Response to Pathogens. Adv. Struct. Saf. Stud. 2019, 1929, 275–290. [Google Scholar] [CrossRef]
- Di Giorgi, N.; Cecchettini, A.; Michelucci, E.; Signore, G.; Ceccherini, E.; Ferro, F.; Elefante, E.; Tani, C.; Baldini, C.; Rocchiccioli, S. Salivary Proteomics Markers for Preclinical Sjögren’s Syndrome: A Pilot Study. Biomolecules 2022, 12, 738. [Google Scholar] [CrossRef]
- Barrera, M.-J.; Aguilera, S.; Veerman, E.; Quest, A.F.G.; Díaz-Jiménez, D.; Urzúa, U.; Cortés, J.; González, S.; Castro, I.; Molina, C.; et al. Salivary mucins induce a Toll-like receptor 4-mediated pro-inflammatory response in human submandibular salivary cells: Are mucins involved in Sjögren’s syndrome? Rheumatology 2015, 54, 1518–1527. [Google Scholar] [CrossRef]
- Seror, R.; Theander, E.; Brun, J.G.; Ramos-Casals, M.; Valim, V.; Dörner, T.; Bootsma, H.; Tzioufas, A.; Solans-Laqué, R.; Mandl, T.; et al. Validation of EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann. Rheum. Dis. 2015, 74, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Garza-García, F.; Delgado-García, G.; Garza-Elizondo, M.; Ceceñas-Falcón, L.Á.; Galarza-Delgado, D.; Riega-Torres, J. Salivary β2-microglobulin positively correlates with ESSPRI in patients with primary Sjögren’s syndrome. Rev. Bras. Reum. 2017, 57, 182–184. [Google Scholar] [CrossRef]
- Delaleu, N.; Mydel, P.; Brun, J.G.; Jonsson, M.V.; Alimonti, A.; Jonsson, R. Sjögren’s syndrome patients with ectopic germinal centers present with a distinct salivary proteome. Rheumatology 2016, 55, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Dee, Z.; Pidcock, K.; Gutierrez, L.S. Thrombospondin-1: Multiple Paths to Inflammation. Mediat. Inflamm. 2011, 2011, 296069. [Google Scholar] [CrossRef]
- Salomonsson, S.; Jonsson, M.V.; Skarstein, K.; Brokstad, K.A.; Hjelmstrom, P.; Wahren-Herlenius, M.; Jonsson, R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003, 48, 3187–3201. [Google Scholar] [CrossRef]
- Cross, T.; Haug, K.B.F.; Brusletto, B.S.; Ommundsen, S.K.; Trøseid, A.-M.S.; Aspelin, T.; Olstad, O.K.; Aass, H.C.D.; Galtung, H.K.; Utheim, T.P.; et al. Non-Coding RNA in Salivary Extracellular Vesicles: A New Frontier in Sjögren’s Syndrome Diagnostics? Int. J. Mol. Sci. 2023, 24, 13409. [Google Scholar] [CrossRef]
- O’brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, P.; Goules, A.V.; Tzioufas, A.G. Epigenetic alterations in Sjögren’s syndrome patient saliva. Clin. Exp. Immunol. 2020, 202, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, T.M.; Mukhlif, M.Y.; Hossein, A.; S, R.J.; Thakur, V.; Mishra, S.; Chauhan, A.S.; Shuhata, M.H.; Alnajar, M.J.; Hammoodi, H.A. lncRNA H19 as a molecular rheostat in autoimmunity: Orchestrating miRNA-mediated gene regulation and immune cell reprogramming. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 14917–14939. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. Int. J. Mol. Sci. 2024, 25, 7666. [Google Scholar] [CrossRef]
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Combined serum anti-SSA/Ro and salivary TRIM29 reveals promising high diagnostic accuracy in patients with primary Sjögren’s syndrome. PLoS ONE 2021, 16, e0258428. [Google Scholar] [CrossRef]
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Distinct microRNA expression profiles in saliva and salivary gland tissue differentiate patients with primary Sjögren’s syndrome from non-Sjögren’s sicca patients. J. Oral Pathol. Med. 2020, 49, 1044–1052. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, R.; Chen, J.; Qi, E.; Zhou, S.; Wang, Y.; Fu, Q.; Chen, R.; Fang, X. Let-7i-5p Regulation of Cell Morphology and Migration Through Distinct Signaling Pathways in Normal and Pathogenic Urethral Fibroblasts. Front. Bioeng. Biotechnol. 2020, 8, 428. [Google Scholar] [CrossRef]
- Zhao, Y. CD26 in autoimmune diseases: The other side of “moonlight protein”. Int. Immunopharmacol. 2019, 75, 105757. [Google Scholar] [CrossRef]
- Najm, A.; Masson, F.; Preuss, P.; Georges, S.; Ory, B.; Quillard, T.; Sood, S.; Goodyear, C.S.; Veale, D.J.; Fearon, U.; et al. MicroRNA-17-5p Reduces Inflammation and Bone Erosions in Mice With Collagen-Induced Arthritis and Directly Targets the JAK/STAT Pathway in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol. 2020, 72, 2030–2039. [Google Scholar] [CrossRef]
- Chiang, S.; Grogan, T.; Kamounah, S.; Wei, F.; Tayob, N.; Kim, J.Y.; Park, J.K.; Akin, D.; A Elashoff, D.; Pedersen, A.M.L.; et al. Distinctive profile of monomeric and polymeric anti-SSA/Ro52 immunoglobulin A1 isoforms in saliva of patients with primary Sjögren’s syndrome and Sicca. RMD Open 2024, 10, e003666. [Google Scholar] [CrossRef]
- Monteiro, R.C. The Role of IgA and IgA Fc Receptors as Anti-Inflammatory Agents. J. Clin. Immunol. 2010, 30, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.M.; Costa, P.A.C.; Diniz, S.Q.; Henriques, P.M.; Kano, F.S.; Tada, M.S.; Pereira, D.B.; Soares, I.S.; Martins-Filho, O.A.; Jankovic, D.; et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog. 2017, 13, e1006484. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jin, Y.; Zhao, R.; Xue, Z.; Ji, J. Expression of ICOS in the salivary glands of patients with primary Sjogren’s syndrome and its molecular mechanism. Mol. Med. Rep. 2022, 26, 348. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Moreno-Quispe, L.A.; Serrano, J.; Virto, L.; Sanz, M.; Ramírez, L.; Fernández-Castro, M.; Hernández, G.; López-Pintor, R.M. Association of salivary inflammatory biomarkers with primary Sjögren’s syndrome. J. Oral Pathol. Med. 2020, 49, 940–947. [Google Scholar] [CrossRef]
- Grisius, M.M.; Bermudez, D.K.; Fox, P.C. Salivary and serum interleukin 6 in primary Sjögren’s syndrome. J. Rheumatol. 1997, 24, 1089–1091. [Google Scholar]
- Tishler, M.; Yaron, I.; Shirazi, I.; Yossipov, Y.; Yaron, M. Increased salivary interleukin-6 levels in patients with primary Sjögren’s syndrome. Rheumatol. Int. 1999, 18, 125–127. [Google Scholar] [CrossRef]
- Maeda, K.; Mehta, H.; Drevets, D.A.; Coggeshall, K.M. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 2010, 115, 4699–4706. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M.; Zerva, L.V. Anti-Ro (SSA)/La (SSB) antibodies and Sjögren’s syndrome. Clin. Rheumatol. 1990, 9, 123–131. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, C.; Wang, T.; Brooks, S.; Ford, R.J.; Lin-Lee, Y.C.; Kasianowicz, A.; Kumar, V.; Martin, L.; Liang, P.; et al. Development of Autoimmunity in IL-14α-Transgenic Mice. J. Immunol. 2006, 177, 5676–5686. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Chen, J.; Shao, M.; Zhang, R.; Liang, Y.; Zhang, X.; Zhang, X.; Zhang, Q.; Li, F.; et al. Tissue-Specific Autoantibodies Improve Diagnosis of Primary Sjögren’s Syndrome in the Early Stage and Indicate Localized Salivary Injury. J. Immunol. Res. 2019, 2019, 3642937. [Google Scholar] [CrossRef]
- Li, Y.-H.; Gao, Y.-P.; Dong, J.; Shi, L.-J.; Sun, X.-L.; Li, R.; Zhang, X.-W.; Liu, Y.; Long, L.; He, J.; et al. Identification of a novel autoantibody against self-vimentin specific in secondary Sjögren’s syndrome. Arthritis Res. Ther. 2018, 20, 30. [Google Scholar] [CrossRef]
- Pepin, M.; Mezouar, S.; Pegon, J.; Muczynski, V.; Adam, F.; Bianchini, E.P.; Bazaa, A.; Proulle, V.; Rupin, A.; Paysant, J.; et al. Soluble Siglec-5 associates to PSGL-1 and displays anti-inflammatory activity. Sci. Rep. 2016, 6, 37953. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Baek, S.; Koh, J.H.; Kim, J.-W.; Kim, S.-Y.; Chung, S.-H.; Choi, S.S.; Cho, M.-L.; Kwok, S.-K.; et al. Soluble siglec-5 is a novel salivary biomarker for primary Sjogren’s syndrome. J. Autoimmun. 2019, 100, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, P.; Christudoss, P.; Kabeerdoss, J.; Mandal, S.K.; Aithala, R.; Mahasampath, G.; Job, V.; Danda, D. Diagnostic accuracy of salivary and serum-free light chain assays in primary Sjögren’s syndrome: A pilot study. Int. J. Rheum. Dis. 2016, 20, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Kormelink, T.G.; Pardo, A.; Knipping, K.; Buendía-Roldán, I.; García-De-Alba, C.; Blokhuis, B.R.; Selman, M.; A Redegeld, F. Immunoglobulin Free Light Chains Are Increased in Hypersensitivity Pneumonitis and Idiopathic Pulmonary Fibrosis. PLoS ONE 2011, 6, e25392. [Google Scholar] [CrossRef]
- Konen, F.F.; Seeliger, T.; Schwenkenbecher, P.; Gingele, S.; Jendretzky, K.F.; Sühs, K.-W.; Ernst, D.; Witte, T.; Skripuletz, T. Saliva Free Light Chains in Patients with Neuro-Sjögren. Biomedicines 2022, 10, 2470. [Google Scholar] [CrossRef]
- Garreto, L.; Charneau, S.; Mandacaru, S.C.; Nóbrega, O.T.; de Araújo, C.N.; Tonet, A.C.; Modesto, F.M.B.; Paula, L.M.; de Sousa, M.V.; Santana, J.M.; et al. Mapping Salivary Proteases in Sjögren’s Syndrome Patients Reveals Overexpression of Dipeptidyl Peptidase-4/CD26. Front. Immunol. 2021, 12, 686480. [Google Scholar] [CrossRef]
- Hopsu-Havu, V.K.; Glenner, G.G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-?-naphthylamide. Histochemie 1966, 7, 197–201. [Google Scholar] [CrossRef]
- Choi, S. (Ed.) Encyclopedia of Signaling Molecules; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Zhang, K.; Yang, J.; Fuchs, H.; Fan, H. Involvement of CD26 in Differentiation and Functions of Th1 and Th17 Subpopulations of T Lymphocytes. J. Immunol. Res. 2021, 2021, 6671410. [Google Scholar] [CrossRef]
- Noll, B.; Mougeot, F.B.; Brennan, M.T.; Mougeot, J.-L.C. Regulation of MMP9 transcription by ETS1 in immortalized salivary gland epithelial cells of patients with salivary hypofunction and primary Sjögren’s syndrome. Sci. Rep. 2022, 12, 14552. [Google Scholar] [CrossRef]
- Zeng, W.; Song, Y.; Wang, R.; He, R.; Wang, T. Neutrophil elastase: From mechanisms to therapeutic potential. J. Pharm. Anal. 2023, 13, 355–366. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, H.; Zuo, X.; Luo, H. Cathepsin G and Its Role in Inflammation and Autoimmune Diseases. Arch. Rheumatol. 2018, 33, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Voyich, J.M.; Burlak, C.; DeLeo, F.R. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. 2005, 53, 505–517. [Google Scholar]
- Wei, P.; Xing, Y.; Li, B.; Chen, F.; Hua, H. Proteomics-Based Analysis Indicating α-Enolase as a Potential Biomarker in Primary Sjögren’s Syndrome. Gland. Surg. 2020, 9, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, J.; Guo, J.; Li, Y.; Lian, S.; Guo, W.; Yang, H.; Kong, F.; Zhen, L.; Guo, L.; et al. Progress in the biological function of alpha-enolase. Anim. Nutr. 2016, 2, 12–17. [Google Scholar] [CrossRef]
- Cui, L.; Elzakra, N.; Xu, S.; Xiao, G.G.; Yang, Y.; Hu, S. Investigation of three potential autoantibodies in Sjogren’s syndrome and associated MALT lymphoma. Oncotarget 2017, 8, 30039–30049. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yanoshita, R. Exosome-Like Vesicles with Dipeptidyl Peptidase IV in Human Saliva. Biol. Pharm. Bull. 2008, 31, 1059–1062. [Google Scholar] [CrossRef]
- Sjoqvist, S.; Otake, K. Saliva and Saliva Extracellular Vesicles for Biomarker Candidate Identification—Assay Development and Pilot Study in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 5237. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, J.; Lu, Y.; Lin, P.; Lin, Y.; Zheng, Y.; Xu, R.; Mai, Z.; Guo, B.; Zhao, X. New frontiers in salivary extracellular vesicles: Transforming diagnostics, monitoring, and therapeutics in oral and systemic diseases. J. Nanobiotechnol. 2024, 22, 171. [Google Scholar] [CrossRef]
- Cecchettini, A.; Finamore, F.; Puxeddu, I.; Ferro, F.; Baldini, C. Salivary extracellular vesicles versus whole saliva: New perspectives for the identification of proteomic biomarkers in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 118), 240–248. [Google Scholar]
- Vyas, B.; Khatiashvili, A.; Galati, L.; Ngo, K.; Gildener-Leapman, N.; Larsen, M.; Lednev, I.K. Raman hyperspectroscopy of saliva and machine learning for Sjögren’s disease diagnostics. Sci. Rep. 2024, 14, 11135. [Google Scholar] [CrossRef]
- Herrala, M.; Mikkonen, J.J.W.; Pesonen, P.; Lappalainen, R.; Tjäderhane, L.; Niemelä, R.K.; Seitsalo, H.; Salo, T.; Myllymaa, S.; Kullaa, A.M. Variability of salivary metabolite levels in patients with Sjögren’s syndrome. J. Oral Sci. 2021, 63, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, G.; Saegusa, J.; Irino, Y.; Tanaka, S.; Tsuda, K.; Takahashi, S.; Sendo, S.; Morinobu, A. Metabolomics analysis of saliva from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 182, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-C.; Guo, C.-L.; Li, Z.; You, X.; Liu, X.-Y.; Su, J.-M.; Zhao, S.-J.; Mu, Y.; Sun, W.; Li, Q. Data-Independent Acquisition-Based Quantitative Proteomic Analysis Reveals Potential Salivary Biomarkers of Primary Sjögren’s Syndrome. Chin. Med Sci. J. 2024, 39, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.; Tashbayev, B.; Utheim, Ø.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Singh, P.B.; Palm, Ø.; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjögren’s syndrome correlate with clinical ocular and oral manifestations. Sci. Rep. 2019, 9, 7319. [Google Scholar] [CrossRef]
- Cecchettini, A.; Finamore, F.; Ucciferri, N.; Donati, V.; Mattii, L.; Polizzi, E.; Ferro, F.; Sernissi, F.; Mosca, M.; Bombardieri, S.; et al. Phenotyping multiple subsets in Sjögren’s syndrome: A salivary proteomic SWATH-MS approach towards precision medicine. Clin. Proteom. 2019, 16, 26. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Guerreiro, E.M.; Øvstebø, R.; Thiede, B.; Utheim, T.P.; Chen, X.; Utheim, Ø.A.; Palm, Ø.; Skarstein, K.; et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 2019, 21, 181. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Øvstebø, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef]
- Deutsch, O.; Krief, G.; Konttinen, Y.T.; Zaks, B.; Wong, D.T.; Aframian, D.J.; Palmon, A. Identification of Sjögren’s syndrome oral fluid biomarker candidates following high-abundance protein depletion. Rheumatology 2014, 54, 884–890. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.A.; Baer, A.N.; Challacombe, S.; Lanfranchi, H.; Schiodt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: A data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheum. 2017, 76, 9–16. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S. Outcome Measures in Primary Sjögren’s Syndrome. Arthritis Care Res. 2020, 72, 134–149. [Google Scholar] [CrossRef]
- Navazesh, M.; Kumar, S.K. Measuring salivary flow. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef]

| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Results of studies on humans; patients age from 18 to 99 years, both genders | Results of studies without human participants (e.g., studies on animals or in vitro) |
| Exposure | Sjögren’s syndrome | Only SICCA |
| Comparison | Not applicable | |
| Outcomes | Saliva components as potential biomarkers | Salivary gland biopsy |
| Study design | Original research articles, pilot studies, and letters to the editor published in English since 2014 | Literature reviews, case reports, commentaries and others in a language other than English |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, V.E.; Skutnik-Radziszewska, A.; Zalewska, E.; Zalewska, A. Salivary Biomarkers for the Diagnosis of Sjögren’s Syndrome: A Review of the Last Decade. Biomedicines 2025, 13, 2664. https://doi.org/10.3390/biomedicines13112664
Lis VE, Skutnik-Radziszewska A, Zalewska E, Zalewska A. Salivary Biomarkers for the Diagnosis of Sjögren’s Syndrome: A Review of the Last Decade. Biomedicines. 2025; 13(11):2664. https://doi.org/10.3390/biomedicines13112664
Chicago/Turabian StyleLis, Virginia Ewa, Anna Skutnik-Radziszewska, Ewa Zalewska, and Anna Zalewska. 2025. "Salivary Biomarkers for the Diagnosis of Sjögren’s Syndrome: A Review of the Last Decade" Biomedicines 13, no. 11: 2664. https://doi.org/10.3390/biomedicines13112664
APA StyleLis, V. E., Skutnik-Radziszewska, A., Zalewska, E., & Zalewska, A. (2025). Salivary Biomarkers for the Diagnosis of Sjögren’s Syndrome: A Review of the Last Decade. Biomedicines, 13(11), 2664. https://doi.org/10.3390/biomedicines13112664









